Abstract
A high level of encephalization is critical to the human adaptive niche and emerged among hominins over the course of the past 2 Myr. Evolving larger brains required important adaptive adjustments, in particular regarding energy allocation and life history. These adaptations included a relatively small brain at birth and a protracted growth of highly dependent offspring within a complex social environment. In turn, the extended period of growth and delayed maturation of the brain structures of humans contribute to their cognitive complexity. The current palaeoanthropological evidence shows that, regarding life history and brain ontogeny, the Pleistocene hominin taxa display different patterns and that one cannot simply contrast an ‘ape-model’ to a ‘human-model’. Large-brained hominins such as Upper Pleistocene Neandertals have evolved along their own evolutionary pathway and can be distinguished from modern humans in terms of growth pattern and brain development. The life-history pattern and brain ontogeny of extant humans emerged only recently in the course of human evolution.
Keywords: hominins, brain, ontogeny, Pleistocene, Homo erectus, Neandertal
1. Introduction
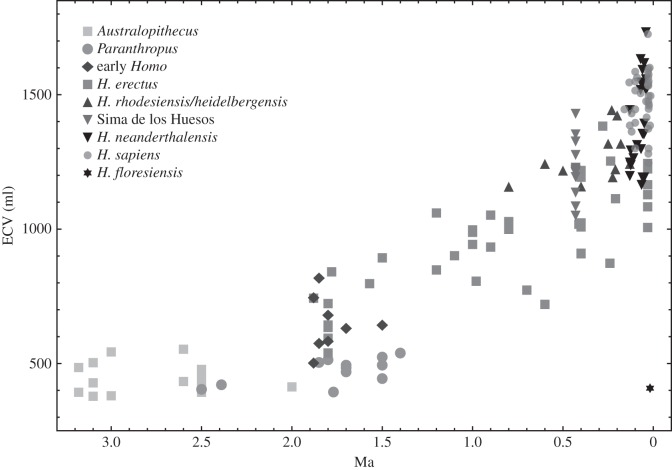
The human adaptive niche is largely shaped around the development of technological and social complexities. These developments allowed our species to extract energy from the environment in a way that is unprecedented among large mammals and subsequently resulted in a dramatic demographic expansion. The complex cognitive abilities required by such adaptive strategies are linked to very high levels of encephalization in hominins, i.e. large brain size relative to body size. Among mammals, the encephalization quotient and number of cortical neurons displayed by humans have only been approached by some cetaceans [1]. In Old World monkeys and apes (Catarrhini), brain size is closely related to body size [2]. The ancestors of the human genus, the australopiths, barely deviated from African apes in terms of brain volume (figure 1), but approximately 2 Ma early Homo started to evolve larger brains and bodies. Until at least 600 000 years before present, the increase of endocranial volume was primarily linked to increases in body mass [9]. It is only after this date that a significant (ca 30%) increase in endocranial volume occurred independently of changes in body mass. Finally, over the course of the past 30 000 years, body size and brain size both declined slightly in recent Homo sapiens.
Figure 1.
Hominin brain size in the past 3.5 Myr. Absolute endocranial volumes of adult hominin fossils are plotted versus their geological ages. The symbols represent different hominin groups according to the legend. Adapted from Holloway et al. [3], Lordkipanidze et al. [4], Arsuaga et al. [5], Falk et al. [6], Berger et al. [7] and Kimbel and Rak [8].
The selective benefits of the very large human brain are counterbalanced by significant energetic costs. The brain is highly thermoregulated and extremely vulnerable to energy shortages, especially during development [10]. An adult brain requires ca 20–25% [11] of the basal metabolic rate for functioning and maintenance. This proportion is much higher during early growth [12] and peaks close to 66% by 4.2–4.4 years, when the developing brain approaches its adult size and synaptic densities are maximal [13]. Several adaptations that are unique to humans among primates have therefore been linked to the need for providing adequate energetic resources to the large human brain. The ‘expensive tissue hypothesis' [14] posits that with increased meat and fat consumption a new source of highly energetic food was available to early Homo. This dietary shift would also have resulted in a reduction of the size of the energy-demanding digestive tract necessary for primarily vegetarian diets, and therefore allowed the reallocation of available energy to the brain. Various other changes in primate body composition have been considered to play a role in reducing the metabolic costs associated with somatic maintenance in humans, in particular, reduced muscularity [15,16]. One of the strengths of this model results from the spectacular increase of meat and marrow consumption observed in early Homo. It has also been proposed that the use of fire for cooking during the Middle Pleistocene or even earlier [17] increased food efficiency and reduced time allocated to foraging, chewing and digesting. Whereas data collected on a wide range of mammalian species [18,19] do not indicate a trade-off between the digestive tract (or any other energetically expensive organ) and the brain, this mechanism might still apply within hominids, which developed by far the largest brains [20].
The ‘maternal energy hypothesis' emphasizes the role of the increased metabolic rate and changes in the life-history pattern in humans for both developing and maintaining a large brain [21]. According to this hypothesis the size of the brain would primarily depend on the amount of energy that a mother can invest during the early ontogeny of her offspring. However, for methodological reasons it was difficult to test this relation adequately [18]. Energy supply is indeed crucial during fetal and early postnatal brain development. Among other human adaptations, the evolution of the highly invasive hemochorial placenta is often considered a response to the needs of the fast growing embryonic brain regarding nutrients and oxygen. The human ability to store energy in adipose tissues and thereby to buffer periodical shortages is also critical during development. In contrast to other primates, humans are born with a high level of adiposity and maintain this during early postnatal life [13]. By oxidation of fatty acids this fat layer provides ketones—the predominant brain fuel in the neonate—and it also contains brain-selective omega-3 fatty acid [22]. It has been proposed that regular exploitation of shore environments allowed early hominins to access aquatic food sources, rich in brain-selective nutrients [22].
Human absolute brain size increases rapidly during early development and, it reaches its adult size later than in other primate species [23]. Importantly, most of the human brain development takes place after birth, with a brain size at birth of around 28% of the adult size [24]. Human offspring are highly dependent on their parents during early development and humans are said to be ‘secondarily altricial’. From an obstetrical perspective, the reduced proportional brain size in humans at birth allowed limitation of the increase in birth canal dimensions over the course of human evolution, as human pelvic anatomy is constrained by postural and biomechanical factors related to bipedalism as well as by thermoregulatory limitations.
Prolonged human development also has important effects on internal brain structures and their maturation. As a result of the low proportional brain size at birth and prolonged development in humans, a large portion of the brain's wiring develops over a long period of time while growing individuals interact with an enriched physical and cultural environment and are exposed to a vast variety of stimuli. Histological studies of brain tissue and magnetic resonance imaging (MRI) revealed that the developmental internal changes of the brain's wiring pattern are not only dramatic during early postnatal ontogeny but also that they extend long past the time of reaching adult brain volume [25–29].
The development of cortical grey matter follows the functional maturation sequence; notably this is also the evolutionary sequence in which these regions were created [30]. Within the cortex, synapses are produced in large numbers during fetal development and infancy. According to experience and function, their density is then reduced and, especially for the regions associated with higher level cognition, does not stabilize until adolescence, which is notably delayed in humans [31]. At the time of birth, human brains are less myelinated than those of chimpanzees [32] and the myelination of the neocortex is developmentally protracted in humans. Humans display a slower myelination of the cortical axons during childhood, and a delayed maturation extending beyond late adolescence [32].
Among anthropoid primates, the human brain is characterized by the highest proportional volume (35%) of white matter formed by long-range axons that underlie the grey matter with its local networks of neurons wired by dendrites and mostly non-myelinated axons [33]. Both chimpanzees and humans differ from macaques (presumed to represent the ancestral primate pattern) in the maturation of the brain's prefrontal portion [34]: white-matter volume increases from infancy to adulthood in chimpanzees as well as humans, but not in macaques. However, during infancy, prefrontal white-matter volume increases at a higher rate in humans than in chimpanzees. Sakai et al. [34,35] suggested that the protracted development enhances the impact of postnatal experiences on neural connectivity and that ‘the rapid development of the human prefrontal white matter during infancy may help the development of complex social interactions’.
The unique human developmental pattern affects and is affected by the economic and social organization of hominin groups. Whereas in apes daily energy production is closely related to daily energy consumption of each individual throughout life, in human foraging societies adults extract energy from the environment at a rate far beyond their individual needs [36]. Meat sharing after a hunt is quite limited in chimpanzees [37]; by contrast, food sharing is systematic among hunter–gatherers. Until late adolescence, developing individuals are net beneficiaries of these inter-individual energy contributions. Transfers occur not only from the mother to her offspring but also from the father, the grandparents and other adults of the group [38]. Humans are therefore said to practise ‘cooperative breeding’ or ‘biocultural reproduction’ [39]. When compared with apes, key aspects of the human adaptive model are a short interbirth interval and an early weaning age: early weaning allows mothers to share the burden of providing energy to the offspring with other adults from the group at an early stage of development. Human and chimpanzee females stop reproducing around the same age, but the first parturition occurs later in humans. However, due to the short interbirth interval, humans can reproduce at a faster pace than apes. Human females have a shorter reproductive life without increasing offspring mortality; this is centred on early adult life when female morbidity is reduced [40]. Importantly, humans can raise several highly dependent children simultaneously. The interactions with siblings and adults that this model entails have far-reaching implications for the psychological development of humans [41]. Finally, humans have a reduced somatic development during the childhood-juvenile period followed by a marked and delayed adolescent growth spurt. Notably, body mass increase is at its slowest and related caloric needs at the lowest at ca 4–5 years when the metabolic expenditure related to brain growth is at its maximum [13]. This growth pattern prioritizes brain development over somatic development and it has been proposed that it also defers the energetic demand on parents with multiple dependents [42].
2. Can we address these issues in the fossil record?
Brains are not preserved in the fossil record but inferences can be made from casts of the internal bony braincase [3,43–45]. Such endocasts sometimes form naturally from sediments that fill the endocranial cavity during fossilization. They can also be generated synthetically, using latex and plaster, or digitally using computed tomography (CT) data of fossil crania. Endocasts provide evidence about brain size and shape; usually they reproduce imprints of surface details such as brain convolutions or meningeal vessels. As brains grow during prenatal and postnatal ontogeny, the bones of the skull accommodate the expanding brain, largely via depositional growth at the cranial sutures [46–49]. The internal shape of the braincase therefore reflects the interplay of tempo and mode of early brain development and the growth of cranial bones. Based on cross-sectional samples of differently aged individuals, it is possible to study brain ontogeny in extinct species and to explore evolutionary changes of the developmental pattern of the brain [23,50–52]. While studies of brain size evolution and ontogenetic brain growth are based on endocranial volumes, quantitative investigations of evolutionary and developmental brain shape changes have been hindered by the difficulty of measuring the smooth endocranial surface. Using geometric morphometrics (GM), a set of methods for the statistical analysis of shape based on Cartesian coordinate data of homologous anatomical landmarks [53–55] and semi-landmarks on curves and surfaces [56], it is now possible to quantify endocranial shape [57–59]. Using GM methods, it is possible to visualize evolutionary and developmental endocranial size and shape changes, and reconstruct fragmentary fossils [60–63].
To understand the evolution of hominin brain ontogeny it is necessary to evaluate the age at death of fossil juvenile individuals. Reconstructing life-history parameters and the exact timing of development in extinct hominins, however, represents a major challenge. Among mammals or within primates, life-history theory relates biological parameters such as body mass or brain size to key aspects of life history such as age at first reproduction, yearly number of offspring, or adult lifespan [64]. However, this approach fails to predict precisely differences between closely related species of similar body/brain size [65]. In extant primates, dental development is also assumed to be tightly correlated with key life-history events. In particular, it has been claimed that the eruption of the mandibular first molar correlates with weaning age and age at sexual maturity, and the eruption of the third molar with age at sexual maturity [66]. These correlations have been discussed in fossils (e.g. [67]), but a major issue resulting from the impossibility of assessing the timing of dental development on fossil specimens has long remained unresolved. Ages at death of immature specimens were classically estimated using modern standards for calcification of the crowns and roots. At best this approach could demonstrate differences in the calcification and/or eruption sequences, or between dental and skeletal development of different taxa. A major breakthrough has resulted from the development of microstructural studies of dental tissues and their application to the fossil record (e.g. [68,69]). The analysis of enamel and dentine from accretional structures developing with a known periodicity opened the possibility of assessing precisely calendar ages for fossil individuals. This new approach revealed differences in the timing of dental and somatic development between hominin taxa. More recently, high-resolution scans captured with synchrotron technology have made it possible to study dental microstructure in a totally non-destructive way [70]. Physical signs of biological stress visible in the dental tissues—in particular, those related to birth and weaning—can now be precisely set within the chronology of individual growth. Similarly, the assessment of changes in the chemical composition of these tissues demonstrated by carbon and nitrogen isotopic composition or enrichments in trace elements such as strontium or barium open new venues for the understanding of the evolution of diet in the early stages of hominin life history [71–73].
3. Before Homo
Fossils from East and South Africa show that members of the hominin genus Australopithecus, who were ancestral to the human lineage, had body sizes and endocranial volumes in the range of extant great apes, in particular comparable with chimpanzees [3,67,74–76]. However, whereas the cognitive evolution of hominins is usually seen in light of this evolutionary increase of endocranial volume, there is mounting evidence that brain size alone is not the only relevant factor contributing to cognitive capacities. Rather, it is the internal organization of the brain and how its parts are connected during ontogeny that contribute to differences in cognitive abilities between species of similar brain size.
Based on fossil endocasts, some authors have argued that several australopith species show signs of brain reorganization that precede the evolutionary increase of brain size in hominins (e.g. [3,45,77,78]). In brains of apes, for example, an impression called the ‘lunate sulcus' marks the anterior limit of the occipital lobe and corresponds roughly to the anterior lateral boundary of the primary visual cortex [79,80]. Early 20th century anatomists argued that a homologous structure can be identified in some modern human brains in a more posterior position than in apes, and suggested that a relative evolutionary expansion of the brain's parietal and temporal cortices had shifted the lunate sulcus posteriorly. As a consequence, the identification of an ‘ape-like’ anteriorly placed lunate sulcus or a ‘human-like’ posteriorly placed lunate sulcus in fossil hominins has been used to infer evolutionary changes to brain organization, in particular the parietotemporo-occipital association cortices [81], and thereby the evolution of hominin cognition. A human-like position of the lunate sulcus was described on the Taung child, the first Australopithecus fossil ever discovered [77] and was used to link this fossil group to the human lineage. This sparked a contentious debate about whether the lunate sulcus can be identified reliably on endocasts, and on its significance to cognitive evolution [82–89]. Importantly, a re-evaluation of the lunate sulcus in a large human sample using MRI demonstrated that this feature is typically not present in modern humans [90]; several authors have therefore questioned the phylogenetic significance of the lunate sulcus. It might, however, be possible to infer the evolutionary changes of the parietal region in australopith endocasts from direct comparisons of the intraparietal area. Recent work on the evolution of the prefrontal cortex [91,92] has emphasized human–ape differences [93] in the ‘middle frontal sulcus', which reproduces well on australopith endocasts [45]. Like humans, several australopith specimens from South Africa differ from the pattern found in apes [45]: the former show a separate ‘middle frontal sulcus' and might therefore indicate an expansion of the prefrontal association cortices.
Several well-preserved fossil skulls of Australopithecus sub-adults make it possible to compare the ontogeny of australopiths to the developmental patterns observed in humans and great apes. Based on dental eruption [94] and tooth microstructure [95], the dental development in Australopithecus has been likened to that in chimpanzees, suggesting a faster life history than in humans. However, based on a comparison of the Dikika child, a 3-year old Australopithecus afarensis, to adult fossils, it has been suggested that despite their ape-like endocranial volumes, members of this species took a longer time to attain the adult brain volume than African apes. This would make the pattern of brain growth in A. afarensis more ‘human-like’ [96]. This protracted pattern of brain development might indicate a longer period of early brain plasticity and an increased period of time during which the offspring remains dependent on parental care. However, to date this remains contentious, in particular due to the fact that the range of interspecific variability within apes is still poorly documented.
4. Homo erectus
The emergence of forms assigned to the genus Homo, in particular of early representatives of H. erectus approximately 1.9 Ma, has long been considered a decisive step in the emergence of several human features fully expressed in recent H. sapiens. These include large body size, human-like body proportions with long legs and reduced abdomen, large brain, high-quality diet including increased carnivory, increased tool-kit complexity and advanced social organization emphasizing food sharing. However, recent work has revealed a more complex mosaic of features, as some of these critical changes took place before or after the appearance of H. erectus [97]. Between 1.8–1.5 Ma, early African members of H. erectus displayed endocranial volumes in the range 800–900 cm3 [3] but some recently discovered representatives of early H. erectus from Dmanisi (Georgia) still display substantially smaller endocranial volumes [4]. When compared with modern humans, H. erectus brains display significant morphological differences. These include low brain height, elongated and wider proportions, less developed temporoparietal areas and narrower frontal lobes, strong posterior projection of the occipital lobes, and anterior positioning of the cerebellar fossa. Building on life-history theory, the larger body size and brain size of early H. erectus prompted some to assign this species’ life-history traits within the modern range of variation (e.g. [98]). However, this prediction is at odds with the fossil record.
Early brain development of H. erectus is best documented by the infant braincase of Mojokerto (Perning 1, Java) [99] and the BSN49/P27 Gona (Ethiopia) pelvis tentatively assigned to a female H. erectus1 [102]. A wide range of estimates for calendar age at death of Perning 1 (18 months to 8 years) has been proposed [99,103,104]. These estimates were based on modern human standards and sometimes on estimates of the brain size itself, which resulted in some level of circular reasoning. Using the pattern of closure of the bregmatic fontanel and of the subarcuate fossa in humans and chimpanzees, Coqueugniot et al. [105] proposed a younger and narrower age range between 0.5 and 1.5 years. Based on CT data, the endocranial volume of Perning 1 has been estimated between 630 and 663 cm3 [105,106]. Leigh [107] pointed out that these values fall within the extant human variation. This is also in line with the estimate of neonatal brain size of 315 cm3 based on the analysis of the 0.9–1.4 Myr old Gona pelvis [102]. Therefore, the rapid early human brain growth was already established in early H. erectus. However, 315 cm3 already represents 34–36% of the corresponding adult H. erectus size, a percentage intermediate between that observed in extant chimpanzees and humans [24]. As the cranial capacity of H. erectus is about 500 cm3 smaller than the modern human mean, these values imply a shorter period of subsequent brain growth in H. erectus than in modern humans.
The geological age of Perning 1 ranges from 1.8 Ma [108] to less than 1.49 Ma [109]. It is critical to the computation of a proportional endocranial volume (PEV) as mean adult endocranial volumes of H. erectus increase by about 20% between 1.8 and 1.0 Ma [110]. Recent discoveries also demonstrate large variations in brain size and body form, reminiscent of those observed in modern populations, among H. erectus and sometimes within limited geographical domains [97,111]. Depending on the chosen adult references, the PEV of Perning 1 could vary between ca 70% and 87%. These values depart from the mean modern values between 0.5 and 1.5 years of age. The former is intermediate between the mean values of humans and chimpanzees and the latter is comparable with ape values [105,112]. It has sometimes been argued that the values of PEV in humans and apes widely overlap (e.g. [107]). However, in most studies intra-specific variation is artificially increased by the use of cross-sectional data. This effect is further amplified by resampling [112], a powerful method to establish mean trajectories but misleading when assessing species-related variation patterns. As shown in figure 2, when longitudinal measurements of the head circumference are taken as proxies for individual brain growth, the distribution of proportional head circumference at a given age is more than three times narrower than that generated by a resampling of the same values.
Figure 2.
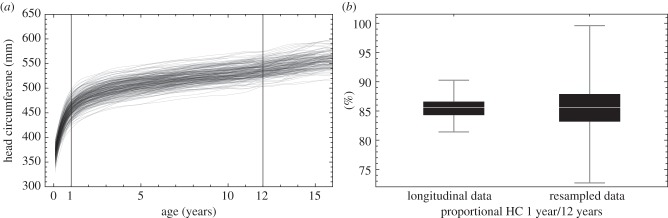
(a) Longitudinal growth of head circumferences (HC) from birth to adolescence in humans (N = 228; 115 males and 113 females). (b) Comparing the proportional HC of 1-year olds with their actual HC at age 12 shows that 86% (range 82–90%) of growth is attained after the first year of life (computed for a subset of 184 individuals for which these two time points were available). Resampling the data by comparing HC at age 1 with the HC of a randomly selected child at age 12 yields the correct median, but increases the range dramatically to 73–100%. Figure based on 5000 random permutations.
Since the first microstructural studies on the dental tissues were implemented to assess growth rates in fossil hominins, KNM-WT 15000 (the so-called ‘Nariokotome Boy’) played a central role in our understanding of the development of early H. erectus [68]. This almost complete skeleton of a sub-adult individual provides us with a unique opportunity to compare calendar age, dental development and skeletal development. KNM-WT 15000 is considered to be a male individual who died at an early stage of adolescence [113,114]. His skeletal developmental age established on the stages of ossification of epiphyses is close to 13–13.5 years by modern standards [115]. For this age, KNM-WT 15000 is a large individual displaying a stature (ca 160 cm) and body mass (ca 48 kg) close to that of a 15–18 year old modern African adolescent [114,116]. By contrast, the dental development, with upper deciduous canines still in place and unerupted third molars, is comparable with that of modern children barely over 10 years old [114]. Assuming a mid-point developmental age ca 12 years old, as well as a stature growth comparable with that of extant humans and assuming an adolescent growth spurt, the likely adult size of such an individual was estimated at 185 cm, but with a possible maximum at 197 cm [115]. However, to date no adult H. erectus of such stature has been described in the entire fossil record. Furthermore, the analysis of enamel and dentine microstructures clearly shows that H. erectus individuals developed much faster than extant humans. At death, KNM-WT 15000 had a calendar age between 7.6 and 8.8 years, and a similarly faster development has been documented in other H. erectus specimens from East Africa and Java [68,114]. When all of these parameters are taken into account, KNM-WT 15000 displays a somatic developmental pattern closer to that of a chimpanzee than to that of a modern human. In particular, the contrast between its skeletal development and actual calendar age seriously challenges the notion that H. erectus growth already exhibited a childhood slowdown and an adolescent spurt.
5. Large-brained Late Pleistocene hominins
In the past 0.5 Ma, hominin brains continued to increase in volume. This evolution is primarily documented by African fossils ancestral to modern humans and by the western Eurasian fossils belonging to the Neandertal clade. This brain size increase is independent from body size changes [9] and occurred separately in the two lineages [57,117]. It has often been claimed that Neandertals developed larger brains than modern humans (e.g. [1]). However, when body mass is taken into account, the encephalization quotient is actually higher in recent H. sapiens, who experienced a recent reduction in both body size and brain size [9].
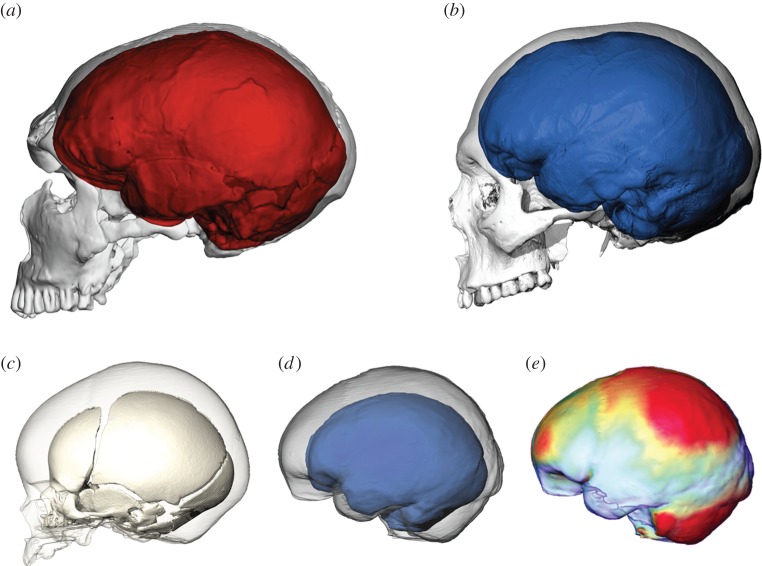
As a result of these independent trajectories, Neandertals and modern humans evolved brains displaying clear anatomical differences. Neandertals developed a larger brain along an allometric trajectory retaining most of the archaic characteristics present in the Middle Pleistocene common ancestor of the two lineages. It resulted in a widening of the frontal lobes, a more elevated shape and a relative reduction of the occipital lobes [57]. By contrast, modern humans developed a more globular shape of the brain primarily resulting from a bulging in the parietal areas and a ventral flexion (figure 3). In addition, modern humans display a proportionally larger cerebellum, larger olfactory bulbs and temporal lobe poles, and a wider orbitofrontal cortex [118]. As the skull develops and evolves as a tightly integrated structure [119], it is currently not well understood to what extent endocranial shape differences primarily result from variation in brain structure, or correlate with species differences in facial and mandibular size and shape. Whereas at least some endocranial changes in the frontal lobe [120] and temporal pole might arguably be related to changes in face and mandible, the posterior cranial fossa has a good correlation with the cerebellar lobes, and the bulging parietals of modern humans have been linked to evolutionary reorganization of deep parietal brain areas such as the precuneus [121].
Figure 3.
Cranial and endocranial shape differences between (a) Neandertal and (b) modern human adults visualized using CT. (c) Like Neandertal babies and adults, modern human newborns (opaque surface) have elongated braincases. Until the eruption of the deciduous dentition (semitransparent surface), the modern human braincase changes its shape during a ‘globularization phase’ (GP); the corresponding virtual endocasts are shown in (d) superimposing a modern human newborn (blue surface) and a child with a 1-year old (semitransparent surface). (e) The shape changes during the GP include a relative expansion of the cerebellum and parietal bulging. Colour gradient (from blue to red) codes the vector length between surface-vertices. Figure redrawn after Gunz et al. [62].
In terms of size, Neandertal brain growth is close to that observed in modern humans. The brain size at birth of the Neandertal neonate from Mezmaiskaya has been estimated to be between 382 and 416 cm3, close to modern values [122]. During infancy and childhood the pattern of PEV increase widely overlaps with that of modern humans, possibly with a slightly higher growth rate in Neandertals [122,123]. Current evidence suggests a comparable level of altriciality in the two groups. However, in terms of shape, the anatomical differences observed between adult Neandertals and modern humans start to develop soon after birth and can be analysed on immature specimens.
Neubauer et al. [59] quantified the ontogenetic shape changes of the endocast between birth and adulthood in recent modern humans and chimpanzees using GM on virtual endocasts. They showed that, whereas modern H. sapiens and Pan differ in endocranial shape throughout postnatal ontogeny, these two groups share a similar pattern of endocranial shape changes following the eruption of the deciduous dentition. In fact, the patterns of endocranial development from late infancy to adulthood are similar among all extant hominoids, suggesting a shared mode of endocranial development [124]. Modern humans, however, depart from this generalized pattern directly after birth: until the eruption of the deciduous dentition, the braincases of recent modern humans undergo a ‘globularization phase’ in which the elongated neurocranial shape found in modern human newborns becomes more globular, with bulging parietal and occipital bones [58,59]. These ontogenetic shape changes seem to mirror the well-known adult shape differences between modern and archaic humans, including Neandertals [125–127]. Based on virtual reconstructions of Neandertal fossils from neonates to adult individuals, Gunz et al. [61,62] tested whether Neandertal sub-adults undergo a postnatal globularization phase. At the time of birth Neandertal and modern human newborns can be distinguished based on facial and dental characteristics [62,122,128,129], yet the shapes of their endocasts are almost identical (figure 3). In early postnatal ontogeny, modern humans and their closest fossil relatives developed their brains differently and Neandertals lack a postnatal globularization phase [61,62]. However, after the eruption of the deciduous dentition modern humans, Neandertals and chimpanzees share a common—presumably ancestral—developmental trajectory with regard to endocranial shape changes [62]. These findings provide an ontogenetic perspective to Bruner's observation [57,130,131] that the neurocranial shape differences between Neandertals and modern humans are prominent in the parietal area. They are also consistent with the findings reported by Bastir et al. [118] based on the shape of the basal brain. These authors demonstrated that the human temporal lobes, involved in language, memory and social functions, as well as the olfactory bulbs, are relatively larger in H. sapiens than in Neandertals.
Some have deduced, from the large size of the Neandertal brain, that the life-history pattern of this group should have been close to that of extant humans (e.g. [122]). However, some differences with H. sapiens have been identified. The rich Neandertal fossil record makes it possible to investigate the somatic and dental development of these hominins, and from histological studies of immature Neandertals one can extract the exact timing of the developmental processes [69,132]. As a whole, the calendar ages obtained by such analyses provide younger ages than those predicted by modern standards for the eruption and calcification pattern of the teeth. These results contrast with those obtained on early forms of H. sapiens already close to the modern conditions [69,70]. This implies that dental development occurred faster in Neandertals and/or that some phases of their somatic development were accelerated in comparison to extant humans. This issue is one of the most contentious among Neandertal studies.
Although Neandertal skeletal development might have slowed down at some point during infancy [133], it has been suggested that Neandertal infants had a faster skeletal growth than extant H. sapiens [134]. Central to this debate is the Neandertal skeleton Le Moustier 1 (France). This rather complete individual displays a skeletal development in the early adolescence somewhere between 9 and 14 years of age with possibly still 15% of its growth to complete before reaching the average stature of an adult male European Neandertal [135,136]. This skeletal development is compatible with the calendar age at death estimated between 11.6 and 12.1 years by the analysis of its dental accretional microstructures [70]. However, both estimates strongly contrast with the dental age of ca 15.5 years based on modern standards. If one emphasizes the correlations between dental development and life-history events [66], an early eruption of the third molar in Le Moustier 1 would imply an early puberty and faster completion of the brain maturation in Neandertals. A reproductive life beginning earlier than in H. sapiens could be related to a high mortality rate in young adults [137]. It should be noted, however, that in Neandertals the primate-wide established correlations between the first molar eruption and some life-history events seem not always to be confirmed. Furthermore, if Le Moustier 1 were representative of the regular Neandertal skeletal development, its early sexual maturation would imply a faster completion of its late skeletal growth.
It has been suggested that Neandertals habitually weaned infants at a later age than recent H. sapiens. However, this conclusion was based on the analyses of dental wear [138] and enamel hypoplasia [139] and did not take into account the faster dental development of Neandertals, which has been demonstrated only recently. Measurements of Ba/Ca ratios, thought to reflect dietary changes, were obtained in the first molar enamel of an immature Neandertal from Scladina (Belgium). These results still need to be replicated but seem consistent with exclusive breastfeeding until seven months followed by supplementation until total cessation of nursing at 1.2 years of age [73]. As a whole, interpreting the Neandertals evidence is quite challenging. When the large adult body and brain size of these hominins and their very high energetic needs [140] are taken into account, the faster development of their offspring, in terms of dentition, as well as possibly brain and later skeletal growth, offer a puzzling picture. Neandertal female adaptations to pregnancy and lactation periods represent a major topic for future investigations.
6. Discussion and conclusion
Debates surrounding the hypotheses proposed to explain how the evolution of large brains relates to life-history patterns and how it can be afforded in energetic terms, highlight the opposition between two approaches. For some, they are the expression of macroevolutionary models arguably applicable to all mammals, including in particular metabolic trade-offs between organs and relations between body size, brain size and life-history patterns. For others, these models do not always match the available fossil evidence and the emphasis should be put on adaptive mechanisms that might be applicable to hominins or to the genus Homo. Even though debate and controversy about the identification of features on fossil endocasts persist, there is a consensus that Australopithecus endocasts show signs of brain reorganization and depart from the sulcal patterns found in apes, despite their ape-like endocranial volumes. It is therefore possible that brain reorganization in australopiths and its cognitive consequences underlie the subsequent brain expansion in the genus Homo. Furthermore, if supported by further investigations, the protracted pattern of brain development in A. afarensis would confirm that one cannot simply contrast a primitive ‘ape-pattern’ to a ‘human-pattern’.
The spectacular development of large brains by later Pleistocene hominins required major anatomical and developmental adjustments, among which improved diet quality, reallocation of the energy budget (including locomotion costs), changes in developmental patterns and reproductive strategies have played key roles. It seems difficult to reduce this evolution to a simple chain of causality [19]. However, as underlined by Aiello [20], of arch importance is the question of why a reallocation of so much of our energy budget to the growth and maintenance of such a costly organ was necessary in the first place.
Human-accelerated sociocultural evolution can be seen as niche construction, in which our large brain plays a central role. Its unparalleled increase depended on the establishment of peculiar developmental and demographic patterns, including protracted growth and delayed reproductive life. As human brain maturation extends over a very long period of time and is shaped by the interactions of the individuals with their environment, the uniquely long human developmental pattern has reciprocally contributed to the increase in our behavioural complexity. Early phases of brain development are critical, but the acquisition of higher cognitive and social skills covers a much longer period of time. For example, during the acquisition of language by modern children, the syntax is not fully mastered before the age of 5 years and the learning of an extended lexicon lasts even longer. Although the brain reaches its adult size at a rather early age in comparison with other organs, its structural and functional maturity is attained only in young adulthood [141].
The fossil evidence provides us with several snapshots highlighting changes in brain development and life history in the course of the past 2 Myr. Simplistic reasoning based on life-history theory have concluded that a human developmental model should have been acquired relatively early during the evolution of the genus Homo [98,122]. The reality is more complex and the four best-documented groups of fossil hominins (Australopithecus, H. erectus, H. neanderthalensis and H. sapiens) display different mosaics of features that cannot simply be seen as phases of a linear evolutionary transition.
In terms of absolute brain size, H. erectus stands in an intermediate position between Australopithecus and Late Pleistocene large-brained hominins. During infancy, its brain experienced a rapid volumetric growth comparable with that of recent humans, but it reached its adult size earlier during childhood. Therefore, during a shorter extra-utero growth phase, a smaller proportion of brain tissue developed under the influences of an enriched environment. Importantly, puberty and sexual maturity occurred at a younger age than in modern humans. A shorter learning period and faster maturation of the brain resulted in an earlier decline of its plasticity. The likely lack of an adolescent spurt in H. erectus also suggests a limited capability for adults to raise multiple dependents and/or an earlier and greater independence of the adolescents. All of these differences have important social, economic and psychological implications. Overall, it is most unlikely that the cognitive abilities and social skills of H. erectus could be comparable with those of recent H. sapiens.
The large brains of H. neanderthalensis and H. sapiens developed along two different evolutionary pathways. The resolution of comparable energetic challenges, especially during early infancy, resulted in some similarities in the life-history patterns of the two groups. Further investigations are still needed to test whether both lineages developed an early weaning of their young in order to allow efficient cooperative breeding. However, clear differences in the growth patterns also appear, notably when the dental development is taken into consideration, and Neandertals might have entered reproductive life earlier than H. sapiens. Early brain development in particular is quite distinct in the two species. While the developmental processes underlying the uniquely modern human globularization phase are not yet known, the timing between birth and the eruption of the deciduous dentition implicates interspecies differences in the tempo and mode of brain development. The Neandertal–modern human differences in early brain ontogeny are most prominent during a critical phase for cognitive development and probably affect the neuronal and synaptic organization of the developing brain, the biological substrate for cognition. These developmental differences are evident in regions of the brain (orbitofrontal cortex, parietal areas and cerebellum) that might be involved in specific cognitive tasks of importance in later H. sapiens [142].
Finally, the exploration of the Neandertal genome, although still in its infancy, also highlights differences between the two groups. In particular, one cannot say much about gene expression. It remains, however, that among the list of genes that were modified in the course of evolution of H. sapiens since the separation from the Neandertal lineage, several are related to brain development and function [143]. In modern children, mutations of the ADSL gene are known to result in varying degrees of psychomotor retardation or autism, mutations of GLDC in glycine encephalopathy and those of SLITRK1 in Tourette syndrome. Some other genes unique to modern humans (CASC5, KIF18A, TKTL1, SPAG5, VCAM1) are expressed in the proliferative layers (ventricular and subventricular zones combined) during mid-fetal development [144]. The activity of CASC5, KIF18A, SPAG5 might influence the number of neurons generated as these genes code proteins that are part of the spindle and kinetochore separating the chromosomes during cell division.
The current evidence strongly suggests that a fully modern life history and cerebral-developmental pattern did not appear until rather recently in the course of human evolution. Moreover, a significant amount of adaptive changes affecting the brain probably also took place in the past few hundred thousand years along the evolutionary line leading to extant modern humans.
Acknowledgements
We are grateful to the organizers of the Royal Society scientific discussion meeting issue 'Human evolution: brain, birthweight and the immune system’ and to Dr Michel Sempé for providing longitudinal data from the Étude Auxologique Française. We also want to thank the editor and two anonymous reviewers for their constructive comments.
Endnote
Funding statement
We are grateful to the Max Planck Society for financial support.
References
- 1.Roth G, Dicke U. 2005. Evolution of the brain and intelligence. Trends Cogn. Sci. 9, 250–257. ( 10.1016/j.tics.2005.03.005) [DOI] [PubMed] [Google Scholar]
- 2.Martin RD. 1983. Human brain evolution in an ecological context. New York, NY: American Museum of Natural History. [Google Scholar]
- 3.Holloway RL, Broadfield DC, Yuan MS. 2004. The human fossil record: brain endocasts—the paleoneurological evidence, p. 315 Hoboken, New Jersey: Wiley-Liss. [Google Scholar]
- 4.Lordkipanidze D, Ponce de León MS, Margvelashvili A, Rak Y, Rightmire GP, Vekua A, Zollikofer CPE. 2013. A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo. Science 342, 326–331. ( 10.1126/science.1238484) [DOI] [PubMed] [Google Scholar]
- 5.Arsuaga JL, et al. 2014. Neandertal roots: cranial and chronological evidence from Sima de los Huesos. Science 344, 1358–1363. ( 10.1126/science.1253958) [DOI] [PubMed] [Google Scholar]
- 6.Falk D, Hildebolt C, Smith K, Morwood MJ, Sutikna T, Brown P, Saptomo EW, Brunsden B, Prior F. 2005. The brain of LB1, Homo floresiensis. Science 308, 242–245. ( 10.1126/science.1109727) [DOI] [PubMed] [Google Scholar]
- 7.Berger LR, de Ruiter DJ, Churchill SE, Schmid P, Carlson KJ, Dirks P, Kibii JM. 2010. Australopithecus sediba: a new species of Homo-like Australopith from South Africa. Science 328, 195–204. ( 10.1126/science.1184944) [DOI] [PubMed] [Google Scholar]
- 8.Kimbel WH, Rak Y. 2010. The cranial base of Australopithecus afarensis: new insights from the female skull. Phil. Trans. R. Soc. B 365, 3365–3376. ( 10.1098/rstb.2010.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruff CB, Trinkaus E, Holliday TW. 1997. Body mass and encephalization in Pleistocene Homo. Nature 387, 173–176. ( 10.1038/387173a0) [DOI] [PubMed] [Google Scholar]
- 10.Skoyles JR. 2012. Human neuromaturation, juvenile extreme energy liability, and adult cognition/cooperation. Nature Preced. See http://hdl.handle.net/10101/npre.2012.7096.1. [Google Scholar]
- 11.Mink JW, Blumenschine RJ, Adams DB. 1981. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Phys. Anthropol. 241, R203–R212. [DOI] [PubMed] [Google Scholar]
- 12.Holliday MA. 1986. Body composition and energy needs during growth. In Human growth: a comprehensive treatise (eds Falkner F, Tanner JM.), pp. 101–107, 2nd edn New York, NY: Plenum. [Google Scholar]
- 13.Kuzawa C. 1998. Adipose tissue in human infancy and childhood: an evolutionary perspective. Yearb. Phys. Anthropol. 41, 177–209. () [DOI] [PubMed] [Google Scholar]
- 14.Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis: the brain and digestive system in human and primate evolution. Curr. Anthropol. 36, 199–221. ( 10.1086/204350) [DOI] [Google Scholar]
- 15.Snodgrass J, Leonard WR, Robertson ML. 2009. The energetics of encephalization in early hominids. In The evolution of hominin diets: integrating approaches to the study of palaeolithic subsistence (eds Hublin J-J, Richards MP.), pp. 15–30. New York, NY: Springer. [Google Scholar]
- 16.Bozek K, et al. 2014. Exceptional evolutionary divergence of human muscle and brain metabolomes parallels human cognitive and physical uniqueness. PLoS Biol. 12, 1–14. ( 10.1371/journal.pbio.1001871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmody RN, Wrangham RW. 2009. The energetic significance of cooking. J. Hum. Evol. 57, 379–391. ( 10.1016/j.jhevol.2009.02.011) [DOI] [PubMed] [Google Scholar]
- 18.Isler K, van Schaik CP. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 57, 392–400. ( 10.1016/j.jhevol.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 19.Navarrete A, van Schaik CP, Isler K. 2011. Energetics and the evolution of human brain size. Nature 480, 91–93. ( 10.1038/nature10629) [DOI] [PubMed] [Google Scholar]
- 20.Aiello LC, Bates N, Joffe T. 2001. In defense of the expensive tissue hypothesis. In Evolutionary anatomy of the primate cerebral cortex (eds Falk D, Gibson K.), pp. 57–78. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 21.Martin RD. 1996. Scaling of the mammalian brain: the maternal energy hypothesis. News Physiol. Sci. 11, 149–156. [Google Scholar]
- 22.Cunnane SC, Crawford MA. In press Energetic and nutritional constraints on infant brain development: implications for brain expansion during human evolution. J. Hum. Evol. ( 10.1016/j.jhevol.2014.05.001) [DOI] [PubMed] [Google Scholar]
- 23.Leigh SR. 2004. Brain growth, life history, and cognition in primate and human evolution. Am. J. Primatol. 62, 139–164. ( 10.1002/ajp.20012) [DOI] [PubMed] [Google Scholar]
- 24.DeSilva J, Lesnik J. 2006. Chimpanzee neonatal brain size: implications for brain growth in Homo erectus. J. Hum. Evol. 51, 207–212. ( 10.1016/j.jhevol.2006.05.006) [DOI] [PubMed] [Google Scholar]
- 25.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863. ( 10.1038/13158) [DOI] [PubMed] [Google Scholar]
- 26.Jernigan TL, Gamst AC. 2005. Changes in volume with age--consistency and interpretation of observed effects. Neurobiol. Aging 26, 1271–1274. ( 10.1016/j.neurobiolaging.2005.05.016) [DOI] [PubMed] [Google Scholar]
- 27.Lenroot RK, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage 36, 1065–1073. ( 10.1016/j.neuroimage.2007.03.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, Meuli R, Thiran J-P, Grant PE. 2010. White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl Acad. Sci. USA 107, 19 067–19 072. 36, 1065–1073. ( 10.1073/pnas.1009073107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paus T. 2010. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 72, 26–35. ( 10.1016/j.bandc.2009.06.002) [DOI] [PubMed] [Google Scholar]
- 30.Gogtay N, et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl Acad. Sci. USA 101, 8174–8179. ( 10.1073/pnas.0402680101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas MSC, Johnson MH. 2008. New advances in understanding sensitive periods in brain development. Curr. Dir. Psychol. Sci. 17, 1–5. ( 10.1111/j.1467-8721.2008.00537.x) [DOI] [Google Scholar]
- 32.Miller DJ, et al. 2012. Prolonged myelination in human neocortical evolution. Proc. Natl Acad. Sci. USA 109, 16 480–16 485. ( 10.1073/pnas.1117943109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofman MA. 2014. Evolution of the human brain: when bigger is better. Front. Neuroanat. 8, 1–12. ( 10.3389/fnana.2014.00015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai T, et al. 2011. Differential prefrontal white matter development in chimpanzees and humans. Curr. Biol. 21, 1397–1402. ( 10.1016/j.cub.2011.07.019) [DOI] [PubMed] [Google Scholar]
- 35.Sakai T, et al. 2013. Developmental patterns of chimpanzee cerebral tissues provide important clues for understanding the remarkable enlargement of the human brain. Proc. R. Soc. B 280, 20122398 ( 10.1098/rspb.2012.2398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. () [DOI] [Google Scholar]
- 37.Fahy GE, Richards M, Riedel J, Hublin J-J, Boesch C. 2013. Stable isotope evidence of meat eating and hunting specialization in adult male chimpanzees. Proc. Natl Acad. Sci. USA 110, 5829–5833. ( 10.1073/pnas.1221991110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurven M, Kaplan H. 2009. Beyond the grandmother hypothesis: evolutionary models of human longevity. In The cultural context of aging: worldwide perspectives (ed. Sokolovsky J.), pp. 53–66, 3rd edn Westport, CT: Praeger. [Google Scholar]
- 39.Bogin B, Bragg J, Kuzawa C. 2014. Humans are not cooperative breeders but practice biocultural reproduction. Ann. Hum. Biol. 41, 368–380. ( 10.3109/03014460.2014.923938) [DOI] [PubMed] [Google Scholar]
- 40.Kachel AF, Premo LS. 2012. Disentangling the evolution of early and late life history traits in humans. Evol. Biol. 39, 638–649. ( 10.1007/s11692-012-9169-4) [DOI] [Google Scholar]
- 41.Hrdy S. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA: Harvard University Press. [Google Scholar]
- 42.Gurven M, Walker R. 2006. Energetic demand of multiple dependents and the evolution of slow human growth. Proc. R. Soc. B 273, 835–841. ( 10.1098/rspb.2005.3380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holloway RL. 1978. The relevance of endocasts for studying primate brain evolution. In Sensory systems of primates (ed. Noback CR.), pp. 181–200. New York, NY: Plenum Press. [Google Scholar]
- 44.Falk D. 1980. Hominid brain evolution: the approach from paleoneurology. Yearb. Phys. Anthropol. 23, 93–107. ( 10.1002/ajpa.1330230507) [DOI] [Google Scholar]
- 45.Falk D. 2014. Interpreting sulci on hominin endocasts: old hypotheses and new findings. Front. Hum. Neurosci. 8, 1–11. ( 10.3389/fnhum.2014.00134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss ML, Young RW. 1960. A functional approach to craniology. Am. J. Phys. Anthropol. 18, 281–292. ( 10.1002/ajpa.1330180406) [DOI] [PubMed] [Google Scholar]
- 47.Enlow DH. 1968. The human face. An account of the postnatal growth and development of the craniofacial skeleton. New York, NY: Hoeber Medical Division, Harper & Row. [Google Scholar]
- 48.Morriss-Kay GM, Wilkie AOM. 2005. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J. Anat. 207, 637–653. ( 10.1111/j.1469-7580.2005.00475.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richtsmeier JT, Aldridge K, DeLeon VB, Panchal J, Kane AA, Marsh JL, Yan P, Cole TM. 2006. Phenotypic integration of neurocranium and brain. J. Exp. Zool. B Mol. Dev. Evol. 306, 360–378. ( 10.1002/jez.b.21092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neubauer S, Hublin J-J. 2012. The evolution of human brain development. Evol. Biol. 39, 568–586. ( 10.1007/s11692-011-9156-1) [DOI] [Google Scholar]
- 51.Leigh SR. 2012. Brain size growth and life history in human evolution. Evol. Biol. 39, 587–599. ( 10.1007/s11692-012-9168-5) [DOI] [Google Scholar]
- 52.Zollikofer CPE, De León MSP. 2013. Pandora's growing box: inferring the evolution and development of hominin brains from endocasts. Evol. Anthropol. 22, 20–33. ( 10.1002/evan.21333) [DOI] [PubMed] [Google Scholar]
- 53.Bookstein FL. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 54.Slice DE. 2007. Geometric morphometrics. Ann. Rev. Anthropol. 36, 261–281. ( 10.1146/annurev.anthro.34.081804.120613) [DOI] [Google Scholar]
- 55.Mitteroecker P, Gunz P. 2009. Advances in geometric morphometrics. Evol. Biol. 36, 235–247. ( 10.1007/s11692-009-9055-x) [DOI] [Google Scholar]
- 56.Gunz P, Mitteroecker P, Bookstein FL. 2005. Semilandmarks in three dimensions. In Modern morphometrics in physical anthropology (ed. Slice DE.), pp. 73–98. Dordrecht, The Netherlands: Kluwer Academic/Plenum. [Google Scholar]
- 57.Bruner E, Manzi G, Arsuaga JL. 2003. Encephalization and allometric trajectories in the genus Homo: evidence from the Neandertal and modern lineages. Proc. Natl Acad. Sci. USA 100, 15 335–15 340. ( 10.1073/pnas.2536671100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neubauer S, Gunz P, Hublin JJ. 2009. The pattern of endocranial ontogenetic shape changes in humans. J. Anat. 215, 240–255. ( 10.1111/j.1469-7580.2009.01106.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neubauer S, Gunz P, Hublin J-J. 2010. Endocranial shape changes during growth in chimpanzees and humans: a morphometric analysis of unique and shared aspects. J. Hum. Evol. 59, 555–566. ( 10.1016/j.jhevol.2010.06.011) [DOI] [PubMed] [Google Scholar]
- 60.Gunz P, Mitteroecker P, Neubauer S, Weber GW, Bookstein FL. 2009. Principles for the virtual reconstruction of hominin crania. J. Hum. Evol. 57, 48–62. ( 10.1016/j.jhevol.2009.04.004) [DOI] [PubMed] [Google Scholar]
- 61.Gunz P, Neubauer S, Maureille B, Hublin J-J. 2010. Brain development after birth differs between Neanderthals and modern humans. Curr. Biol. 20, R921–R922. ( 10.1016/j.cub.2010.10.018) [DOI] [PubMed] [Google Scholar]
- 62.Gunz P, Neubauer S, Golovanova L, Doronichev V, Maureille B, Hublin J-J. 2012. A uniquely modern human pattern of endocranial development. Insights from a new cranial reconstruction of the Neandertal newborn from Mezmaiskaya. J. Hum. Evol. 62, 300–313. ( 10.1016/j.jhevol.2011.11.013) [DOI] [PubMed] [Google Scholar]
- 63.Neubauer S, Gunz P, Weber GW, Hublin J-J. 2012. Endocranial volume of Australopithecus africanus: new CT-based estimates and the effects of missing data and small sample size. J. Hum. Evol. 62, 498–510. ( 10.1016/j.jhevol.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 64.Charnov EL, Berrigan D. 1993. Why do female primates have such long lifespans and so few babies? or life in the slow lane. Evol. Anthropol. 1, 191–194. ( 10.1002/evan.1360010604) [DOI] [Google Scholar]
- 65.Leigh SR, Blomquist GE. 2007. Life history. In Primates in perspective (eds Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK.), pp. 396–407. Oxford, UK: Oxford University Press. [Google Scholar]
- 66.Smith BH. 1989. Dental development as a measure of life history in primates. Evolution 43, 683–688. ( 10.2307/2409073) [DOI] [PubMed] [Google Scholar]
- 67.Robson SL, Wood B. 2008. Hominin life history: reconstruction and evolution. J. Anat, 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean C, Leakey MG, Reid D, Schrenk F, Schwartz GT, Stringer C, Walker A. 2001. Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature 414, 628–631. ( 10.1038/414628a) [DOI] [PubMed] [Google Scholar]
- 69.Smith TM, et al. 2010. Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proc. Natl Acad. Sci. USA 107, 20 923–20 928. ( 10.1073/pnas.1010906107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith TM, Tafforeau P, Reid DJ, Grün R, Eggins S, Boutaiout M, Hublin J-J. 2007. Earliest evidence of modern human life history in North African early Homo sapiens. Proc. Natl Acad. Sci. USA 104, 6128–6133. ( 10.1073/pnas.0700747104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Humphrey LT. 2014. Isotopic and trace element evidence of dietary transitions in early life. Ann. Hum. Biol. 41, 348–357. ( 10.3109/03014460.2014.923939) [DOI] [PubMed] [Google Scholar]
- 72.Fahy GE, Richards MP, Fuller BT, Deschner T, Hublin J-J, Boesch C. 2014. Stable nitrogen isotope analysis of dentine serial sections elucidate sex differences in weaning patterns of wild chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 153, 635–642. ( 10.1002/ajpa.22464) [DOI] [PubMed] [Google Scholar]
- 73.Austin C, et al. 2013. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature 498, 216–219. ( 10.1038/nature12169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mchenry HM. 1994. Behavioral ecological implications of early hominid body-size. J. Hum. Evol. 27, 77–87. ( 10.1006/jhev.1994.1036) [DOI] [Google Scholar]
- 75.Kimbel WH, Rak Y, Johanson DC. 2004. The skull of Australopithecus afarensis. Oxford, UK: Oxford University Press. [Google Scholar]
- 76.Kimbel WH, Delezene LK. 2009. ‘Lucy’ redux: a review of research on Australopithecus afarensis. Am. J. Phys. Anthropol. 140(Suppl. 49), 2–48. ( 10.1002/ajpa.21183) [DOI] [PubMed] [Google Scholar]
- 77.Dart RA. 1925. Australopithecus africanus: the man-ape of South Africa. Nature 115, 195–199. ( 10.1038/115195a0) [DOI] [Google Scholar]
- 78.Carlson KJ, Stout D, Jashashvili T, de Ruiter DJ, Tafforeau P, Carlson K, Berger LR. 2011. The endocast of MH1, Australopithecus sediba. Science 333, 1402–1407. ( 10.1126/science.1203922) [DOI] [PubMed] [Google Scholar]
- 79.Smith GE. 1903. The so-called affenspalte in the human (Egyptian) brain. Anat. Anz. 24, 74–83. [Google Scholar]
- 80.Smith GE. 1904. The morphology of the occipital region of the cerebral hemisphere in man and the apes. Anat. Anz. 24, 436–451. [Google Scholar]
- 81.Holloway RL, Clarke RJ, Tobias PV. 2004. Posterior lunate sulcus in Australopithecus africanus: was Dart right? C. R. Palevol 3, 287–293. ( 10.1016/j.crpv.2003.09.030) [DOI] [Google Scholar]
- 82.Falk D. 1980. A reanalysis of the South African australopithecine natural endocasts. Am. J. Phys. Anthropol. 53, 525–539. ( 10.1002/ajpa.1330530409) [DOI] [PubMed] [Google Scholar]
- 83.Falk D. 1983. The Taung endocast: a reply to Holloway. Am. J. Phys. Anthropol. 60, 479–489. ( 10.1002/ajpa.1330600410) [DOI] [PubMed] [Google Scholar]
- 84.Falk D. 1985. Apples, oranges, and the lunate sulcus. Am. J. Phys. Anthropol. 67, 313–315. ( 10.1002/ajpa.1330670403) [DOI] [PubMed] [Google Scholar]
- 85.Falk D. 1989. Ape-like endocast of ‘ape-man’ Taung. Am. J. Phys. Anthropol. 80, 335–339. ( 10.1002/ajpa.1330800307). [DOI] [PubMed] [Google Scholar]
- 86.Holloway RL. 1981. Revisiting the South African Taung australopithecine endocast: the position of the lunate sulcus as determined by the stereoplotting technique. Am. J. Phys. Anthropol. 56, 43–58. ( 10.1002/ajpa.1330560105) [DOI] [Google Scholar]
- 87.Holloway RL. 1984. The Taung endocast and the lunate sulcus: a rejection of the hypothesis of its anterior position. Am. J. Phys. Anthropol. 64, 285–287. ( 10.1002/ajpa.1330640310) [DOI] [PubMed] [Google Scholar]
- 88.Holloway RL. 1985. The past, present, and future significance of the lunate sulcus in early hominid evolution. In Hominid evolution: past, present and future: Proc. Taung Diamond Jubilee Int. Symp., Johannesburg and Mmabatho, Southern Africa, 27th January–4th February 1985 (ed. Tobias PV.), pp. 47–64. Johannesburg, South Africa: Alan R. Liss. [Google Scholar]
- 89.Holloway RL. 1991. On Falk's 1989 accusations regarding Holloway's study of the Taung endocast: a reply. Am. J. Phys. Anthropol. 84, 87–88. ( 10.1002/ajpa.1330840108) [DOI] [PubMed] [Google Scholar]
- 90.Allen JS, Bruss J, Damasio H. 2006. Looking for the lunate sulcus: a magnetic resonance imaging study in modern humans. Anat. Rec. A 288A, 867–876. ( 10.1002/ar.a.20362) [DOI] [PubMed] [Google Scholar]
- 91.Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, Travis K, Buckwalter J. 2011. Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb. Cortex 21, 1485–1497. ( 10.1093/cercor/bhq191) [DOI] [PubMed] [Google Scholar]
- 92.Teffer K, Semendeferi K. 2012. Human prefrontal cortex: evolution, development, and pathology. In Progress in brain research (eds Hofman MA, Falk D.), pp. 191–218. Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed] [Google Scholar]
- 93.Connolly CJ. 1950. External morphology of the primate brain. Springfield, IL: Charles C. Thomas. [Google Scholar]
- 94.Dean MC. 1985. The eruption pattern of the permanent incisors and first permanent molars in Australopithecus (Paranthropus) robustus. Am. J. Phys. Anthropol. 67, 251–257. ( 10.1002/ajpa.1330670310) [DOI] [PubMed] [Google Scholar]
- 95.Bromage TG, Dean MC. 1985. Re-evaluation of the age at death of immature fossil hominids. Nature 317, 525–527. ( 10.1038/317525a0) [DOI] [PubMed] [Google Scholar]
- 96.Alemseged Z, Spoor F, Kimbel WH, Bobe R, Geraads D, Reed D, Wynn JG. 2006. A juvenile early hominin skeleton from Dikika, Ethiopia. Nature 443, 296–301. ( 10.1038/nature05047) [DOI] [PubMed] [Google Scholar]
- 97.Antón SC, Potts R, Aiello LC. 2014. Evolution of early Homo: an integrated biological perspective. Science 345, 1236828(1–13) ( 10.1126/science.1236828) [DOI] [PubMed] [Google Scholar]
- 98.Hawkes K. 2003. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 15, 380–400. ( 10.1002/ajhb.10156) [DOI] [PubMed] [Google Scholar]
- 99.Von Koenigswald GHR. 1936. Ein fossiler Hominide aus dem Altpleistocän Ostjavas. De Ingenieur in Nederlandsch-Indie. Mijnb. Geol. Mijningenieur 4, 149–157. [Google Scholar]
- 100.Ruff C. 2010. Body size and body shape in early hominins—implications of the Gona pelvis. J. Hum. Evol. 58, 166–178. ( 10.1016/j.jhevol.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 101.Simpson SW, Quade J, Levin NE, Semaw S. 2014. The female Homo pelvis from Gona: response to Ruff (2010). J. Hum. Evol. 68, 32–35. ( 10.1016/j.jhevol.2013.12.004) [DOI] [PubMed] [Google Scholar]
- 102.Simpson SW, Quade J, Levin NE, Butler R, Dupont-Nivet G, Everett M, Semaw S. 2008. A female Homo erectus pelvis from Gona, Ethiopia. Science 322, 1089–1092. ( 10.1126/science.1163592) [DOI] [PubMed] [Google Scholar]
- 103.Von Koenigswald GHR. 1940. Neue Pithecanthropus-Funde 1936–1938. Wetenschapelijke Mededeelingen van den Dienst. van den Mijnb. 28, 1–232. [Google Scholar]
- 104.Antón SC. 1997. Developmental age and taxonomic affinity of the Mojokerto child, Java, Indonesia. Am. J. Phys. Anthropol. 102, 497–514. () [DOI] [PubMed] [Google Scholar]
- 105.Coqueugniot H, Hublin J-J, Veillon F, Houët F, Jacob T. 2004. Early brain growth in Homo erectus and implications for cognitive ability. Nature 431, 299–302. ( 10.1038/nature02852) [DOI] [PubMed] [Google Scholar]
- 106.Balzeau A, Grimaud-Herve D, Jacob T. 2005. Internal cranial features of the Mojokerto child fossil (East Java, Indonesia). J. Hum. Evol. 48, 535–553. ( 10.1016/j.jhevol.2005.01.002) [DOI] [PubMed] [Google Scholar]
- 107.Leigh SR. 2006. Brain ontogeny and life history in Homo erectus. J. Hum. Evol. 50, 104–108. ( 10.1016/j.jhevol.2005.02.008) [DOI] [PubMed] [Google Scholar]
- 108.Swisher CC, Curtis GH, Jacob T, Getty AG, Suprijo A. 1994. Age of the earliest known hominids in Java, Indonesia. Science 263, 1118–1121. ( 10.1126/science.8108729) [DOI] [PubMed] [Google Scholar]
- 109.Morwood MJ, O'Sullivan P, Susanto EE, Aziz F. 2003. Revised age for Mojokerto 1, an early Homo erectus cranium from East Java, Indonesia. Aust. Archaeol. 57, 1–4. [Google Scholar]
- 110.Hublin J-J, Coqueugniot H. 2006. Absolute or proportional brain size: that is the question. A reply to Leigh's (2006) comments. J. Hum. Evol. 50, 109–113. ( 10.1016/j.jhevol.2005.08.009) [DOI] [Google Scholar]
- 111.Spoor F, Leakey MG, Gathogo PN, Brown FH, Antón SC, McDougall I, Kiarie C, Manthi FK, Leakey LN. 2007. Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya. Nature 448, 688–691. ( 10.1038/nature05986) [DOI] [PubMed] [Google Scholar]
- 112.O'Connell CA, DeSilva JM. 2013. Mojokerto revisited: evidence for an intermediate pattern of brain growth in Homo erectus. J. Hum. Evol. 65, 156–161. ( 10.1016/j.jhevol.2013.04.007) [DOI] [PubMed] [Google Scholar]
- 113.Walker A, Leakey R. 1993. The Nariokotome Homo erectus skeleton, p. 457 Cambridge, MA: Harvard University Press. [Google Scholar]
- 114.Dean MC, Smith BH. 2009. Growth and development of the Nariokotome Youth, KNM-WT 15000. In The first humans: origin and early evolution of the genus Homo (eds Grine FE, Fleagle JG, Leakey RE.), pp. 101–120. New York, NY: Springer. [Google Scholar]
- 115.Ruff CB, Walker A. 1993. Body size and body shape. In The Nariokotome Homo erectus skeleton (eds Walker A, Leakey R.), pp. 234–263. Cambridge, MA: Harvard University Press. [Google Scholar]
- 116.Ruff C. 2007. Body size prediction from juvenile skeletal remains. Am. J. Phys. Anthropol. 133, 698–716. ( 10.1002/ajpa.20568) [DOI] [PubMed] [Google Scholar]
- 117.Hublin J-J. 2014. How to build a Neandertal. Science 344, 1338–1339. ( 10.1126/science.1255554) [DOI] [PubMed] [Google Scholar]
- 118.Bastir M, Rosas A, Gunz P, Pena-Melian A, Manzi G, Harvati K, Kruszynski R, Stringer C, Hublin J-J. 2011. Evolution of the base of the brain in highly encephalized human species. Nat. Commun. 2, 588 ( 10.1038/ncomms1593) [DOI] [PubMed] [Google Scholar]
- 119.Bruner E, de la Cuétara JM, Masters M, Amano H, Ogihara N. 2014. Functional craniology and brain evolution: from paleontology to biomedicine. Front. Neuroanat. 8, 19 ( 10.3389/fnana.2014.00019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bruner E, Holloway R. 2010. Bivariate approach to the widening of the frontal lobes in the genus Homo. J. Hum. Evol. 58, 138–146. ( 10.1016/j.jhevol.2009.10.005) [DOI] [PubMed] [Google Scholar]
- 121.Bruner E, de Lázaro GR, de la Cuétara JM, Martín-Loeches M, Colom R, Jacobs HIL. 2014. Midsagittal brain variation and MRI shape analysis of the precuneus in adult individuals. J Anat. 224, 367–376. ( 10.1111/joa.12155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ponce de Leon MS, Golovanova L, Doronichev V, Romanova G, Akazawa T, Kondo O, Ishida H, Zollikofer CPE. 2008. Neanderthal brain size at birth provides insights into the evolution of human life history. Proc. Natl Acad. Sci. USA 105, 13 764–13 768. ( 10.1073/pnas.0803917105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coqueugniot H, Hublin J-J. 2007. Endocranial volume and brain growth in immature Neandertals. Period. Biolog. 109, 379–385. [Google Scholar]
- 124.Scott N, Neubauer S, Hublin JJ, Gunz P. 2014. A shared pattern of postnatal endocranial development in extant hominoids. Evol. Biol. 41, 572–594. ( 10.1007/s11692-014-9290-7) [DOI] [Google Scholar]
- 125.Lieberman DE, McBratney BM, Krovitz G. 2002. The evolution and development of cranial form in Homo sapiens. Proc. Natl Acad. Sci. USA 99, 1134–1139. ( 10.1073/pnas.022440799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lieberman DE, Krovitz GE, McBratney-Owen B. 2004. Testing hypotheses about tinkering in the fossil record: the case of the human skull. J. Exp. Zool. B 302, 284–301. ( 10.1002/jez.b.21004) [DOI] [PubMed] [Google Scholar]
- 127.Gunz P, Bookstein FL, Mitteroecker P, Stadlmayr A, Seidler H, Weber GW. 2009. Early modern human diversity suggests subdivided population structure and a complex out-of-Africa scenario. Proc. Natl Acad. Sci. USA 106, 6094–6098. ( 10.1073/pnas.0808160106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tillier AM. 1996. The Pech de l'Azé and Roc de Marsal children (middle paleolithic, France): skeletal evidence for variation in Neanderthal ontogeny. Hum. Evol. 11, 113–119. ( 10.1007/BF02437394) [DOI] [Google Scholar]
- 129.Zollikofer CPE, Ponce de León MS. 2010. The evolution of hominin ontogenies. Semin. Cell Dev. Biol. 21, 441–452. ( 10.1016/j.semcdb.2009.10.012) [DOI] [PubMed] [Google Scholar]
- 130.Bruner E. 2004. Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J. Hum. Evol. 47, 279–303. ( 10.1016/j.jhevol.2004.03.009) [DOI] [PubMed] [Google Scholar]
- 131.Bruner E. 2010. Morphological differences in the parietal lobes within the human genus: a neurofunctional perspective. Curr. Anthropol. 51, S77–S88. ( 10.1086/650729) [DOI] [Google Scholar]
- 132.Smith TM, Toussaint M, Reid DJ, Olejniczak AJ, Hublin J-J. 2007. Rapid dental development in a Middle Paleolithic Belgian Neandertal. Proc. Natl Acad. Sci. USA 104, 20 220–20 225. ( 10.1073/pnas.0707051104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martín-González JA, Mateos A, Goikoetxea I, Leonard WR, Rodríguez J. 2012. Differences between Neandertal and modern human infant and child growth models. J. Hum. Evol. 63, 140–149. ( 10.1016/j.jhevol.2012.04.005) [DOI] [PubMed] [Google Scholar]
- 134.Sasaki C, Suzuki K, Mishima H, Kozawa Y. 2003. Age determination of the Dederiyeh 1 Neanderthal child using enamel cross-striations. In Neanderthal burials: excavations of the Dederiyeh Cave, Afrin, Syria; studies in honour of Hisashi Suzuki (eds Akazawa T, Muhesen S.), pp. 263–270. Auckland: KW Publications. [Google Scholar]
- 135.Thompson JL, Nelson AJ. 1999. Le Moustier 1, limb proportions and the ontogeny of the Neandertal form. J. Hum. Evol. 36, A22–A23. [Google Scholar]
- 136.Nelson AJ, Thompson JL. 2005. Le Moustier 1 and the interpretation of stages in Neandertal growth and development. In The Neandertal adolescent Le Moustier 1—new aspects, new results (ed. Ullrich H.), pp. 328–338. Berlin, Germany: Staatliche Museen Zu Berlin - Preußischer Kulturbesiz. [Google Scholar]
- 137.Trinkaus E. 1995. Neanderthal mortality patterns. J. Archaeol. Sci. 22, 121–142. ( 10.1016/S0305-4403(95)80170-7) [DOI] [Google Scholar]
- 138.Skinner M. 1997. Dental wear in immature late Pleistocene European hominines. J. Archaeol. Sci. 24, 677–700. ( 10.1006/jasc.1996.0151) [DOI] [Google Scholar]
- 139.Ogilvie MD, Curran BK, Trinkaus E. 1989. Incidence and patterning of dental enamel hypoplasia among the Neanderthals. Am. J. Phys. Anthropol. 79, 25–41. ( 10.1002/ajpa.1330790104) [DOI] [PubMed] [Google Scholar]
- 140.Froehle AW, Churchill SE. 2009. Energetic competition between Neandertals and anatomically modern humans. PaleoAnthropology, 140, 96–116. [Google Scholar]
- 141.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. 1999. Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283, 1908–1911. ( 10.1126/science.283.5409.1908) [DOI] [PubMed] [Google Scholar]
- 142.Bruner E, Martin-Loeches M, Burgaleta M, Colom R. 2011. Midsagittal brain shape correlation with intelligence and cognitive performance. Intelligence 39, 141–147. ( 10.1016/j.intell.2011.02.004) [DOI] [Google Scholar]
- 143.Castellano S, et al. 2014. Patterns of coding variation in the complete exomes of three Neandertals. Proc. Natl Acad. Sci. USA 111, 6666–6671. ( 10.1073/pnas.1405138111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prüfer K, et al. 2014. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505, 43–49. ( 10.1038/nature12886) [DOI] [PMC free article] [PubMed] [Google Scholar]