We show that the per-act risk of HIV transmission through condom-unprotected sex with HIV-infected individuals on combination antiretroviral therapy (cART) in comprehensive care for >6 months is <13:100 000. Available data do not support zero HIV transmission risk under cART.
Keywords: per-act risk of HIV transmission, combined antiretroviral treatment, HIV prevention, viral load, condom use
Abstract
Background. Although essential for patient counseling and quality of life of human immunodeficiency virus (HIV)–infected individuals, the risk of HIV transmission during 1 unprotected sex act with an HIV-infected person under combination antiretroviral therapy (cART) remains unknown.
Methods. We reviewed systematically the literature for studies on HIV transmission among heterosexual HIV-serodiscordant couples, where the infected partner was on cART, with regular virological monitoring, reporting on condom use and sexual activity. We used Bayesian statistics to combine data from selected studies, to investigate the per-act risk of HIV transmission through unprotected sex with an HIV-infected person on cART for >6 months.
Results. At most, 1 HIV transmission, over an estimated 113 480 sex acts, of which 17% were not condom protected, was reported within 1672 HIV-serodiscordant couples where the index partner had been treated for >6 months. Data were insufficient to determine whether the reported transmission occurred before or after 6 months of cART. We estimated the upper-bound per-act risk of HIV transmission at either 8.7 or 13:100 000, depending on whether the transmission occurred before or after 6 months of cART. These estimates applied whether or not index partners were virally suppressed. Estimating an upper-bound risk <1:100 000 would require observing no HIV transmission while collecting >12 times the available amount of data.
Conclusions. Available data do not support zero risk of HIV transmission under cART. The per-act risk of HIV transmission through unprotected sex with HIV-infected individuals on cART in comprehensive care for >6 months (whether or not virally suppressed) is <13:100 000. Estimating a 10-fold lower upper-bound risk may be unfeasible due to high condom use among HIV-serodiscordant couples in most research studies.
Human immunodeficiency virus (HIV) transmission under combined antiretroviral therapy (cART) is a fundamental issue for the quality of life of HIV-infected individuals. Evidence supporting a low risk of transmission under cART could reduce stigma, discrimination, fear of transmission, and reluctance to disclose HIV status. Furthermore, risk estimates are indispensable for HIV prevention counseling and family planning in serodiscordant couples. Regarding public health, evidence supporting low risk of transmission is the basis of the treatment as prevention strategy, involving widespread HIV testing and early cART [1].
Studies showed that HIV transmission in HIV-serodiscordant couples decreased by 26%–96% when the HIV-infected partner was on cART [2–5]; higher reductions were observed in studies where viral load was regularly monitored [2–4]. Meta-analyses combined data from these studies to estimate HIV incidence under cART [6–9]—that is, the number of new cases per susceptible individual per unit time. HIV incidence is an aggregate epidemiological measure, which depends on the probability of transmission per unprotected sex act, sexual activity, and prevention measures (eg, condom use). The last 2 factors have strong behavioral components and are highly variable among individuals, but could not be factored out in the meta-analyses. Therefore, HIV incidence estimates resulting from these meta-analyses may not be appropriate for personalized HIV prevention counseling and evaluating the potential impact of the treatment as prevention strategy. A more appropriate measure of HIV transmission is the probability of transmission per unprotected sex act, because it may be combined with any pattern of sexual activity and prevention to calculate an individual's rate of HIV transmission.
In 2008, the Swiss Commission on AIDS asserted that HIV-infected persons on effective cART—defined as an HIV RNA level ≤40 copies/mL—for at least 6 months and without other sexually transmitted infections (STIs) cannot transmit HIV through unprotected sex; specifically, they reported that, in this case, the risk to transmit HIV per unprotected sex act is much lower than 1:100 000 [10]. This statement is an expert opinion based on reviewing 3 studies [11–13] of heterosexual HIV-serodiscordant couples. Later, it has been suggested that these studies do not provide evidence to defend the Swiss statement [6].
Here, we reviewed systematically the literature on HIV transmission under cART among serodiscordant couples. We combined data from the retrieved studies to estimate the per-act risk of heterosexual HIV transmission through unprotected sex with an infected partner on cART in clinical care for >6 months. We restricted our search to studies including virological monitoring in the care protocol, because regular viral load testing, as a means to monitor HIV treatment uptake, is a key component of successful treatment programs aiming to reduce HIV transmission [2–4]. We chose a 6-month duration because, in comprehensive care, most patients achieve viral suppression after 6 months of cART. We did not address directly the risk of HIV transmission of virally suppressed individuals. Instead, we focused on a more tangible clinical concept: the risk of HIV transmission of individuals on cART for >6 months in comprehensive care, including regular virological monitoring.
METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14, 15] to conduct our systematic review. Supplementary Table 1 provides the PRISMA checklist.
Study Search Strategy
No protocol for this review has been published. A comprehensive and systematic literature search was conducted in electronic databases and archives of abstracts from conferences (see Supplementary Section 1). The search was updated on 2 August 2013.
Study Selection
Duplicate citations were removed. Then, one author (V. S.) excluded the references that were clearly irrelevant. Two authors (V. S. and R. B.) independently assessed the reduced reference list for potential inclusion in the review. Disagreements were resolved by consensus-based discussion. Full-text reports of selected studies were assessed independently for inclusion. To be included, a study had to (1) examine HIV transmission among heterosexual couples where the HIV-infected partner was on cART, and provide information on (2) incident HIV infections among seronegative partners, (3) viral load of the treated index partner, (4) condom use, and (5) sexual activity. Among studies following the same population, only the most complete were considered. We contacted study authors for additional information when necessary, and relied solely on the published data when the authors did not reply.
Data Collection
A data collection form was developed to retrieve information from the included studies. Two authors (V. S. and R. B.) independently extracted data; discrepancies were resolved by consensus. Data included study characteristics (number of couples with follow-up, total follow-up duration, location and time period of the study), care protocols (duration between follow-up visits, frequency of viral load and CD4 determinations, detection limit of viral load assay, type of cART, cART adherence, management of virologic failure, fraction of virally suppressed participants, treatment of other STIs, HIV testing frequency, couples’ counseling), couples' characteristics (frequency of sex acts, condom use, cART status of the index partner, male circumcision status, type of sex act) and clinical outcomes (number of HIV transmissions on and off cART, time from cART initiation to HIV transmission, whether HIV transmission was genetically linked to the index partner).
Risk of Bias Assessment
Two authors (V. S. and R. B.) independently assessed the risk of bias in the included studies (Supplementary Section 2). Publication bias was not formally assessed due to the low number of studies.
Data Analysis and Statistical Methods
Bayesian statistics [16, 17] was used to infer the risk of HIV transmission per unprotected sex act with an infected person on cART in clinical care for >6 months. We assumed a low-informative prior distribution for the risk of transmission under cART, uniform between 0 and the value of the risk of HIV transmission off ART, reflecting lack of knowledge and ensuring that the results are data driven. This prior distribution was updated with observed data on serodiscordant couples where the index partner was on cART, via a likelihood model, to obtain the posterior distribution, used for summary statistics (Supplementary Section 3).
The likelihood model required values for the following parameters regarding serodiscordant couples where the index partner had been on cART for >6 months: (1) number of couples with follow-up data, (2) number of linked HIV transmissions, (3) number of sex acts that occurred during follow-up, (4) fraction of couples reporting 100% condom use and (5) per-act efficacy of condom. The prior distribution required estimating the per-act risk of HIV transmission off ART. Values for parameters 1–4 were either collected or estimated from sexual activity data provided by the included studies (Supplementary Section 4). The condom efficacy and risk of HIV transmission off ART were estimated through an auxiliary Bayesian analysis using data on serodiscordant couples where the index partner was off ART, collected from the same included studies (Supplementary Section 4.1).
Using the per-act risk of transmission, we calculated the cumulative risk of acquiring HIV after numerous sex acts with partners who had been on cART for >6 months (Supplementary Section 5). We also compared cART vs condom for HIV prevention by calculating the probability that the risk of HIV transmission under cART is lower than that off ART when condoms are used (Supplementary Section 6). We performed sensitivity analyses to investigate the potential impact of overreporting condom use on our results.
RESULTS
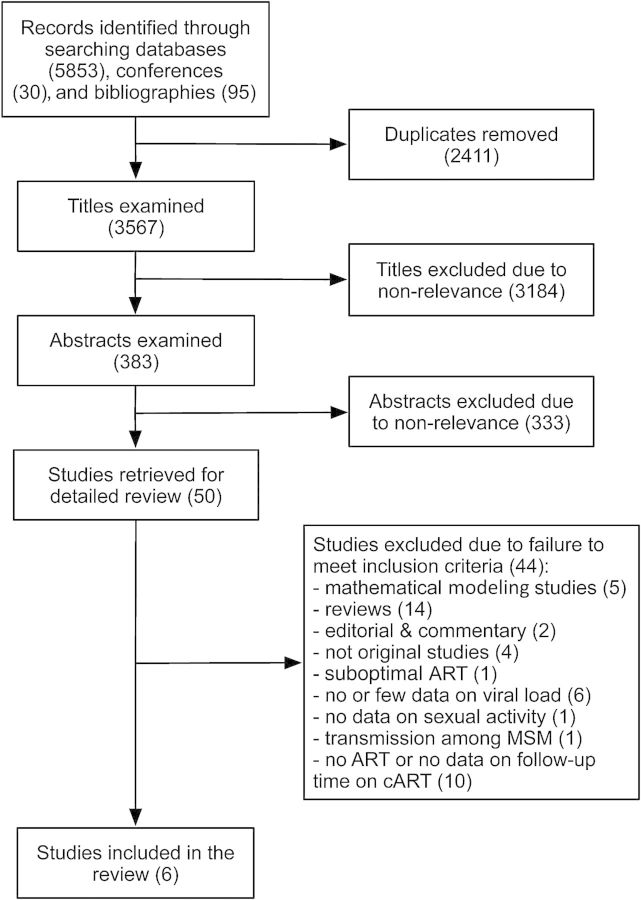
The search produced 5978 titles, of which 2411 were removed as duplicates (Figure 1). After screening the titles, 383 publications qualified for further review. Of these, 50 were eligible for full-text review based on screening the abstract. Six studies met all the criteria to be included in our analysis [2–4, 12, 18, 19]. All included studies had low risk of bias (Supplementary Tables 2 and 3). We received unpublished data from the authors of 3 included studies [2, 4, 12]. Articles excluded after full-text review are listed, by reason for exclusion, in Supplementary Section 7.
Figure 1.
Flow diagram summarizing the study selection process. Further details regarding reasons for exclusion are provided in the Supplementary Data for the 10 studies excluded because of no antiretroviral therapy or no data on follow-up time on combination antiretroviral therapy. Abbreviations: ART, antiretroviral therapy; cART, combination antiretroviral therapy; MSM, men who have sex with men.
Extracted data are presented in Tables 1 and 2 and Supplementary Table 4. Table 1 presents a summary of the care protocols followed in the included studies. The serodiscordant couples attended regular checkups (every 3–6 months) when the viral loads and CD4 counts of the index partners were determined. If needed, couples were treated for STIs (in 5 of 6 studies). The index partners were provided with adherence support (in 3 of 6 studies) and management of virologic failure (in 4 of 6 studies). The fraction of virally suppressed patients varied from 70% to 100%. Table 2 and Supplementary Table 4 list couples' characteristics and clinical outcomes when index partners were on and off cART, respectively.
Table 1.
Summary of the Protocols of Care Provided to the HIV-Serodiscordant Couples in the 6 Selected Clinical Studies
| Study, First Author (Year) | Time Interval Between Visits, mo | CD4 and VL Measurements | Viral Suppression Threshold, Copies/mL | First-line Regimen | Management of Virologic Failure | Adherence to cART | Fraction of Virally Suppressed Participants | Treatment of STI (Other Than HIV) | Other Information |
|---|---|---|---|---|---|---|---|---|---|
| Melo (2008) [12] | ≤6 | At each visit | Not specified <78 | AZT/3TC/NFV; AZT/3TC/EFV; |
Provided | Not specified | 100%a | Provided (diagnosed in 23.6% of the couples) | STI test for seronegative partners |
| Donnell (2010) [4] | 3 | CD4 every 6 mo VL (enrollment, 3, 6, 12, and 24 mo) |
240 | D4T/3TC/NVP (61%); AZT/3TC/NVP (13%): PI-containing regimen; (3%); others (16%); insufficient data to establish full regimen (7%) |
Not specified | 13% of the patients on cART reported no ART use at a subsequent visit | 70% by the final study visit | Provided | Quarterly HIV antibody test for seronegative partners; couples’ counseling on HIV risk |
| Del Romero (2010) [3] | 6 | At each visit | 500 (until 1999) 50 (after) |
Combined therapy with at least 3 active drugs | Provided | Not specified | 92%a | Provided (diagnosed in 6% of the couples) | HIV antibody test for seronegative partners; both partners attended the follow-up visits |
| Cohen (2011) [2] | 3 | At each visit | 400 | AZT/3TC/EFV (72%); AZT/3TC/ATV (9%); FTC/TDF/EFV (9%); AZT/3TC/LPV/RTV (6%); others (3%) | Second-line treatment regimens provided | Adherence counseling and self-reporting pill counts (79% with >95% adherence) | 89% by 3 mo and 91% by 24 mo | Provided | Quarterly HIV antibody test for seronegative partners; couple counseling on HIV risk |
| Reynolds (2011) [19] | 6 | VL at each visit, CD4 not specified | 400 | Not specified | Not specified | Adherence counseling and support | 71% by 6 mo, 85% by 12 mo and 100% by 24 mo | Provided (diagnosed in 2% of the couples) | HIV antibody test for seronegative partners; couple counseling |
| Apondi (2011) [18] | 3 | At each visit | 400 | D4T/3TC/NVP; D4T/3TC/EFV |
Provided | Adherence interventions and high adherence based on pill counts | >90% at 36 mo | Not provided | Annually HIV antibody test for seronegative partners; couple counseling |
Abbreviations: 3TC, lamivudine; ATV, atazanavir; AZT, zidovudine; cART, combined antiretroviral therapy; D4T, stavudine; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; LPV, lopinavir; NFV, nelfinavir; NVP, nevirapine; PI, protease inhibitor; RTV, ritonavir; STI, sexually transmitted infection; TDF, tenofovir; VL, viral load.
a The authors of the study did not mention at what time point the fraction of virally suppressed individuals was measured.
Table 2.
Characteristics of Serodiscordant Couples Under Combined Antiretroviral Therapy in the 6 Selected Clinical Studies
| Study, First Author (Year) | Region of Study and Time Period | No. of Couples With Follow-up | Length of Follow-up, Person-years | No. of Sex Actsa | Condom Usea | No. of HIV Transmissions Linked to Index Partners | Time From cART Initiation to HIV Transmission | Other Information |
|---|---|---|---|---|---|---|---|---|
| Melo (2008) [12] | Brazil, 2000–2006 | 41 | 90.4 | Median 12 per mo per couple | 77% of index partners reported unprotected sexual intercourse | 0 | N/A | No men were circumcised |
| Donnell (2010) [4] | 7 African countriesb, 2004–2008 | 349 | 273 | Median 4 per mo per couple | Unprotected sex acts were reported by index partners at 4% of all clinical visits | 1 | ≤3 mo | 55% of uninfected male partners were circumcised |
| Del Romero (2010) [3] | Spain, 1989–2008 | 144 | 417 | 28 000 over all follow-up | 7400 risky sexual exposures over all follow-up | 0 | N/A | Unprotected penile-anal contacts in 8% of couples |
| Cohen (2011) [2]c | Botswana, Kenya, Malawi, South Africa, Brazil, India, Thailand, US, 2007–2011 | 860 | 1585.3 | Median 1 per week per couple | A mean of 96% of index partners reported 100% condom use during the study | 1 | ≤3 mo | 19% of uninfected male partners were circumcised |
| 184 | 169.5 | Median 1 per week per couple | A mean of 95% of index partners reported 100% condom use during the study | 1 | ≤1 mo | 14% of uninfected male partners were circumcised | ||
| Reynolds (2011) [19] | Uganda, 2004–2009 | 32 | 53.6 | Median 3 per mo per couple | 54% of time periods at which 100% condom use was reported | 0 | N/A | 21% of uninfected male partners were circumcised |
| Apondi (2011) [18] | Uganda, 2003–2007 | 62 | 184 | Mean 16.6 per 3 mo per couple | 77% of clinical visits at which 100% condom use was reported | 1 | ≤12 mo | |
| Total | 1672 | 2772.8d | 4 |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; N/A, not applicable.
a Data using comparable units can be found in the Supplementary Table 5.
b Botswana, Kenya, Rwanda, South Africa, Tanzania, Uganda, Zambia.
c The top and bottom rows report on the early and delayed therapy arms of the trial, respectively.
d Follow-up time after 6 months of cART can be found in Supplementary Table 5.
HIV Transmissions and Sexual Activity on cART
Four incident HIV transmissions, genetically linked to the index partner, occurred within 1672 serodiscordant couples (during 2772.8 person-years) where the index partner was on cART (Table 2). At least 3 transmissions occurred when the index partner had been on cART for <6 months. The fourth transmission, reported by Apondi et al [18], occurred within the first year of cART; the index partner took 6 months to achieve viral suppression. Data were, however, insufficient to determine whether this infection occurred before or after 6 months of cART because the partner, who was HIV negative at baseline, was retested only at 12 months. Baseline median number of sexual acts per couple was 3–12 per month; 2 studies reported on the average or total number of sex acts that occurred during the study. Condom use was high in most studies. These data provided parameter estimates needed for the likelihood model of the Bayesian analysis (Supplementary Section 8 and Supplementary Table 5). The rest of the parameter estimates were obtained from data on couples where the infected partner was off ART.
Condom Efficacy and Per-Act Risk of HIV Transmission off ART
In total, 182 HIV incident transmissions occurred within 4817 serodiscordant couples where the index partner was off ART during 7384.1 person-years of follow-up (Supplementary Table 4). With the exception of 1 study [19], sexual activity and condom use were similar to those of couples where the index partner was on cART. Using these data, we estimated the parameters required for the auxiliary Bayesian analysis (Supplementary Table 6) and obtained 75% (95% credible interval, 63%–83%) for the per-act condom efficacy, and 0.0014 (0.0010–0.0018) for the per-act risk of transmission off ART during condom-unprotected sex (Supplementary Section 8.1).
Per-Act Risk of HIV Transmission Beyond 6 Months of cART
We estimated that a total of 113 480 sex acts, of which 17% were not condom protected, occurred in couples when the index partner had been on cART for >6 months. Because at most 1 HIV transmission occurred during these sex acts, we focused on estimating the upper bound of the per-act risk. We considered 2 exclusive cases regarding the transmission event reported by Apondi et al [18]. In case 1, the transmission occurred within the first 6 months of cART (ie, no transmission occurred after 6 months of cART), whereas in case 2, the transmission occurred after 6 months of cART, (ie, 1 transmission occurred after 6 months of cART). Our Bayesian analyses (Supplementary Sections 8.2 and 8.3) showed that the upper-bound risk was 8.7:100 000 in case 1 and 13:100 000 in case 2.
Data Needed for the Estimated Upper-Bound Risk on cART to Be at 1:100 000
We evaluated how many more sex acts with HIV-infected partners on cART for >6 months, without any incident HIV infection, should be observed for estimating an upper-bound per-act risk of HIV transmission at 1:100 000, the figure provided by the Swiss commission (Supplementary Section 9). In case 1, we found that one should monitor between 326 000 (if the sex acts are unprotected; ie, 0% condom use) and 1 185 000 (if 96% of the couples use condoms 100% of the time) sex acts. By comparison, this represents 27 years of follow-up of the early-therapy arm of HIV Prevention Trials Network 052 (ie, 860 couples), or approximately 12 times the amount of the current data.
Cumulative Risk of HIV Transmission on cART
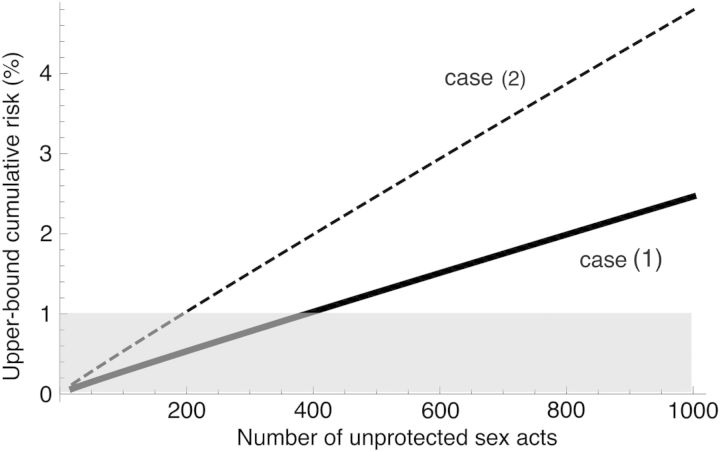
The risk of HIV transmission cumulates over repeated exposures. Using the per-act risk of HIV transmission after 6 months of cART, we calculated the cumulative probability of acquiring HIV as a function of the number of sex acts (Supplementary Section 5). We found that the upper bound of the cumulative probability (Figure 2) would exceed 1% after 389 sex acts in case 1 and 195 sex acts in case 2.
Figure 2.
Upper-bound cumulative risk of human immunodeficiency virus (HIV) transmission within a heterosexual serodiscordant couple where the partner had been on combination antiretroviral therapy (cART) in care for >6 months vs the number of condom-unprotected sexual acts. We considered 2 interpretations of current data. In case 1, the HIV transmission reported by Apondi et al [18] occurred within the first 6 months of cART (continuous line), whereas in case 2, the HIV transmission reported by Apondi et al [18] occurred after 6 months of cART (dashed line). Hence, in case 1, no HIV transmission occurred beyond 6 months of cART, whereas in case 2, 1 HIV transmission occurred beyond 6 months of cART. The gray area indicates upper-bound cumulative risk <1%.
HIV Prevention: cART Versus Condom Use
We used the distribution of the per-act risk of HIV transmission under cART and the distribution of the per-act risk of HIV transmission off ART when using a condom to calculate the probability that condom use is more protective than cART against HIV transmission. This probability was <0.01% in case 1 and 0.02% in case 2 (Supplementary Sections 6 and 10).
Sensitivity Analyses
To arrive at condom efficacy estimates in agreement with previously published estimates of 90%–95% [20], we assumed that 20% of couples reporting 100% condom use did not in fact use condoms, whether or not the index partner was on cART (Supplementary Section 10). Rerunning the analyses, we found that our results did not change significantly (Supplementary Table 8), except for the per-act condom efficacy, from 75% (63%–83%) to 91% (77%–99%), and the probability that condom use is more protective than cART against HIV transmission, from <0.01% to 10% in case 1 and from 0.02% to 20% in case 2.
DISCUSSION
Using a Bayesian approach, we combined data from 6 studies to obtain the first estimate of the risk of heterosexual HIV transmission per unprotected sex act with an HIV-infected individual under cART in comprehensive care for >6 months. The reported number of HIV transmissions caused by index partners on cART for >6 months could be zero; data were insufficient to determine when the HIV transmission event reported by Apondi et al [18] occurred. Failure to observe transmission events does not exclude the possibility of HIV transmission, yet indicates a low risk, which in theory, could be even zero. The number of monitored sex acts can be used for estimating an upper bound of the risk, a concept widely spread in toxicology and oncology.
We estimated the upper-bound per-act risk to be between 8.7:100 000 and 13:100 000, depending on how the transmission event was considered. We also estimated that the upper-bound cumulative risk would exceed 1% after 195–389 sex acts. Furthermore, according to our findings, cART is likely more protective than condom use against HIV transmission.
We used data originated from studies providing comprehensive care programs to serodiscordant couples, including regular viral load and CD4 monitoring, management of virologic failure, adherence support, and STI treatment. These programs led to viral suppression for >70% of the patients, in line with the performance of the standard of care in some high-income countries (eg, 86% for France [21] and 80% for the United States [22]). Hence, our results are relevant for patients undergoing cART for >6 months under such comprehensive care.
Although not directly derived for virally suppressed individuals, some of our findings apply to them. We estimated the upper-bound risk by combining all data on couples where the index partner had been on cART for >6 months, whether or not virally suppressed. At most, 1 transmission event occurred, caused by an index partner on cART for >6 months who reached viral suppression at 6 months of cART [18]. Restricting the data to virally suppressed index partners (representing 70%–100% of the total number of index partners in each study) would have resulted in the same number of transmission events but fewer sex acts. Consequently, this would yield less statistical power and higher upper-bound risks. Therefore, data do not support lower upper-bound risks for virally suppressed individuals. In addition, further restricting the data to couples where the index partner had been on effective cART for at least 6 months (ie, satisfying one of the Swiss statement requirements) would have resulted in zero transmission events (as in our case 1) but many fewer sex acts. Consequently, this would yield even less statistical power and higher upper-bound risk than in our case 1. Therefore, data do not support a lower upper-bound risk than in case 1 (ie, 8.7:100 000), for individuals on effective cART for >6 months, hence the Swiss statement. Furthermore, we found it would require collecting 12 times the available amount of data while observing no incident HIV case for the estimated upper-bound risk to be at 1:100 000, the figure provided by the Swiss statement. A data collection effort of this magnitude may be unfeasible because of the considerable duration of follow-up and/or monitoring costs.
The main strengths of our approach are as follows. First, all parameters needed for estimating the per-act risk were inferred from data of the included studies. Second, our modeling framework is validated by the agreement between our estimates for condom efficacy and risk of HIV transmission off ART and those reported by previous work. We obtained 0.0014 (0.0010–0.0018) for the per-act risk off ART, in the range of previous estimates (0.0001–0.0110) [23]. Relying on self-reports of condom use, we estimated the per-act condom efficacy at 75% (63%–83%), in agreement with recent estimates [24], but lower than older estimates [20]. However, sensitivity analyses showed that overreporting of condom use might explain this discrepancy, without affecting other results.
Some limitations should be acknowledged. First, our calculations rely on data from studies that enrolled exclusively [3, 4, 12, 18, 19] or almost exclusively [2] (ie, 97%) heterosexual couples, and may have limited applicability for men having sex with men. Second, we could not distinguish between male-to-female and female-to-male transmission risks due to lack of data. However, these 2 risks were found similar after adjustment for viral load [24]. Third, we could not examine the effect of HIV genotype, host genetics, circumcision status, type of intercourse, or STIs on the HIV transmission risk [24, 25]. Hence, our results remain averaged over these population characteristics.
Our findings are essential for evaluating the population-level impact of treatment as prevention policies. Based on evidence that cART reduces HIV incidence, the United States and France adopted new guidelines, recommending clinicians to offer cART to all patients regardless of CD4 count [26, 27]. Furthermore, the World Health Organization now recommends offering cART when the CD4 count falls below 500 cells/µL (rather than 350 cells/µL) [28]. However, HIV incidence depends on not only the reduction in the per-act risk of HIV transmission due to cART, but also sexual activity and prevention methods other than cART. The per-act risk under cART quantifies the contribution of treatment alone to preventing HIV transmission, independent of sexual activity and other prevention methods. Hence, knowing the per-act risk under cART is essential to support treatment as prevention policies.
CONCLUSIONS
Our findings provide key information for patient counseling on HIV transmission risk and preventive care. Available data do not support zero risk of HIV transmission under cART. The per-act risk of HIV transmission through unprotected sex with HIV-infected individuals on cART in comprehensive care for >6 months (whether or not virally suppressed) is <13:100 000. Estimating a 10-fold lower upper-bound risk may be unfeasible due to high condom use among serodiscordant couples in most research studies. Even though it is small, the risk of transmission cumulates with the number of exposure and may represent a major long-term concern. Combining several prevention methods may offer optimal protection against HIV transmission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Breno Riegel Santos, Karin Nielsen, Lei Wang, Myron Cohen, and Deborah Donnell for providing additional data.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by Sidaction and Agence Nationale de Recherche sur le SIDA et les Hépatites Virales, in the form of postdoctoral research fellowships to V. S.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Romero J, Castilla J, Hernando V, Rodriguez C, Garcia S. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ. 2010;340:c2205. doi: 10.1136/bmj.c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Z, Ruan Y, Li Q, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet. 2013;382:1195–203. doi: 10.1016/S0140-6736(12)61898-4. [DOI] [PubMed] [Google Scholar]
- 6.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 7.Baggaley RF, White RG, Hollingsworth TD, Boily MC. Heterosexual HIV-1 infectiousness and antiretroviral use: systematic review of prospective studies of discordant couples. Epidemiology. 2013;24:110–21. doi: 10.1097/EDE.0b013e318276cad7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loutfy MR, Wu W, Letchumanan M, et al. Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PLoS One. 2013;8:e55747. doi: 10.1371/journal.pone.0055747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev. 2013;4:CD009153. doi: 10.1002/14651858.CD009153.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernazza P, Hirschel B, Bernasconi E, Flepp M. Les personnes séropositives ne souffrant d'aucune autre MST et suivant un traitement antirétroviral efficace ne transmettent pas le VIH par voie sexuelle. Bulletin des Médecins Suisses. 2008;89:165–9. [Google Scholar]
- 11.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40:96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 12.Melo MG, Santos BR, De Cassia Lira R, et al. Sexual transmission of HIV-1 among serodiscordant couples in Porto Alegre, southern Brazil. Sex Transm Dis. 2008;35:912–5. doi: 10.1097/OLQ.0b013e31817e2491. [DOI] [PubMed] [Google Scholar]
- 13.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 16.Lilford RJ, Braunholtz D. The statistical basis of public policy: a paradigm shift is overdue. BMJ. 1996;313:603–7. doi: 10.1136/bmj.313.7057.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narsky I. Estimation of upper limits using a Poisson statistic. Nucl Instrum Meth Phys Res Sect A. 2000;450:444–55. [Google Scholar]
- 18.Apondi R, Bunnell R, Ekwaru JP, et al. Sexual behavior and HIV transmission risk of Ugandan adults taking antiretroviral therapy: 3 year follow-up. AIDS. 2011;25:1317–27. doi: 10.1097/QAD.0b013e328347f775. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–7. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinkerton SD, Abramson PR, Turk ME. Effectiveness of condoms in preventing HIV transmission. Soc Sci Med. 1997;44:1303–12. doi: 10.1016/s0277-9536(96)00258-4. [DOI] [PubMed] [Google Scholar]
- 21.Supervie V, Costagliola D. The spectrum of engagement in HIV care in France: strengths and gaps. 20th Conference on Retroviruses and Opportunistic Infections; March 2013; Atlanta, GA,. Paper 1030. [Google Scholar]
- 22.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarzebowski W, Caumes E, Dupin N, et al. Effect of early syphilis infection on plasma viral load and CD4 cell count in human immunodeficiency virus-infected men: results from the FHDH-ANRS CO4 cohort. Arch Intern Med. 2012;172:1237–43. doi: 10.1001/archinternmed.2012.2706. [DOI] [PubMed] [Google Scholar]
- 26.Ministère des Affaires Sociales et de la Santé, Conseil National du Sida, Agence Nationale de Recherche sur le SIDA et les Hépatites Virales. Rapport d'experts sur la prise en charge médicale de l'infection par le VIH. 2013. Available at: http://www.sante.gouv.fr/IMG/pdf/Rapport_Morlat_2013_Mise_en_ligne.pdf . Accessed 19 April 2014.
- 27.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Geneva, Switzerland: WHO; 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.