In this double-blind, randomized, multicenter study, ceftobiprole was shown to be noninferior to combined ceftazidime/linezolid in patients with hospital-acquired pneumonia (HAP) for clinical cure. Ceftobiprole is considered a safe and effective bactericidal antibiotic for empiric treatment of HAP (excluding ventilator-associated pneumonia).
Keywords: ceftazidime, ceftobiprole, linezolid, hospital-acquired pneumonia, ventilator-associated pneumonia
Abstract
Background. Ceftobiprole, the active moiety of ceftobiprole medocaril, is a novel broad-spectrum cephalosporin, with bactericidal activity against a wide range of gram-positive bacteria, including Staphylococcus aureus (including methicillin-resistant strains) and penicillin- and ceftriaxone-resistant pneumococci, and gram-negative bacteria, including Enterobacteriaceae and Pseudomonas aeruginosa.
Methods. This was a double-blind, randomized, multicenter study of 781 patients with hospital-acquired pneumonia (HAP), including 210 with ventilator-associated pneumonia (VAP). Treatment was intravenous ceftobiprole 500 mg every 8 hours, or ceftazidime 2 g every 8 hours plus linezolid 600 mg every 12 hours; primary outcome was clinical cure at the test-of-cure visit.
Results. Overall cure rates for ceftobiprole vs ceftazidime/linezolid were 49.9% vs 52.8% (intent-to-treat [ITT], 95% confidence interval [CI] for the difference, −10.0 to 4.1) and 69.3% vs 71.3% (clinically evaluable [CE], 95% CI, −10.0 to 6.1). Cure rates in HAP (excluding VAP) patients were 59.6% vs 58.8% (ITT, 95% CI, −7.3 to 8.8), and 77.8% vs 76.2% (CE, 95% CI, −6.9 to 10.0). Cure rates in VAP patients were 23.1% vs 36.8% (ITT, 95% CI, −26.0 to −1.5) and 37.7% vs 55.9% (CE, 95% CI, −36.4 to 0). Microbiological eradication rates in HAP (excluding VAP) patients were, respectively, 62.9% vs 67.5% (microbiologically evaluable [ME], 95% CI, −16.7 to 7.6), and in VAP patients 30.4% vs 50.0% (ME, 95% CI, −38.8 to −0.4). Treatment-related adverse events were comparable for ceftobiprole (24.9%) and ceftazidime/linezolid (25.4%).
Conclusions. Ceftobiprole is a safe and effective bactericidal antibiotic for the empiric treatment of HAP (excluding VAP). Further investigations are needed before recommending the use of ceftobiprole in VAP patients.
Clinical Trials Registration. NCT00210964, NCT00229008.
Hospital-acquired pneumonia (HAP) is the second most frequent hospital-acquired infection in adults, and a leading cause of death in hospitalized patients [1, 2]. Common pathogens in HAP are Staphylococcus aureus (including methicillin-resistant S. aureus [MRSA]), Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella species, Escherichia coli, and Enterobacter species; Streptococcus pneumoniae may also play a role in HAP [3].
There is a significant unmet need for new antibiotics effective against bacterial pathogens responsible for HAP. Treatment guidelines for HAP recommend rapid empiric therapy with combinations of antibiotics based on local resistance patterns and patient risk factors [1].
Ceftobiprole, the active moiety of its prodrug ceftobiprole medocaril, is a novel cephalosporin that binds tightly to the penicillin-binding proteins (PBPs), including those responsible for β-lactam resistance in staphylococci (PBP2a) and pneumococci (PBP2x), with a low propensity for resistance development [4–6]. It demonstrates potent activity against gram-positive pathogens, including MRSA and penicillin-resistant S. pneumoniae, together with activity against gram-negative pathogens commonly associated with pneumonia [7–14]. Ceftobiprole demonstrates in vitro activity against S. aureus resistant to linezolid, vancomycin, and daptomycin [15], and ceftriaxone-resistant S. pneumoniae [16, 17]. It also demonstrates superior bactericidal activity to daptomycin, linezolid, and vancomycin in several animal models of endocarditis [18, 19], osteomyelitis [20, 21], meningitis [22], mediastinitis [23], and peritonitis [24], and good efficacy in animal models of pneumonia and skin infections [25, 26]. Ceftobiprole is therefore a promising agent with potential utility for difficult-to-treat infections.
Ceftobiprole has been shown to be a safe and efficacious option in the treatment of patients with severe community-acquired pneumonia [27].The aim of this study was to demonstrate that in patients with HAP, ceftobiprole is noninferior to combined treatment with ceftazidime and linezolid for the primary endpoint of clinical cure at the test-of-cure (TOC) visit.
METHODS
Study Design
This was a multicenter, double-blind, randomized controlled trial conducted between 6 April 2005 and 22 May 2007 at 157 sites in Europe, North and South America, and the Asia-Pacific region, following approval by independent ethics committees/institutional review boards for each site. Signed written informed consent was obtained for all participants prior to enrollment.
Participants
Men and women aged 18 years or older were eligible for enrollment if they had a clinical diagnosis of pneumonia after at least 72 hours of hospitalization or stay in a chronic care facility, clinical signs or symptoms of pneumonia (at least 2 of purulent respiratory secretion, tachypnea, or hypoxemia), fever or leukocytosis/leukopenia, new or persistent radiographic infiltrates, and an Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥8 and ≤25. Ventilator-associated pneumonia (VAP) was defined as pneumonia developing >48 hours after onset of mechanical ventilation. Of the 781 HAP patients enrolled, 210 had VAP.
The main exclusion criteria included severe renal impairment (calculated creatinine clearance rate <30 mL/minute or oliguria <20 mL/hour unresponsive to fluid challenge) or hepatic dysfunction (at least 3 times the upper limit of normal for total bilirubin, alanine aminotransferase or aspartate aminotransferase), evidence of infection with ceftazidime- or ceftobiprole-resistant pathogens, and clinical conditions that would interfere with efficacy assessment, such as sustained shock, active tuberculosis, lung abscess, or post-obstructive pneumonia. With predefined exceptions, participants must not have had systemic antibiotic treatment for >24 hours in the 48 hours before enrollment.
Interventions
Eligible patients were randomized 1:1 to ceftobiprole 500 mg every 8 hours as a 120-minute intravenous infusion, plus placebo every 12 hours as a 60-minute intravenous infusion, or ceftazidime 2 g every 8 hours as a 120-minute intravenous infusion plus linezolid 600 mg every 12 hours as a 60-minute intravenous infusion. For blinding reasons, the 120-minute infusion time was longer than the recommended infusion time in the ceftazidime label. Planned treatment duration was 7 days, to a maximum of 14 days. Additional open-label treatment with a fluoroquinolone or an aminoglycoside was allowed for patients at risk of pseudomonal infection.
Assessments
Clinical assessments were performed at baseline, at days 4, 8, and 14, and at the end of treatment (EOT, within 24 hours after last study drug infusion). The TOC visit was 7–14 days, and the late follow-up visit 28–35 days, after EOT. Mandatory laboratory safety tests were performed at baseline and EOT, and mandatory chest radiographs were performed at baseline and TOC. Samples for bacterial culture and Gram stain were obtained predose, and on days 4, 8, and 14 if sputum or other respiratory tract specimens were available. Blood samples for bacterial culture were obtained at baseline, and on days 4, 8, and 14 if clinically indicated. Pathogen identification and susceptibility testing was confirmed by a central laboratory according to Clinical and Laboratory Standards Institute methodology.
Safety and tolerability were assessed from treatment-emergent adverse events (AEs), changes in pre- to posttreatment physical examination results, vital signs, 12-lead electrocardiogram results, and clinical laboratory results. An independent data monitoring committee assessed blinded safety-related data during the study to ensure the safety of study participants.
Sample Size and Noninferiority Margin
Assuming a clinical cure rate of 50% and a clinically evaluable rate of 60%, a sample size of 770 patients (ITT) was calculated to provide 90% power at a 2-sided significance level (type 1 error) of 5% to demonstrate the noninferiority of ceftobiprole to ceftazidime/linezolid within a margin of 15% for the primary endpoint of clinical cure rate at TOC in the overall study population.
Randomization and Blinding
Participants were randomly assigned to treatment via a central interactive voice response system based on a computer-generated randomization schedule. Randomization was stratified by disease entity (patients with and without VAP) using randomly permuted blocks for each of the 2 strata, and by APACHE II score (8–19; 20–25). Patients with VAP were further stratified by time to randomization after onset of mechanical ventilation (<5 days or ≥5 days of ventilation). The study was conducted in a double-blind fashion.
Statistical Analysis
The primary endpoint was clinical cure (resolution of signs and symptoms of infection, or improvement to such an extent that no further antimicrobial therapy was necessary) at TOC, in the coprimary ITT (all patients randomly assigned to treatment) and clinically evaluable (CE) (patients who received at least 1 dose of study medication and were clinically evaluable at the TOC visit) analysis sets. Patients who received systemic nonstudy antibiotics between baseline and the TOC visit for the treatment of pneumonia were considered to have clinical failure; patients who received systemic nonstudy antibiotics for indications other than pneumonia were excluded from the CE analysis set.
The main secondary endpoint was microbiological eradication at TOC in the microbiologically evaluable analysis set (all patients in the CE analysis set who had a valid pathogen at baseline and were microbiologically evaluable at the TOC visit) and microbiological ITT analysis set (all patients in the ITT analysis set who had a valid pathogen at baseline). Endpoints were analyzed with a 2-sided 95% confidence interval (CI) for treatment difference. Noninferiority of ceftobiprole to ceftazidime/linezolid was to be concluded if the lower limit of this interval was ≥ −15%.
A post hoc multivariate logistic regression analysis (MVLRA) was performed on the VAP subgroup to explore individual factors (eg, baseline pathogens, sex, age, comorbidities, vasopressor use) or a combination of factors that might explain differences in clinical and microbiological outcomes between the 2 treatment groups in VAP patients. A total of 37 factors were included in multivariate analyses (using stepwise, forward, and backward factor selection with and without consideration of treatment by factor interactions) to assess their impact on clinical cure, microbiological eradication, and 30-day all-cause-mortality.
RESULTS
Recruitment
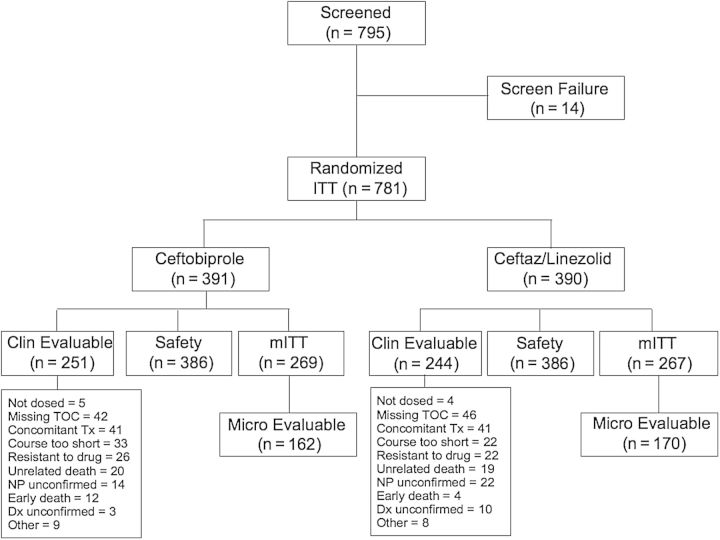
Of 795 patients screened (Figure 1), 781were randomized, with 391 assigned to ceftobiprole and 390 to ceftazidime/linezolid (ITT analysis set). The CE analysis set comprised 251 ceftobiprole and 244 ceftazidime/linezolid patients. Three hundred eighty-six patients in each treatment group were available for safety assessment.
Figure 1.
Patient disposition. Abbreviations: Ceftaz, ceftazidime; Dx, diagnosis; mITT, microbiological intent-to-treat; NP, nosocomial pneumonia; TOC, test-of-cure; Tx, treatment.
Of the 247 patients who discontinued the study, 126 patients (32%) were from the ceftobiprole group, and 121 (31%) were from the ceftazidime/linezolid group. The most common reasons for discontinuation were death (77 ceftobiprole [20%] and 74 ceftazidime/linezolid [19%]), and AEs (14 ceftobiprole [4%], and 6 ceftazidime/linezolid [2%]).
Baseline Data
Baseline demographic and clinical characteristics are provided in Table 1. The study recruited patients in 32 countries; 56% in Europe, 12% in the United States, 32% in other regions. 71% vs 62% of the patients in the ceftobiprole and the comparator groups, respectively, were men, and 45% vs 47% were aged >65 years. A substantial proportion of the patient population was severely ill: 41% of the patients in each treatment group had an APACHE II score ≥15, and 12% of ceftobiprole patients and 13% of ceftazidime/linezolid patients had an APACHE II score ≥20. Seventy-two percent and 73% of the patients, respectively, presented with systemic inflammatory response syndrome, 10% and 12%, respectively, had bacteremia, and 58% and 62% had received prior antibiotics within 24 hours of enrollment. A pathogen considered to cause pneumonia was found at baseline in 69% of the patients in the ceftobiprole group and in 68% of the patients in the ceftazidime/linezolid group. Ten percent and 12% of the patients, respectively, were infected with MRSA. In patients with VAP, 73% in the ceftobiprole and 81% in the ceftazidime/linezolid group had been ventilated for ≥5 days prior to enrollment.
Table 1.
Baseline Demographic and Clinical Characteristics (Intent-to-Treat Analysis Set)
| Variable | All Patients, No. (%) |
HAP (Excluding VAP), No. (%) |
VAP, No. (%) |
|||
|---|---|---|---|---|---|---|
| Ceftobiprole (n = 391) | Ceftazidime/Linezolid (n = 390) | Ceftobiprole (n = 287) | Ceftazidime/Linezolid (n = 284) | Ceftobiprole (n = 104) | Ceftazidime/Linezolid (n = 106) | |
| Male sex | 278 (71) | 243 (62) | 202 (70) | 170 (60) | 76 (73) | 73 (69) |
| Age >65 y | 177 (45) | 185 (47) | 148 (52) | 147 (52) | 29 (28) | 38 (36) |
| Age < 45 y | 81 (21) | 65 (17) | 42 (15) | 34 (12) | 39 (38) | 31 (29) |
| European Union | 220 (56) | 219 (56) | 165 (57) | 165 (58) | 55 (53) | 54 (51) |
| United States | 48 (12) | 47 (12) | 34 (12) | 34 (12) | 14 (13) | 13 (12) |
| Other region | 123 (31) | 124 (32) | 88 (31) | 85 (30) | 35 (34) | 39 (37) |
| Smoking | 113 (29) | 116 (30) | 83 (29) | 85 (30) | 30 (29) | 31 (29) |
| Diabetes mellitus | 79 (20) | 77 (20) | 59 (21) | 60 (21) | 20 (19) | 17 (16) |
| Chronic care | 45 (12) | 44 (11) | 41 (14) | 42 (15) | 4 (4) | 2 (2) |
| Emphysema | 34 (9) | 43 (11) | 33 (11) | 33 (12) | 1 (1) | 10 (9) |
| Asthma | 21 (5) | 28 (7) | 15 (5) | 23 (8) | 6 (6) | 5 (5) |
| SIRS | 283 (72) | 286 (73) | 217 (76) | 226 (80) | 66 (63) | 60 (57) |
| CRP >100 mg/L | 240 (61) | 218 (56) | 169 (59) | 153 (54) | 71 (68) | 65 (61) |
| Prior AB within 24 h | 225 (58) | 243 (62) | 169 (59) | 176 (62) | 56 (54) | 67 (63) |
| APACHE II ≥15 | 162 (41) | 160 (41) | 101 (35) | 104 (37) | 61 (59) | 56 (53) |
| APACHE II ≥20 | 47 (12) | 51 (13) | 25 (9) | 26 (9) | 22 (21) | 25 (24) |
| Baseline ventilation | 145 (37) | 150 (38) | 41 (14) | 44 (15) | 104 (100) | 106 (100) |
| Ventilated ≥5 d | 76 (19) | 86 (22) | 0 (0) | 0 (0) | 76 (73) | 86 (81) |
| Valid baseline pathogen | 269 (69) | 267 (68) | 179 (62) | 181 (64) | 90 (87) | 86 (81) |
| Valid gram-positive pathogen | 136 (35) | 149 (38) | 85 (30) | 102 (36) | 51 (49) | 47 (44) |
| Valid gram-negative pathogen | 196 (50) | 177 (45) | 122 (43) | 116 (41) | 74 (71) | 61 (58) |
| Polymicrobial | 95 (24) | 92 (24) | 50 (17) | 56 (20) | 45 (43) | 36 (34) |
| Pseudomonas | 49 (13) | 52 (13) | 29 (10) | 30 (11) | 20 (19) | 22 (21) |
| MRSA | 41 (10) | 48 (12) | 28 (10) | 32 (11) | 13 (13) | 16 (15) |
| MSSA | 55 (14) | 72 (18) | 27 (9) | 44 (15) | 28 (27) | 28 (26) |
| Bacteremia | 41 (10) | 45 (12) | 24 (8) | 27 (10) | 17 (16) | 18 (17) |
| CrCl <50 mL/min | 63 (16) | 65 (17) | 56 (20) | 55 (19) | 7 (7) | 10 (9) |
| CrCl ≥150 mL/min | 67 (17) | 66 (17) | 37 (13) | 38 (13) | 30 (29) | 28 (26) |
Abbreviations: AB, antibiotics; APACHE, Acute Physiology and Chronic Health Evaluation; CrCl, creatinine clearance; CRP, C-reactive protein; HAP, hospital-acquired pneumonia; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; SIRS, systemic inflammatory response syndrome; VAP, ventilator-associated pneumonia.
Outcomes
Results for the primary endpoint are provided in Table 2. The study achieved its primary objective demonstrating noninferiority of ceftobiprole to ceftazidime/linezolid for clinical cure rate at the TOC visit within the protocol-defined margin of 15% in the coprimary ITT and CE analysis sets. The cure rates in the ITT analysis set were 49.9% and 52.8% for ceftobiprole and ceftazidime/linezolid, respectively (difference, −2.9% [95% CI, −10.0 to 4.1]), and 69.3% and 71.3%, respectively (−2.0% [95% CI, −10.0 to 6.1]), in the CE analysis set.
Table 2.
Primary Endpoint: Clinical Cure at Test of Cure (Intent-to-Treat and Clinically Evaluable Analysis Sets)
| Analysis Set Group | Ceftobiprole |
Ceftazidime/Linezolid |
||||
|---|---|---|---|---|---|---|
| No. | No.a (%) | No. | No.a (%) | Difference (%)b | (95% CI)c | |
| Intent-to-treat | ||||||
| All patients | 391 | 195 (49.9) | 390 | 206 (52.8) | −2.9 | (−10.0 to 4.1) |
| HAP (excluding VAP) | 287 | 171 (59.6) | 284 | 167 (58.8) | 0.8 | (−7.3 to 8.8) |
| VAP | 104 | 24 (23.1) | 106 | 39 (36.8) | −13.7 | (−26.0 to −1.5) |
| HAP, mechanically ventilated | 69 | 21 (30.4) | 70 | 19 (27.1) | 3.3 | (−11.8 to 18.3) |
| Clinically evaluable | ||||||
| All patients | 251 | 174 (69.3) | 244 | 174 (71.3) | −2.0 | (−10.0 to 6.1) |
| HAP (excluding VAP) | 198 | 154 (77.8) | 185 | 141 (76.2) | 1.6 | (−6.9 to 10.0) |
| VAP | 53 | 20 (37.7) | 59 | 33 (55.9) | −18.2 | (−36.4 to −.0) |
| HAP (excluding VAP), mechanically ventilated | 38 | 21 (55.3) | 37 | 15 (40.5) | 14.7 | (−7.6 to 37.1) |
Abbreviations: CI, confidence interval; HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia.
a No. of patients with clinical cure at test of cure.
b Difference ceftobiprole minus ceftazidime/linezolid.
c Two-sided 95% CI is based on the normal approximation to the difference of the 2 proportions.
Consistent with regulatory guidance distinguishing HAP (excluding VAP) and VAP [28, 29], further efficacy analyses were conducted of the HAP (excluding VAP) and VAP patient populations. The results for HAP (excluding VAP) patients and VAP patients are therefore discussed separately below.
Subgroup Analyses of the Primary Endpoint
In the ITT analysis set, 73% of all patients enrolled were HAP (excluding VAP) patients (287 in the ceftobiprole group and 284 in the ceftazidime/linezolid group). Baseline characteristics of HAP (excluding VAP) patients were comparable to those of the overall study population (Table 1). Noninferiority of ceftobiprole to ceftazidime/linezolid for clinical cure at TOC was demonstrated in patients with HAP (excluding VAP) within the predefined noninferiority margin of −15%. The cure rates at TOC for HAP (excluding VAP) patients in the ITT analysis set were 59.6% for ceftobiprole and 58.8% for ceftazidime/linezolid (difference, 0.8 [95% CI, −7.3 to 8.8]), and 77.8% and 76.2%, respectively, in the CE analysis set (difference, 1.6 [95% CI, −6.9 to 10.0]) (Table 2). Results for the primary endpoint in HAP (excluding VAP) patients were also comparable for ceftobiprole and ceftazidime/linezolid when analyzed by baseline demographic and clinical characteristics. Clinical cure rates for HAP (excluding VAP) patients were comparable in subgroup analyses by sex, geographical region, age, and disease severity (APACHE II score) (Table 3).
Table 3.
Clinical Cure Rates at Test of Cure by Demographic and Clinical Characteristics (Clinically Evaluable Analysis Set) in Patients With Hospital-Acquired Pneumonia (Excluding Ventilator-Associated Pneumonia)
| Ceftobiprole |
Ceftazidime/Linezolid |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. | n | % | No. | n | % | Difference (%)a | (95% CI)b | |
| HAP (excluding VAP) | 198 | 154 | 77.8 | 185 | 141 | 76.2 | 1.6 | (−6.9 to 10.0) |
| Age | ||||||||
| 18–44 y | 27 | 24 | 88.9 | 22 | 18 | 81.8 | 7.1 | (−12.9 to 27.1) |
| 45–64 y | 60 | 48 | 80.0 | 66 | 52 | 78.8 | 1.2 | (−12.9 to 15.3) |
| ≥65 y | 111 | 82 | 73.9 | 97 | 71 | 73.2 | 0.7 | (−11.3 to 12.7) |
| Sex | ||||||||
| Male | 139 | 107 | 77.0 | 112 | 86 | 76.8 | 0.2 | (−10.3 to 10.7) |
| Female | 59 | 47 | 79.7 | 73 | 55 | 75.3 | 4.3 | (−9.9 to 18.6) |
| Geographical region | ||||||||
| United States | 27 | 20 | 74.1 | 24 | 14 | 58.3 | 15.7 | (−10.0 to 41.5) |
| Europec | 112 | 93 | 83.0 | 104 | 90 | 86.5 | −3.5 | (−13.1 to 6.1) |
| Otherd | 59 | 41 | 69.5 | 57 | 37 | 64.9 | 4.6 | (−12.5 to 21.7) |
| APACHE II score | ||||||||
| 8–19 | 185 | 146 | 78.9 | 171 | 134 | 78.4 | 0.6 | (−8.0 to 9.1) |
| 20–25 | 13 | 8 | 61.5 | 14 | 7 | 50.0 | 11.5 | (−25.7 to 48.8) |
| Care facility | ||||||||
| ICU | 73 | 51 | 69.9 | 59 | 39 | 66.1 | 3.8 | (−12.3 to 19.8) |
| Non-ICU | 125 | 103 | 82.4 | 126 | 102 | 81.0 | 1.4 | (−8.1 to 11.0) |
| Prestudy antibioticse | ||||||||
| No antibiotics | 53 | 44 | 83.0 | 59 | 49 | 83.1 | −0.0 | (−14.0 to 13.9) |
| Using ≤24 h | 65 | 52 | 80.0 | 59 | 45 | 76.3 | 3.7 | (−10.8 to 18.3) |
| Using >24 h | 80 | 58 | 72.5 | 67 | 47 | 70.1 | 2.4 | (−12.3 to 17.0) |
| Antipseudomonal antibioticsf | ||||||||
| Yes | 27 | 15 | 55.6 | 19 | 10 | 52.6 | 2.9 | (−26.3 to 32.2) |
| No | 171 | 139 | 81.3 | 166 | 131 | 78.9 | 2.4 | (−6.2 to 10.9) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; HAP, hospital-acquired pneumonia; n, number of patients with a clinical outcome of cure; ICU, intensive care unit; VAP, ventilator-associated pneumonia.
a Ceftobiprole minus ceftazidime/linezolid.
b Two-sided 95% CI is based on normal approximation to the difference of the 2 proportions.
c Europe includes Belgium, Bulgaria, Czech Republic, France, Germany, Great Britain, Hungary, Latvia, Lithuania, Poland, Romania, Russia, Serbia, Montenegro, Spain, Switzerland, and Ukraine.
d Other region includes Argentina, Australia, Brazil, Canada, Chile, Democratic People's Republic of Korea, Honduras, India, Israel, Mexico, People's Republic of China, Peru, South Africa, Taiwan, and Thailand.
e The window for prestudy antibiotics was limited to 72 hours prior to baseline.
f Empirical treatment with antibiotic therapy was added to the study drug regimen for 48 hours in patients with a suspected infection due to Pseudomonas aeruginosa or for 5–7 days for patients with proven infection due to P. aeruginosa.
In the CE analysis set, a higher proportion of HAP (excluding VAP) patients in the ceftobiprole group than in the ceftazidime/linezolid group (CE, 86.9% vs 78.4%; difference 8.5 [95% CI, .9–16.1]) showed early improvement (4 days after onset of therapy) as assessed by the investigator based on the resolution of clinical signs and symptoms. The largest difference was for patients with a baseline culture positive for MRSA, with 94.7% in the ceftobiprole group having early improvement vs 52.6% in the ceftazidime/linezolid group (difference, 42.1 [95% CI, 17.5–66.7]) (Table 4).
Table 4.
Clinical Improvement at Day 4 (Intent-to-Treat and Clinically Evaluable Analysis Sets) in Patients With Hospital-Acquired Pneumonia (Excluding Ventilator-Associated Pneumonia)
| Analysis Set Group | Ceftobiprole |
Ceftazidime/Linezolid |
||||
|---|---|---|---|---|---|---|
| No. | n (%) | No. | n (%) | Difference (%)a | (95% CI)b | |
| ITT | ||||||
| HAP (excluding VAP) | 287 | 221 (77.0) | 284 | 214 (75.4) | 1.7 | (−5.3 to 8.6) |
| Any valid gram-positive | 85 | 69 (81.2) | 102 | 75 (73.5) | 7.6 | (−4.3 to 19.6) |
| Any Staphylococcus aureus | 55 | 45 (81.8) | 76 | 55 (72.4) | 9.4 | (−4.9 to 23.8) |
| Any MRSA | 28 | 22 (78.6) | 32 | 19 (59.4) | 19.2 | (−3.6 to 42.0) |
| Clinically evaluable | ||||||
| HAP (excluding VAP) | 198 | 172 (86.9) | 185 | 145 (78.4) | 8.5 | (.9–16.1) |
| Any valid gram-positive | 61 | 53 (86.9) | 69 | 51 (73.9) | 13.0 | (−.4 to 26.4) |
| Any S. aureus | 39 | 36 (92.3) | 49 | 35 (71.4) | 20.9 | (5.7–36.0) |
| Any MRSA | 19 | 18 (94.7) | 19 | 10 (52.6) | 42.1 | (17.5–66.7) |
Clinical improvement was assessed by the investigator. All categories include monomicrobial and polymicrobial infections.
Abbreviations: CI, confidence interval; HAP, hospital-acquired pneumonia; ITT, intent-to-treat; MRSA, methicillin-resistant Staphylococcus aureus; n, number of patients with clinical improvement at Day 4; VAP, ventilator-associated pneumonia.
a Difference for ceftobiprole minus ceftazidime/linezolid.
b Two-sided 95% CI is based on the normal approximation to the difference of the 2 proportions.
Of the 198 ceftobiprole and 185 ceftazidime/linezolid HAP (excluding VAP) patients in the CE analysis set, 38 (19%) and 37 (20%), respectively, required mechanical ventilation during treatment, or developed pneumonia within 48 hours after the start of ventilation (ie, did not fall within the definition of VAP). For these ventilated HAP (excluding VAP) patients, higher cure rates were observed in the ceftobiprole group than in the ceftazidime/linezolid group: 55.3% vs 40.5%, respectively (CE; difference, 14.7 [95% CI, −7.6 to 37.1]) (Table 2).
Noninferiority was not demonstrated in VAP patients. The cure rates at TOC for VAP patients in the ITT analysis set were 23.1% for ceftobiprole and 36.8% for ceftazidime/linezolid (difference, −13.7 [95% CI, −26.0 to −1.5]) (Table 2). An MVLRA did not reveal any specific patient factors in VAP patients that could explain the differential clinical and microbiological outcome between the treatment arms in this subgroup. The subgroup of VAP patients was relatively small, and was characterized by a substantial heterogeneity in baseline characteristics (Table 1).
Subgroup Analyses of Secondary Endpoints
Microbiological Eradication
For the main secondary endpoint, the microbiological eradication rates at TOC for HAP (excluding VAP) patients in the microbiologically evaluable analysis set were 62.9% for ceftobiprole and 67.5% for ceftazidime/linezolid (difference, −4.6% [95% CI, −16.7 to 7.6]) (Table 5). For patients with VAP, the rates were 30.4% for ceftobiprole and 50.0% for ceftazidime/linezolid (difference, −19.6% [95% CI, −38.8 to −0.4]) (Table 5).
Table 5.
Microbiological Eradication at Test of Cure (Microbiological Intent-to-Treat and Microbiologically Evaluable Analysis Sets)
| Analysis Set Group | Ceftobiprole |
Ceftazidime/Linezolid |
||||
|---|---|---|---|---|---|---|
| No. | n (%) | No. | n (%) | Difference (%)a | (95% CI)b | |
| Microbiological ITT | ||||||
| All patients | 269 | 105 (39.0) | 267 | 127 (47.6) | −8.5 | (−16.9 to −.2) |
| HAP (excluding VAP) | 179 | 87 (48.6) | 181 | 97 (53.6) | −5.0 | (−15.3 to 5.3) |
| VAP | 90 | 18 (20.0) | 86 | 30 (34.9) | −14.9 | (−27.9 to −1.9) |
| Microbiologically evaluable | ||||||
| All patients | 162 | 87 (53.7) | 170 | 106 (62.4) | −8.6 | (−19.2 to 1.9) |
| HAP (excluding VAP) | 116 | 73 (62.9) | 120 | 81 (67.5) | −4.6 | (−16.7 to 7.6) |
| VAP | 46 | 14 (30.4) | 50 | 25 (50.0) | −19.6 | (−38.8 to −0.4) |
Abbreviations: CI, confidence interval; HAP, hospital-acquired pneumonia; ITT, intent-to-treat; n, number of patients with microbiological eradication at test of cure; VAP, ventilator-associated pneumonia.
a Difference for ceftobiprole minus ceftazidime/linezolid.
b Two-sided 95% CI is based on the normal approximation to the difference of the 2 proportions.
Clinical Cure and Microbiological Eradication by Pathogen
Clinical cure and microbiological eradication rates by pathogen in patients with HAP (excluding VAP) were similar for gram-positive and most gram-negative pathogens (Table 6). For the most prevalent Enterobacteriaceae and for P. aeruginosa, clinical cure and microbiological eradication rates were similar between treatment groups. For the relatively small number of patients with Haemophilus and Acinetobacter species as baseline pathogens, numerically lower clinical cure and microbiological eradication rates were observed in the ceftobiprole group.
Table 6.
Clinical Cure and Microbiological Eradication, by Pathogen (Microbiologically Evaluable Analysis Set)
| Pathogen | HAP (excluding VAP), No. (%) |
VAP, No. (%) |
All Patients, No. (%) |
|||
|---|---|---|---|---|---|---|
| Ceftobiprole (n = 116) | Ceftazidime/Linezolid (n = 120) | Ceftobiprole (n = 46) | Ceftazidime/ Linezolid (n = 50) | Ceftobiprole (n = 162) | Ceftazidime/ Linezolid (n = 170) | |
| Staphylococcus aureus | 39 | 49 | 25 | 28 | 64 | 77 |
| Clinical cure | 28 (72) | 36 (73) | 9 (36) | 16 (57) | 37 (58) | 52 (68) |
| Microbiological eradication | 23 (59) | 31 (63) | 10 (40) | 18 (64) | 33 (52) | 49 (64) |
| MSSA | 20 | 30 | 17 | 19 | 37 | 49 |
| Clinical cure | 15 (75) | 24 (80) | 5 (29) | 10 (53) | 20 (54) | 34 (69) |
| Microbiological eradication | 15 (75) | 21 (70) | 5 (29) | 12 (63) | 20 (54) | 33 (67) |
| MRSA | 19 | 19 | 8 | 9 | 27 | 28 |
| Clinical cure | 13 (68) | 12 (63) | 4 (50) | 6 (67) | 17 (63) | 18 (64) |
| Microbiological eradication | 8 (42) | 10 (53) | 5 (63) | 6 (67) | 13 (48) | 16 (57) |
| Streptococcus pneumoniae | 7 | 14 | 4 | 1 | 11 | 15 |
| Clinical cure | 7 (100) | 13 (93) | 0 (0) | 1 (100) | 7 (64) | 14 (93) |
| Microbiological eradication | 7 (100) | 13 (93) | 0 (0) | 1 (100) | 7 (64) | 14 (93) |
| Enterobacteriaceae | 46a | 45b | 18 | 16 | 64 | 61 |
| Clinical cure | 33 (72) | 32 (71) | 5 (28) | 6 (38) | 38 (59) | 38 (62) |
| Microbiological eradication | 29 (63) | 32 (71) | 6 (33) | 7 (44) | 35 (55) | 39 (64) |
| Escherichia coli | 14 | 11 | 6 | 3 | 20 | 14 |
| Clinical cure | 8 (57) | 7 (64) | 2 (33) | 1 (33) | 10 (50) | 8 (57) |
| Microbiological eradication | 8 (57) | 7 (64) | 2 (33) | 1 (33) | 10 (50) | 8 (57) |
| Klebsiella pneumoniae | 12 | 19 | 4 | 4 | 16 | 23 |
| Clinical cure | 11 (92) | 15 (79) | 0 (0) | 1 (25) | 11 (69) | 16 (70) |
| Microbiological eradication | 10 (83) | 15 (79) | 0 (0) | 1 (25) | 10 (63) | 16 (70) |
| Enterobacter species | 9 | 7 | 3 | 2 | 12 | 9 |
| Clinical cure | 7 (78) | 3 (43) | 1 (33) | 0 (0) | 8 (75) | 3 (33) |
| Microbiological eradication | 6 (67) | 3 (43) | 2 (66) | 0 (0) | 8 (75) | 3 (33) |
| Proteus species | 5 | 5 | 2 | 5 | 7 | 10 |
| Clinical cure | 4 (80) | 2 (40) | 0 (0) | 1 (20) | 4 (57) | 3 (30) |
| Microbiological eradication | 3 (60) | 2 (40) | 0 (0) | 2 (40) | 3 (43) | 4 (40) |
| Serratia species | 5 | 4 | 3 | 3 | 8 | 7 |
| Clinical cure | 3 (60) | 2 (50) | 1 (33) | 3 (100) | 4 (50) | 5 (71) |
| Microbiological eradication | 2 (40) | 2 (50) | 1 (33) | 3 (100) | 3 (38) | 5 (71) |
| Pseudomonas aeruginosa | 16 | 20 | 11 | 14 | 27 | 34 |
| Clinical cure | 12 (75) | 14 (70) | 5 (45) | 10 (71) | 17 (63) | 24 (71) |
| Microbiological eradication | 9 (56) | 11 (55) | 4 (36) | 8 (57) | 13 (48) | 19 (56) |
| Acinetobacter baumannii | 8 | 12 | 6 | 5 | 14 | 17 |
| Clinical cure | 4 (50) | 9 (75) | 3 (50) | 4 (80) | 7 (50) | 13 (77) |
| Microbiological eradication | 4 (50) | 9 (75) | 3 (50) | 3 (60) | 7 (50) | 12 (71) |
| Haemophilus | 5 | 9 | 4 | 0 | 9 | 9 |
| Clinical cure | 2 (40) | 9 (100) | 1 (25) | na | 3 (33) | 9 (100) |
| Microbiological eradication | 2 (40) | 9 (100) | 1 (25) | na | 3 (33) | 9 (100) |
Numbers in bold refer to the number of patients with a pathogen in the respective group.
Abbreviations: HAP, hospital-acquired pneumonia; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; n, number of patients with an outcome of clinical cure or eradication for the respective pathogen at test of cure; VAP, ventilator-associated pneumonia.
a Forty-six patients with Enterobacteriaceae isolated at baseline, including E. coli (monomicrobial), n = 11; E. coli plus Klebsiella spp, n = 1; E. coli plus Proteus spp, n = 1; E. coli plus Providencia spp, n = 1; K. pneumoniae (monomicrobial), n = 9; Klebsiella oxytoca, n = 1; K. pneumoniae plus Proteus spp, n = 1; K. pneumoniae plus Serratia spp, n = 1; K. pneumoniae plus Enterobacter spp, n = 1; Enterobacter spp, n = 8; Serratia spp, n = 5; Proteus mirabilis, n = 3; Citrobacter spp, n = 2; Providencia spp, n = 1.
b Forty-five patients with Enterobacteriaceae isolated at baseline, including E. coli (monomicrobial), n = 8; E. coli plus K. pneumoniae, n = 1; E. coli plus Proteus spp, n = 1; E. coli plus K. pneumoniae plus Serratia spp, n = 1; K. pneumoniae (monomicrobial), n = 14; Klebsiella oxytoca, n = 1; Klebsiella spp, n = 2; K. pneumoniae plus Proteus spp, n = 2; K. pneumoniae plus Proteus spp plus Serratia spp, n = 1; Enterobacter spp, n = 7; Serratia spp, n = 2; Proteus mirabilis, n = 3; Citrobacter spp, n = 1; Hafnia alvei, n = 1.
Mortality
Thirty-day all-cause mortality (ACM) and 30-day pneumonia-specific mortality were similar between the ceftobiprole and ceftazidime/linezolid treatment groups. For HAP (excluding VAP) patients in the ITT analysis set, 30-day ACM was 16.7% for ceftobiprole and 18.0% for ceftazidime/linezolid (difference, −1.2 [95% CI, −7.4 to 5.0]), and pneumonia-specific mortality was 5.9% and 5.6%, respectively (difference, 0.3 [95% CI, −3.5 to 4.1]). For VAP patients, 30-day ACM was 26.9% for ceftobiprole and 19.8% for ceftazidime/linezolid (difference, 7.1 [95% CI, −4.3 to 18.5]), and pneumonia-specific mortality was 8.7% and 7.5%, respectively (difference, 1.1 [95% CI, −6.3 to 8.5]).
Safety and Tolerability
Treatment-related AEs were reported for 96 ceftobiprole patients (24.9%) and 98 ceftazidime/linezolid patients (25.4%) (Table 7). Ceftobiprole patients had fewer treatment-related events of diarrhea than patients treated with ceftazidime/linezolid (3.1% and 6.5%, respectively), whereas hyponatremia was observed more frequently with ceftobiprole than with ceftazidime/linezolid (4.4% and 2.6%, respectively). Dysgeusia occurred only in patients treated with ceftobiprole (1.3%), as ceftobiprole medocaril is known to arouse a caramel taste. There were 15 treatment-related serious AEs reported for ceftobiprole (3.9%), and 12 (3.1%) for ceftazidime/linezolid. No clinically relevant differences in other laboratory values, vital signs, physical examinations, or electrocardiograms were observed between treatment groups.
Table 7.
Treatment-Related Adverse Events Reported in ≥1% of Patients in at Least 1 Treatment Group (Safety Analysis Set)
| Adverse Event | Ceftobiprole (n = 386), No. (%) | Ceftazidime/Linezolid (n = 386), No. (%) |
|---|---|---|
| Total No. of subjects with at least 1 related adverse event | 96 (24.9) | 98 (25.4) |
| Hyponatremia | 17 (4.4) | 10 (2.6) |
| Diarrhea | 12 (3.1) | 25 (6.5) |
| Nausea | 8 (2.1) | 8 (2.1) |
| Phlebitis | 8 (2.1) | 5 (1.3) |
| Oral candidiasis | 6 (1.6) | 4 (1.0) |
| Hypokalemia | 6 (1.6) | 3 (0.8) |
| Vomiting | 6 (1.6) | 3 (0.8) |
| Dysgeusia | 5 (1.3) | 0 |
| Pyrexia | 4 (1.0) | 2 (0.5) |
| Rash | 3 (0.8) | 6 (1.6) |
| Alanine aminotransferase increased | 3 (0.8) | 6 (1.6) |
| Aspartate aminotransferase increased | 3 (0.8) | 4 (1.0) |
DISCUSSION
The results of this large global study demonstrate that ceftobiprole is noninferior to ceftazidime plus linezolid for clinical cure at the TOC visit in treating patients with HAP. Noninferiority of ceftobiprole to ceftazidime/linezolid was also demonstrated in the large subgroup of HAP (excluding VAP) patients but not in the smaller subgroup of VAP patients. The overall safety profile of ceftobiprole was consistent with that of other cephalosporins.
The population of this study was representative of nosocomial pneumonia patients in terms of age, underlying conditions, and severity of disease, which is reflected in the mortality rate of approximately 19% in the overall study population (17% in HAP [excluding VAP] and 23% in VAP) [30–33]. Patients in the study had a broad range of gram-positive and gram-negative bacteria typically causing HAP [3].
Differences in clinical cure and eradication rates by pathogen between treatment groups, with a tendency toward higher cure and eradication rates in the ceftazidime/linezolid group, were observed overall (all patients, see Table 6). However, for gram-positive pathogens and most of the gram-negative pathogens, these differences were driven by the inferior outcome of ceftobiprole in the subgroup of VAP patients. For pathogens in the larger group of HAP (excluding VAP) patients, clinical cure and microbiological eradication rates were similar for gram-positive pathogens and also most gram-negative pathogens. Analyses of clinical and microbiological outcome by baseline pathogen show comparable results between treatment groups for gram-positive pathogens, E. coli, Klebsiella pneumoniae, Enterobacter species, Proteus mirabilis, and P. aeruginosa. Only for Acinetobacter baumannii and Haemophilus species were numerically lower clinical cure and microbiological eradication rates observed in the ceftobiprole group, but this has to be interpreted with caution due to the small sample size.
Noninferiority of ceftobiprole compared with ceftazidime/linezolid was not demonstrated in VAP patients. The fact that clinical cure and mortality rates in mechanically ventilated HAP (excluding VAP) patients (ie, ventilated patients who did not have VAP) either favored ceftobiprole or were comparable to those for ceftazidime/linezolid suggests that mechanical ventilation itself does not account for the outcome in VAP patients. A multivariate analysis did not reveal any specific patient factors that could explain the differential outcome in VAP patients in both treatment arms. The substantial heterogeneity in baseline characteristics of VAP patients [34–36] is the most likely explanation for the differential outcome in VAP patients. Moreover, a population pharmacokinetic model showed that ceftobiprole plasma concentrations were sufficient for a targeted minimum inhibitory concentration of 4 mg/L in 92% of all patients, suggesting that plasma concentrations of ceftobiprole were also adequate in VAP patients [37].
In summary, ceftobiprole is a novel cephalosporin with broad-spectrum and bactericidal activity against gram-positive and gram-negative pathogens typically found in HAP, including MRSA and P. aeruginosa. This large, double-blind, randomized study demonstrates that ceftobiprole is noninferior to the combination of ceftazidime and linezolid, and is therefore a safe and effective monotherapy for the empiric treatment of HAP (excluding VAP). Whether the early improvement observed in a higher proportion of HAP (excluding VAP) patients in the ceftobiprole group translates into additional benefits from the use of ceftobiprole requires further investigation.
Notes
Acknowledgments. The authors acknowledge the assistance of Anne Thérèse Witschi, Basilea Pharmaceutica International Ltd, and the provision of medical writing services by David Main, Basilea Pharmaceutica International Ltd, and Richard S. Perry, PharmD, RP Consulting.
Financial support. This work was supported by Basilea Pharmaceutica International Ltd, Basel, Switzerland.
Potential conflicts of interest. M. E. is a full-time employee of Basilea Pharmaceutica International Ltd. M. S. is a full-time employee of Aptiv Solutions, providing biostatistical and data management services to Basilea Pharmaceutica International Ltd. A. H. R. has received honoraria for participating in speakers’ bureaus from MSD, Pfizer, Novartis, Thermo Fisher, Astellas, and Gilead Sciences. T. W. L. S. reports receiving compensation for costs of recruiting patients that was paid to University Hospital Rostock. G. R. has a National Institutes of Health grant pending, and has received a research grant from Basilea Pharmaceutica. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010;51(suppl 1):S48–53. doi: 10.1086/653049. [DOI] [PubMed] [Google Scholar]
- 2.Masterton R. The place of guidelines in hospital-acquired pneumonia. J Hosp Infect. 2007;66:116–22. doi: 10.1016/j.jhin.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(suppl 1):S81–7. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 4.Lovering AL, Gretes MC, Safadi SS, et al. Structural insights into the anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of ceftobiprole. J Biol Chem. 2012;287:32096–102. doi: 10.1074/jbc.M112.355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies TA, Page MG, Shang W, Andrew T, Kania M, Bush K. Binding of ceftobiprole and comparators to the penicillin binding proteins of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Antimicrob Agents Chemother. 2007;51:2621–4. doi: 10.1128/AAC.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdanovich T, Ednie LM, Appelbaum PC, Shapiro S. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 2005;49:4210–9. doi: 10.1128/AAC.49.10.4210-4219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flamm RK, Sader HS, Streit JM, Jones RN. Activity of ceftobiprole tested against gram-negative clinical isolates from European medical centres [abstract P1627]. Programme and abstracts of the 23rd European Congress of Clinical Microbiology and Infectious Diseases; Berlin, Germany. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2013. [Google Scholar]
- 8.Flamm RK, Sader HS, Streit JM, Jones RN. Activity of ceftobiprole tested against clinical isolates of staphylococci and streptococci from European surveillance (2008–2010) [abstract P1628]. Programme and abstracts of the 23rd European Congress of Clinical Microbiology and Infectious Diseases; Berlin, Germany. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2013. [Google Scholar]
- 9.Germel C, Haag A, Söderquist B. In vitro activity of beta-lactam antibiotics to community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Eur J Clin Microbiol Infect Dis. 2012;31:475–80. doi: 10.1007/s10096-011-1333-8. [DOI] [PubMed] [Google Scholar]
- 10.Denis O, Deplano A, Nonhoff C, et al. In vitro activities of ceftobiprole, tigecycline, daptomycin, and 19 other antimicrobials against methicillin-resistant Staphylococcus aureus strains from a national survey of Belgian hospitals. Antimicrob Agents Chemother. 2006;50:2680–5. doi: 10.1128/AAC.00272-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amsler KM, Davies TA, Shang W, Bush K, Jacobs MR. In vitro activity of ceftobiprole against pathogens from two phase 3 clinical trials of complicated skin and skin structure infections. Antimicrob Agents Chemother. 2008;52:3418–23. doi: 10.1128/AAC.00336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhanel GG, Lam A, Thomson K, et al. Ceftobiprole: a review of a broad-spectrum and anti-MRSA cephalosporin. Am J Clin Dermatol. 2008;9:245–54. doi: 10.2165/00128071-200809040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Betriu C, Culebras E, Gómez M, et al. Comparative in vitro activity of ceftobiprole against gram-positive cocci. Int J Antimicrob Agents. 2010;36:111–13. doi: 10.1016/j.ijantimicag.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Flamm RK, Sader HS, Streit JM, Jones RN. Activity of ceftobiprole tested against pathogens associated with hospital-acquired bacterial pneumonia in Europe [abstract P1625]. Programme and abstracts of the 23rd European Congress of Clinical Microbiology and Infectious Diseases; Berlin, Germany. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2013. [Google Scholar]
- 15.Flamm RK, Sader HS, Streit JM, Jones RN. Activity of ceftobiprole against methicillin-resistant Staphylococcus aureus including strains with reduced susceptibility to daptomycin, linezolid, and vancomycin [abstract P1629]. Programme and abstracts of the 23rd European Congress of Clinical Microbiology and Infectious Diseases; Berlin, Germany. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2013. [Google Scholar]
- 16.Davies TA, Flamm RK, Lynch AS. Activity of ceftobiprole against Streptococcus pneumoniae isolates exhibiting high-level resistance to ceftriaxone. Int J Antimicrob Agents. 2012;39:534–8. doi: 10.1016/j.ijantimicag.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Davies TA, Shang W, Bush K. Activities of ceftobiprole and other beta-lactams against Streptococcus pneumoniae clinical isolates from the United States with defined substitutions in penicillin-binding proteins PBP 1a, PBP 2b, and PBP 2x. Antimicrob Agents Chemother. 2006;50:2530–2. doi: 10.1128/AAC.00238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tattevin P, Basuino L, Bauer D, Diep BA, Chambers HF. Ceftobiprole is superior to vancomycin, daptomycin, and linezolid for treatment of experimental endocarditis in rabbits caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:610–3. doi: 10.1128/AAC.00886-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers HF. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:884–8. doi: 10.1128/AAC.49.3.884-888.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saleh-Mghir A, Dumitrescu O, Dinh A, et al. Ceftobiprole efficacy in vitro against Panton-Valentine leukocidin production and in vivo against community-associated methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob Agents Chemother. 2012;56:6291–7. doi: 10.1128/AAC.00926-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin LY, Calhoun JH, Thomas JK, Shapiro S, Schmitt-Hoffmann A. Efficacies of ceftobiprole medocaril and comparators in a rabbit model of osteomyelitis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52:1618–22. doi: 10.1128/AAC.00638-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stucki A, Cottagnoud M, Acosta F, Egerman U, Läuffer J, Cottagnoud P. Evaluation of ceftobiprole activity against a variety of gram-negative pathogens, including Escherichia coli, Haemophilus influenzae (β-lactamase positive and β-lactamase negative), and Klebsiella pneumoniae, in a rabbit meningitis model. Antimicrob Agents Chemother. 2012;56:921–5. doi: 10.1128/AAC.01537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnea Y, Navon-Venezia S, Kuzmenko B, Artzi N, Carmeli Y. Ceftobiprole medocaril is an effective treatment against methicillin-resistant Staphylococcus aureus (MRSA) mediastinitis in a rat model. Eur J Clin Microbiol Infect Dis. 2014;33:325–9. doi: 10.1007/s10096-013-1959-9. [DOI] [PubMed] [Google Scholar]
- 24.Arias CA, Singh KV, Murray BE, et al. Evaluation of ceftobiprole medocaril against Enterococcus faecalis in a mouse peritonitis model. J Antimicrob Chemother. 2007;60:594–8. doi: 10.1093/jac/dkm237. [DOI] [PubMed] [Google Scholar]
- 25.Hilliard JJ, Melton JL, Flamm RK, Bush K. Efficacy of ceftobiprole against Streptococcus pneumoniae in a murine lower respiratory tract infection model [abstract A-036]. Programme and abstracts of the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy,; 2008; Washington, DC. Raritan, NJ: Johnson & Johnson Pharmaceutical Research and Devevelopment; [Google Scholar]
- 26.Fernandez J, Hilliard JJ, Abbanat D, et al. In vivo activity of ceftobiprole in murine skin infections due to Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:116–25. doi: 10.1128/AAC.00642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson SC, Welte T, File TM, Jr, et al. A double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int J Antimicrob Agents. 2012;39:240–6. doi: 10.1016/j.ijantimicag.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Committee for Medicinal Products for Human Use (CHMP) Addendum to the note for guidance on evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 REV 2) to address indication-specific clinical data. 2012. European Medicines Agency EMA/CHMP/776609/2011.
- 29.Center for Drug Evaluation and Research. US Food and Drug Administration; 2010. Guidance for industry: hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: developing drugs for treatment. Revision 1. [Google Scholar]
- 30.Rubinstein E, Lalani T, Corey GR, et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis. 2011;52:31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Réa-Neto A, Niederman M, Lobo SM, et al. Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter study. Curr Med Res Opin. 2008;24:2113–26. doi: 10.1185/03007990802179255. [DOI] [PubMed] [Google Scholar]
- 32.Freire AT, Melnyk V, Kim MJ, et al. the 311 Study Group. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68:140–51. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Zanetti G, Bally F, Greub G, et al. Cefepime Study Group. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob Agents Chemother. 2003;47:3442–7. doi: 10.1128/AAC.47.11.3442-3447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51(suppl 1):S120–5. doi: 10.1086/653060. [DOI] [PubMed] [Google Scholar]
- 35.Napolitano LM. Use of severity scoring and stratification factors in clinical trials of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010;51(suppl 1):67–80. doi: 10.1086/653052. [DOI] [PubMed] [Google Scholar]
- 36.Nguile-Makao M, Zahar JR, Français A, et al. Attributable mortality of ventilator-associated pneumonia: respective impact of main characteristics at ICU admission and VAP onset using conditional logistic regression and multi-state models. Intensive Care Med. 2010;36:781–9. doi: 10.1007/s00134-010-1824-6. [DOI] [PubMed] [Google Scholar]
- 37.Muller AE, Schmitt-Hoffmann AH, Punt N, Mouton JW. Monte Carlo simulations based on phase 1 studies predict target attainment of ceftobiprole in nosocomial pneumonia patients: a validation study. Antimicrob Agents Chemother. 2013;57:2047–53. doi: 10.1128/AAC.02292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]