Abstract
High histaminase [amine:oxygen oxidoreductase (deaminating) (pyridoxal-containing), EC 1.4.3.6] activity is found in certain human tumors and in the placenta of most mammals. The present study explores the relationship of tumor histaminase to histaminases found in placenta and other human, pig, and rat tissues. The electrophoretic mobility and Michaelis constants for the deamination of histimine and putrescine were identical for histaminases from human placenta and from medullary thyroid carcinoma. An antibody was raised in rabbits against human placental histaminase that was highly purified by a new affinity procedure. In separate studies, using inhibitory concentrations of antibody and a second antibody precipitation technique, identical patterns of immunoreactivity were found for histaminases from human placenta, kidney, medullary thyroid carcinoma, and small cell lung carcinoma; human intestinal histaminase crossreacted well but less strongly than did enzymes from these other tissues. Histaminases from pig kidney, pig intestine, and rat intestine showed no crossreaction; histaminases from rat thymus and adrenal gland showed minimal crossreactivity. The findings suggest that placental histaminase activity is not a unique product of a fetal or trophoblastic genome. The presence of histaminase in malignancies does not appear to be an example of ectopic tumor production of a placental trophoblastic protein.
Full text
PDF

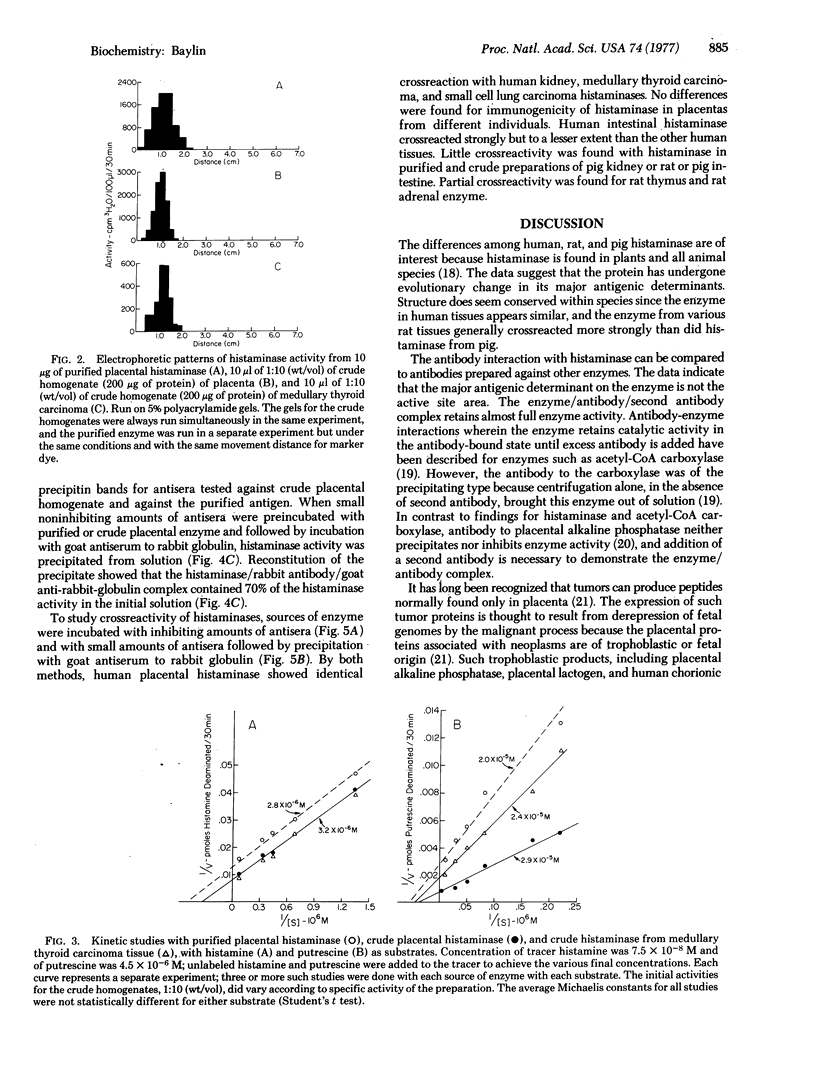
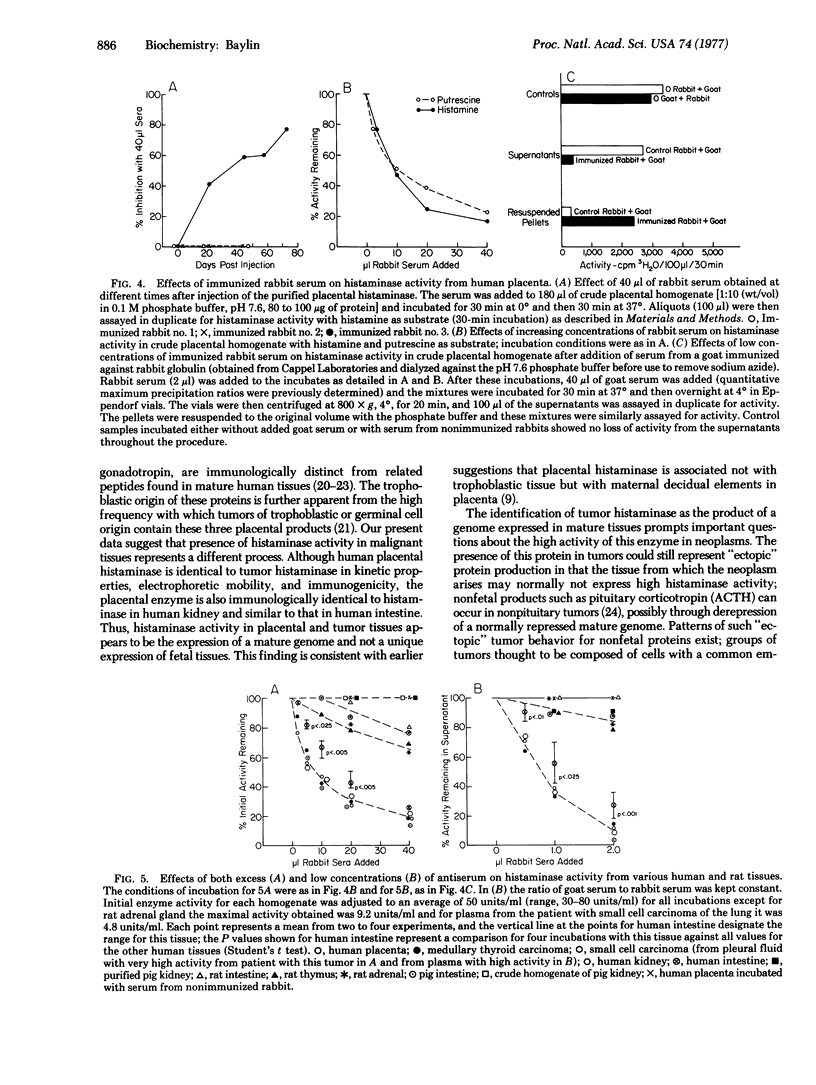

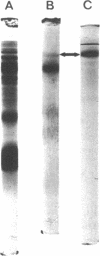
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORGLIN N. E., WILLERT B. Increased histaminolytic power of plasma in endometrial adenocarcinoma. Cancer. 1962 Mar-Apr;15:271–275. doi: 10.1002/1097-0142(196203/04)15:2<271::aid-cncr2820150210>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- BOYER S. H. Human organ alkaline phosphatases: discrimination by several means including starch gel electrophoresis of antienzyme-enzyme supernatant fluids. Ann N Y Acad Sci. 1963 May 8;103:938–951. doi: 10.1111/j.1749-6632.1963.tb53746.x. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Abeloff M. D., Wieman K. C., Tomford J. W., Ettinger D. S. Elevated histaminase (diamine oxidase) activity in small-cell carcinoma of the lung. N Engl J Med. 1975 Dec 18;293(25):1286–1290. doi: 10.1056/NEJM197512182932504. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Beaven M. A., Buja L. M., Keiser H. R. Histaminase activity: a biochemical marker for medullary carcinoma of the thyroid. Am J Med. 1972 Dec;53(6):723–733. doi: 10.1016/0002-9343(72)90189-1. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Beaven M. A., Engelman K., Sjoerdsma A. Elevated histaminase activity in medullary carcinoma of the thyroid gland. N Engl J Med. 1970 Dec 3;283(23):1239–1244. doi: 10.1056/NEJM197012032832301. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Beaven M. A., Keiser H. R., Tashjian A. H., Jr, Melvin K. E. Serum histaminase and calcitonin levels in medullary carcinoma of the thyroid. Lancet. 1972 Feb 26;1(7748):455–458. doi: 10.1016/s0140-6736(72)90120-1. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Margolis S. Purification of histaminase (diamine oxidase) from human pregnancy plasma by affinity chromatography. Biochim Biophys Acta. 1975 Aug 26;397(2):294–306. doi: 10.1016/0005-2744(75)90119-9. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Jacobsen S. A new assay for histaminase activity: measurement of tritiated water from beta (side chain label)-H 3-histamine. J Pharmacol Exp Ther. 1971 Jan;176(1):52–64. [PubMed] [Google Scholar]
- Beaven M. A., Shaff R. E. Study of the relationship of histaminase and diamine oxidase activities in various rat tissues and plasma by sensitive isotopic assay procedures. Biochem Pharmacol. 1975 May 1;24(9):979–984. doi: 10.1016/0006-2952(75)90431-1. [DOI] [PubMed] [Google Scholar]
- Boyer S. H. Alkaline Phosphatase in Human Sera and Placentae: Starch gel electrophoresis reveals many phosphatase components including a polymorphism in placentae. Science. 1961 Oct 6;134(3484):1002–1004. doi: 10.1126/science.134.3484.1002. [DOI] [PubMed] [Google Scholar]
- CASATI G., CARBONE M., LOPS M., ITALIA R. [Values of serum histaminase in respiratory pathology]. G Ital Tuberc Mal Torace. 1963 Jan-Feb;17:8–10. [PubMed] [Google Scholar]
- Clark J. L., Duffy P. Polyamine metabolism, RNA synthesis, and proliferation in density-Inhibited 3T3 cells. Arch Biochem Biophys. 1976 Feb;172(2):551–557. doi: 10.1016/0003-9861(76)90107-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gunther R. E., Glick D. Determination of histaminase activity in histologic samples and its quantitative distribution in intact human placenta and uterus. J Histochem Cytochem. 1967 Aug;15(8):431–435. doi: 10.1177/15.8.431. [DOI] [PubMed] [Google Scholar]
- Keiser H. R., Beaven M. A., Doppman J., Wells S., Jr, Buja L. M. Sipple's syndrome: medullary thyroid carcinoma, pheochromocytoma, and parathyroid disease. Studies in a large family. NIH conference. Ann Intern Med. 1973 Apr;78(4):561–579. doi: 10.7326/0003-4819-78-4-561. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liddle G. W., Givens J. R., Nicholson W. E., Island D. P. The ectopic ACTH syndrome. Cancer Res. 1965 Aug;25(7):1057–1061. [PubMed] [Google Scholar]
- Lin C. W., Orcutt M. L., Stolbach L. L. Elevation of histaminase and its concurrence with Regan isoenzyme in ovarian cancer. Cancer Res. 1975 Oct;35(10):2762–2765. [PubMed] [Google Scholar]
- Majerus P. W., Kilburn E. Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver. J Biol Chem. 1969 Nov 25;244(22):6254–6262. [PubMed] [Google Scholar]
- OKUYAMA T., KOBAYASHI Y. Determination of diamine oxidase activity by liquid scintillation counting. Arch Biochem Biophys. 1961 Nov;95:242–250. doi: 10.1016/0003-9861(61)90141-2. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Placental proteins and their subunits as tumor markers. Ann Intern Med. 1975 Jan;82(1):71–83. doi: 10.7326/0003-4819-82-1-71. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Stambrook P. J. Cell cycle specific fluctuations in adenosine 3':5'-cyclic monophosphate and polyamines of Chinese hamster cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1482–1486. doi: 10.1073/pnas.72.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANBERG H. Histaminase in pregnancy: with special reference to its origin and formation; an experimental and clinical study. Acta Physiol Scand Suppl. 1950;23(79):1–69. [PubMed] [Google Scholar]
- Sussman H. H., Small P. A., Jr, Cotlove E. Human alkaline phosphatase. Immunochemical identification of organ-specific isoenzymes. J Biol Chem. 1968 Jan 10;243(1):160–166. [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Welbourn R. B., Pearse A. G., Polak J. M., Bloom S. R., Joffe S. N. The APUD cells of the alimentary tract in health and disease. Med Clin North Am. 1974 Nov;58(6):1359–1374. doi: 10.1016/s0025-7125(16)32077-6. [DOI] [PubMed] [Google Scholar]