Abstract
Hominin evolution saw the emergence of two traits—bipedality and encephalization—that are fundamentally linked because the fetal head must pass through the maternal pelvis at birth, a scenario termed the ‘obstetric dilemma’. While adaptive explanations for bipedality and large brains address adult phenotype, it is brain and pelvic growth that are subject to the obstetric dilemma. Many contemporary populations experience substantial maternal and perinatal morbidity/mortality from obstructed labour, yet there is increasing recognition that the obstetric dilemma is not fixed and is affected by ecological change. Ecological trends may affect growth of the pelvis and offspring brain to different extents, while the two traits also differ by a generation in the timing of their exposure. Two key questions arise: how can the fit between the maternal pelvis and the offspring brain be ‘renegotiated’ as the environment changes, and what nutritional signals regulate this process? I argue that the potential for maternal size to change across generations precludes birthweight being under strong genetic influence. Instead, fetal growth tracks maternal phenotype, which buffers short-term ecological perturbations. Nevertheless, rapid changes in nutritional supply between generations can generate antagonistic influences on maternal and offspring traits, increasing the risk of obstructed labour.
Keywords: nutrition transition, birthweight, encephalization, adaptation, obstetric dilemma, fistula
1. Introduction
Bipedal locomotion is a defining feature of the hominin lineage, though it has varied in its anatomical basis over time [1]. The genus Homo further evolved large brain size. Since these traits have both persisted for millions of years, they have clearly been strongly favoured by selective pressures in ancestral environments [2,3], though what exactly were the adaptive benefits remains debated. Understanding their evolutionary emergence is central to understanding the long-term history of our own species. This is especially the case because the two traits are fundamentally linked: the neonatal head must pass, at the time of birth, through the maternal pelvis. For decades, the challenge posed by this interaction has been known as the ‘obstetric dilemma’ [4] and has been broadly attributed to contrasting selective pressures acting on locomotion and brain size, favouring a large neonatal head relative to the dimensions of the maternal pelvis [4–6].
There is no doubt that pelvic structure changed substantially through hominin evolutionary history, and that the tight fit between its dimensions and those of the offspring brain is reflected in an unusually complex birth process in contemporary humans, as elegantly described by Trevathan and co-workers [5,6]. The duration of delivery is longer in our species than other apes, and the norm is for the fetus to rotate as it passes through the pelvis, resulting in it emerging facing away from the mother. Human mothers therefore benefit from the assistance of others to minimize the risk of injury to the neonate, though solitary births have been recorded. To aid delivery, the fetal head is compressible, and the pelvic diameter can also expand slightly [7]. Collectively, therefore, these traits represent a generic ‘resolution’ to the obstetric dilemma, and yet in contemporary populations, this resolution often appears to be only partial.
Many contemporary human populations experience high levels of maternal and neonatal mortality as a consequence of obstructed labour, which accounts for approximately 12% of the total global burden of maternal mortality, as well as a substantial proportion of perinatal mortality [8,9]. Beyond the immediate risks, fistula injuries to the mother cause debilitating conditions such as incontinence. Thus, as discussed by Arrowsmith et al. [10], ‘women who have experienced prolonged obstructed labour often develop serious social problems, including divorce, exclusion from religious activities, separation from their families, worsening poverty, malnutrition and almost unendurable suffering’. Recognizing the morbidity and mortality burden of obstructed labour, in 1951 Krogman described human birth as a ‘scar’ of our evolutionary history [11].
It is worth considering, however, whether the obstetric dilemma has been uniform in hominin evolution over the long term, or whether the risk of obstructed labour has been exacerbated by recent secular trends in behaviour and biology. It is increasingly recognized that many hominin traits have a complex mosaic evolutionary history [12]. For example, the manifestation of bipedal locomotion has altered across earlier and later hominins [1], while encephalization also occurred incrementally across 2 Myr in the genus Homo [7]. The implication is that the magnitude of the obstetric dilemma must have changed over the long-term during hominin evolutionary history. Consistent with that hypothesis, Australopithecines and archaic humans appear to have had a higher degree of cephalo-pelvic disproportion than Homo erectus [7]. In the past few hundred thousand years, both body size and cranial capacity changed in the genus Homo, though in contrasting ways, as illustrated in figure 1 [13]. Likewise, few biologists are ignorant of the rapid changes in body size that have occurred in recent centuries, where many populations have become both taller and also relatively heavier [14,15].
Figure 1.
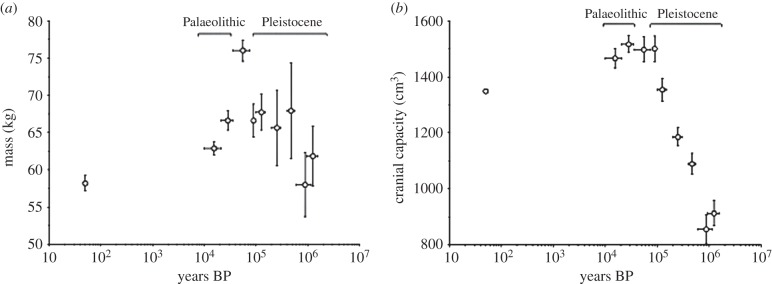
Long-term secular trends in (a) adult body mass and (b) adult cranial capacity over the past 1.2 Myr in the genus Homo. The trends do not match, indicating that the relationship between adult body mass and brain size has shifted during this period. This suggests that the obstetric dilemma may also have undergone renegotiation during the same period. Adapted from Ruff et al. [13].
While these trends in body size and proportions are generally described in terms of adult data, they also have major implications for the obstetric dilemma, and in this context the importance of bipedal locomotion to the obstetric dilemma is undergoing reconsideration. One alternative perspective is that the magnitude of fetal growth is constrained not by maternal locomotory anatomy, but by maternal metabolism being unable to support longer gestation of large-brained offspring [16]. However, this offers little explanation for obstructed labour, for which a key risk factor is the fetus growing beyond the size at which delivery is possible without complications. An alternative argument is that both pelvic dimensions and offspring brain size may change across generations in response to ecological trends, and that discordant responses of pelvic versus brain growth to such trends may exacerbate the risk of obstructed labour [7].
The aim of this review is to develop the latter perspective in more detail, focusing on a specific question: as body size and shape evolve in response to changing ecological conditions, how can the fit between the maternal pelvis and offspring size be ‘renegotiated’?
2. Resolution of the dilemma by genetic adaptation
The classic concept of Darwinian adaptation assumes that organisms acquire the phenotypic traits that improve their ability to survive and breed in their habitual environment. To understand how skeletal dimensions are shaped by ecological stresses, we can learn much by considering how growth responds to climate.
In the nineteenth century, for example, two classic ‘ecological laws’ were proposed, regarding the adaptation of body size and shape to climatic stresses. Bergmann [17] hypothesized that total body size within warm-blooded species would increase as temperatures fell, while Allen [18] hypothesized that the size of body extremities would decrease in accordance with physical thermodynamic theory. A substantial body of work has subsequently supported these hypotheses, both in humans [19–23] and in other species [24–27]. These ecological laws are likewise widely used to interpret evolutionary trends in hominin body shape [28–30] and have become influential in evolutionary anthropology as examples of a more general capacity for morphological adaptation.
Given the lengthy timescale of hominin and human evolution, and also the wide range of latitude occupied by Homo sapiens, adaptation to stresses such as the thermal environment was widely assumed to have occurred through genetic change. The neo-Darwinian synthesis, which consolidated around the 1940s, ordained that phenotypic change over time arises through the accumulation of small genetic changes driven by the differential reproductive success achieved by some alleles relative to others [31]. The statistician Ronald Fisher suggested that the normal distribution of phenotypic traits arose through many genes each exerting a small effect, a scenario now supported from genome-wide association studies for indices of body size such as stature [32,33]. Genetic variants associated with infant head circumference are now emerging [34], although minimal information is yet available for the dimensions of the pelvis.
The obstetric dilemma might therefore be considered as the consequence of two traits being forced into a ‘genetic compromise’ because each is exposed to the other through the process of birth. The contemporary dilemma might be seen as the end result of a long-term ‘genetic negotiation’, optimizing the response to contrasting selective pressures favouring large adult brains and efficient adult locomotion, further impacted by trends in body size. As the environment changed, and different modes of bipedal locomotion and encephalization were favoured, so might the fit between the neonatal head and the maternal pelvis have shifted adaptively.
Recently, Grabowski [35] explored how the capacity for evolutionary change in a given trait may be constrained by stabilizing selection across a suite of covarying traits. In relation to the obstetric dilemma, he suggested that such constraints may have reduced the overall evolvability of the birth canal in earlier hominins, but that these constraints became weaker in later hominins. A study of regional skeletal variability in recent human populations found that the pelvic canal was, in fact, the most variable trait, suggesting that stabilizing selection is no longer a major constraint [36].
While such genetic change can be assumed to have played a role in the evolving obstetric dilemma, it also appears insufficient as an explanation for the contemporary burden of mortality from obstructed labour. Whatever the selective pressures acting on adult encephalization and locomotion, the obstetric dilemma is, in fact, the consequence of a clash not between two adult traits, but between two developmental traits—growth of the maternal pelvis and fetal growth. Their interaction at the time of birth makes resolution of the dilemma a complex two-party process, and selection furthermore acts not only on the traits per se, but also on their coordination. Since maternal growth occurs a generation ahead of offspring growth, their co-adaptation to ecological stresses takes on the form of a ‘three-legged race’ (figure 2), in which the two traits are linked without the possibility of perfect phenotypic integration.
Figure 2.
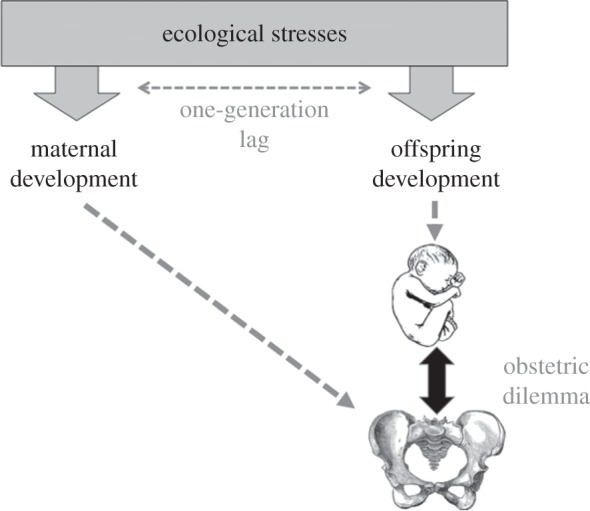
Schematic diagram illustrating how the obstetric dilemma emerges from the interaction between two traits, the maternal pelvis and offspring neonatal size, which are shaped by ecological stresses that are characterized by a one-generation time-lag in the timing of their exposure.
We need to gain greater insight into the non-genetic mechanisms whereby the obstetric dilemma can be renegotiated in response to changing ecological conditions. We can therefore make the focus of our enquiry more specific: what ecological signals do the maternal pelvis and fetal growth respond to, and how exactly can their adaptive responses be coordinated given that they are shaped in different time periods?
3. Adaptation as a trans-generational process
The notion that adult morphology is determined by growth trajectories prompts re-evaluation of how phenotype responds to ecological stresses. To provide insight into this process, let us reconsider the ecogeographical distributions described by Bergmann's and Allen's laws. Contrary to the notion of traits adapting directly to external ecological stresses such as climate, it is now clear that the adaptive process begins in utero, which means that many of the key stresses acting on the offspring are mediated by maternal phenotype [37,38]. For example, environmental heat stress and birthweight are associated across populations, with lower birthweight in hotter climates [39]. Although the mother herself is directly exposed to the thermal environment, fetal heat loss can occur only through maternal tissues, hence the immediate influence on fetal thermodynamics comprises maternal metabolism and homeostatic capacity [40].
More generally, therefore, adaptation in growth traits must be considered a trans-generational process, and the phenotype of each generation has already been exposed to maternal traits before the external environment itself is experienced. Since mortality risk is greatest in the first few years of life [41], the way that the fetus responds to maternal influences is crucial for early survival. Both the pelvis and the brain are subject to stresses early in development, long before adult cognition and locomotion are themselves exposed to selective pressures.
For example, Aiello & Wheeler [42] suggested that the metabolic costs of the large Homo brain may have been met in part by decreasing investment in gut mass, through improving dietary quality. Since in relative terms the energy costs of the brain are greatest in early life (approx. 85% of basal metabolism at birth, versus approx. 25% by adulthood) [43], and since both adult brain and gut mass are determined by their growth patterns, infant or childhood nutrition may have been a key selective pressure shaping this trade-off. Humans are uniquely characterized by the use of ‘weaning foods’ that allow ‘complementary feeding’ following exclusive breastfeeding [44]. Thus, it can be seen that the ecological stresses acting on brain development may be very different from those acting on brain function in adult life.
Moreover, beyond any genetic determinants, there is now substantial evidence that body size and proportions are also characterized by plasticity prior to adulthood and are strongly shaped by experience early in the life-course. Through developmental plasticity, growth patterns are subject to reaction norms, and adult phenotype bears the cumulative influence of multiple developmental stresses [45]. For example, classic studies of rats showed that variation in nutrition in early life exerted lifelong effects on body size and proportions [46,47]. Observational data on humans are consistent, showing that early growth variability tracks on into later life [48], while rickets during development is well established to constrain growth of the pelvis [49].
Indeed, the primary period of human growth plasticity comprises fetal life and infancy, and these developmental stages also represent the period of maternal care. Maternal phenotype is thus the primary source of ecological signals to which the developing offspring adapts [37,38,50]. I have argued that this overlap is no coincidence, and that offspring traits retain plasticity for as long as they remain within the protective umbrella of maternal buffering [37,51]. When this buffering is withdrawn (at birth for some traits, and at the end of lactation for others), many traits become canalized and track over time. Absolute size of body components may continue to increase, but relative differences between individuals persist.
Any renegotiated resolution of the obstetric dilemma is therefore sensitive to the regulation of offspring development by maternal phenotype. Compounding the one-generation time-lag between maternal and offspring growth, the complexity of this process of adaptation increases when we consider that the selective pressures acting on the two parties are not identical.
4. Adaptation in early life as a tug-of-war
The developmental trajectory of each individual can be addressed through the lens of life-history theory, which assumes that energy is invested optimally across competing functions to maximize early survival and adult reproductive fitness [52]. However, the process of maternal care (placental nutrition and lactation) brings two different life histories together, where the mother is exposed to environmental stresses and the fetus/infant is exposed to maternal phenotype. Since mothers and their offspring share only 50% of their genes, the maternal investment strategies that maximize maternal fitness are not necessarily identical to those that maximize offspring fitness. This scenario leads to what Trivers [53] termed ‘parent–offspring conflict’ over the investment of parental resources in each offspring.
It is clear from non-mammals, e.g. birds, that parent–offspring conflict is ubiquitous when parents feed their offspring [54], but the conflict has particular relevance to the obstetric dilemma because the magnitude of investment during fetal life directly impacts size at birth. In relation to the obstetric dilemma, we can focus on two outcomes in particular: the amount of energy available for investment in the offspring per unit time, and the duration of pregnancy.
The notion of parent–offspring conflict has been criticized—for example, Bateson [55] has suggested that the interests of mother and offspring are closely aligned. This criticism misses two key points. First, rather than outright conflict, it is a conflict of interest over when the mother should divert investment from one offspring to other offspring. This is where the concept of negotiation becomes so important, as demonstrated in studies of parent–offspring interactions among birds [56]. Second, in the absence of such a conflict of interest, it would be impossible for the offspring, enclosed within the umbrella of maternal care, to adapt to ecological signals at all. Moore & Haig [57] elegantly described parent–offspring conflict as a ‘tug-of-war’ over the pool of maternal resources potentially available for investment in the offspring. Haig [58] further described how offspring hormones manipulate metabolism in order to increase the supply of nutrients through the placenta, and maternal physiology responds by reducing nutrient transfer, while a similar behavioural tug-of-war characterizes lactation [59]. The same scenario applies to the duration of gestation, which introduces an additional axis of variability into the resolution of the obstetric dilemma [60].
The fetus has no direct exposure to the environment, so what makes it adapt is the tension applied by maternal physiology within the tug-of-war, and if there were no tension, no adaptation could occur. The fact that the fit between the neonatal head and the maternal pelvis is generically tight in Homo sapiens suggests that the tug-of-war over size at birth has been especially strongly contested. What therefore is it that prevents each party from ‘surrendering its position’?
5. Why cannot the offspring be smaller?
Recognizing that the obstetric dilemma as classically described results from the interaction of a two-dimensional area (the birth canal) and a three-dimensional volume (the head), Epstein [61] calculated that even very moderate increments in pelvic diameter or decrements in neonatal head girth would make delivery much easier. These values lie well within the range of within- and between-population variability evident in archaeological and skeletal data [7,36]. The paradox is therefore that for almost every neonate whose head must pass through the constraining pelvis of its mother, there are other women whose larger pelvic dimensions would have made delivery of the neonate easier, and other offspring whose smaller heads would have provided the same benefit [7]. What is it that keeps the fit so tight within individual mother–offspring dyads?
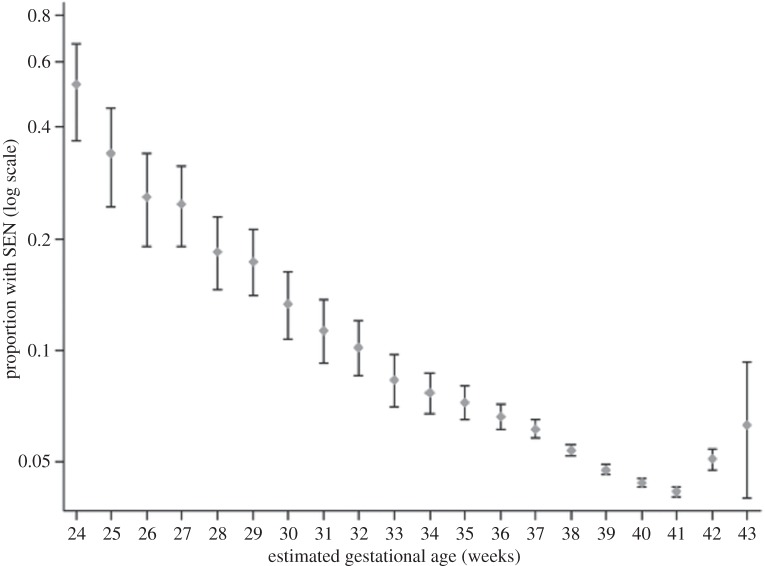
The first question is, why cannot the offspring be smaller? In this respect, most attention has focused on the dimensions of the offspring brain. In the human fetus gestated to term, approximately 30% of adult brain size has been completed by birth [7]. Although there is greater variability across mammals in general in the proportion of brain growth achieved in fetal life [62], at least 30% appears to be achieved in all primate species, suggesting that this degree of brain development represents the minimum for a viable primate infant [7]. Consistent with that hypothesis, a systematic shortening of gestation in humans, which would be one potential solution to producing smaller brained neonates, appears to be non-viable: figure 3 shows a strong dose–response association between delivery before term and the likelihood of impaired cognitive function in later life [63]. Clearly, selection favours 40 weeks of fetal brain growth in our species, though gestation length still varies within and between populations.
Figure 3.
Prevalence of special educational need (SEN) by gestation at delivery, showing an inverse dose–response association with the lowest level of detrimental outcome for offspring born at 41 weeks post-conception. Reproduced with permission from MacKay et al. [63].
However, it is also misleading to focus only on fetal head dimensions. A significant proportion of obstructed labour arises from difficulties in delivering the fetal body, with shoulder dystocia a leading cause of birth injury to both mother (maternal tearing and post-partum haemorrhage) and offspring (muscular or spinal damage) [7]. Indeed, difficulties during delivery are not unique to humans and have been observed in other primates [64,65] and mammals [66]. Classic analyses by Leutenegger indicated that humans do not actually produce unusually encephalized neonates, rather they produce unexpectedly heavy neonates relative to maternal body mass, and these heavier neonates have large brains, but not disproportionately so compared with other primates [67]. Re-investigation of this issue using a more comprehensive dataset suggests that Leutenegger slightly underestimated the magnitude of encephalization in the human neonate, but was correct in concluding that increased neonatal mass is the main outlying characteristic of our species [7].
We therefore need to consider whether body size, as well as head size, could potentially be lower in human neonates. Of all the dimensions of the neonate, the coefficient of variation is lowest for head girth and length (approx. 30% of the coefficient of variation of weight), whereas it is higher for body girths (approx. 50–60%) and greatest for subcutaneous skinfold thicknesses (approx. 100%) [68]. These data indicate greater plasticity in offspring weight and adiposity than in linear growth or brain growth [69].
Human offspring could therefore potentially be smaller at birth, thereby reducing the magnitude of the obstetric dilemma, by reducing non-brain tissues more than the brain. Nevertheless, birthweight is the single biggest predictor of survival during early life [70]; hence any reduction in non-brain tissues must still impose fitness penalties on the offspring, independently of the cognitive penalties associated with shorter gestation described above. A recent study emphasized the contribution of adiposity to infant survival, by showing that low levels of leptin, the hormone secreted by adipose tissue, were strongly associated with mortality risk in malnourished African infants [71]. This may explain why, in a comparison of infants weighing on average approximately 2.7 kg in India, and approximately 3.5 kg in the UK, the difference in birthweight and length was approximately 1.5 z-scores, in head circumference approximately 1.2 z-scores, but in subscapular skinfold was only approximately 0.3 z-scores. The greatest reduction was in abdominal girths (−2.3 z-scores), indicating preferential sacrifice of the visceral organs [69,72].
It seems therefore that a gestation of 40 weeks is favoured for optimal brain growth in our species, and that humans also stand out from other species in delivering relatively large-bodied neonates. From this perspective, it is clear that selection favours fetuses developing large bodies and brains, and that if compromise is necessary, the offspring protects the brain at the expense of other organs and tissues, a phenomenon known as brain-sparing [73,74]. If selection strongly favours fetal growth, could the stress of delivery be reduced by increasing the dimensions of the maternal pelvis?
6. Why cannot the maternal pelvis be larger?
It is now clear that there is substantial variability in maternal pelvic dimensions, as summarized previously [7,36]. The coefficient of variation is approximately 7% for the anterior–posterior and transverse diameters of the pelvic inlet, approximately 11% for the transverse diameter of the outlet and approximately 13.5% for the anterior–posterior diameter of the outlet.
This variability is also associated with ecological variables. Most notably, there is a strong association of bi-iliac diameter with the thermal environment [7,75]. This indicates that locomotion per se is not the only factor impacting pelvic shape, and that wider pelvic dimensions per se do not preclude efficient locomotion. As discussed in §3, heat stress is an established constraint on human growth and physique, and narrow pelves in populations exposed to hot and humid environments are a plausible factor contributing to increased rates of maternal and perinatal mortality in African and Asian populations. Notably, gestation is slightly shorter in African and South Asian women relative to European women [76–79]. This might indicate a modest fetal co-adaptation to smaller birth canal in hot-adapted populations, but although acute heat stress has been linked with preterm delivery [80], robust support for a link between mean gestation duration and climate remains lacking.
Nevertheless, the influence of the thermal environment on pelvic proportions appears an incomplete explanation for population variability in the prevalence of obstructed labour, for a key risk factor in diverse populations is short maternal stature [81–83]. Within African populations characterized on average by narrow pelves, it is short mothers who have the highest risk of obstructed labour. In other words, the maternal pelvis could be larger, and the risk of obstructed labour could be lower, if the mother experienced greater growth during her development. This directs attention instead to additional ecological stresses affecting growth, implicating nutrition in particular.
Indeed, nutrition is likely to be especially relevant to the obstetric dilemma, first because nutritional stresses impact all age groups, second because secular trends are now well described in both maternal and fetal size and third because metabolic fuel is the primary target of the tug-of-war between mother and offspring during fetal life [57], the outcome of which is size at birth.
7. Secular trends in body size
There are now substantial data on secular trends in adult body size in many populations, indicating that shifting ecological conditions impact growth trajectories. In Holland, for example, average female stature increased from around 154 cm in 1840 to 171 cm in the 1997, an average gain of 1.26 cm per decade [84,85]. In turn, increases in female stature are associated with larger pelvic dimensions and a reduced risk of cephalo-pelvic disproportion and caesarean section [81–83,86,87]. However, whilst much attention has been given to recent upward trends in size in European populations, negative trends have been observed in many populations. In India, for example, stature has declined by almost 20 cm over the past 10 000 years, a decline attributed to the cumulative impact of the origins of agriculture, increasing population density, and exposure to regular droughts, famines and epidemics of disease [38,88]. A very modest upward trend in female stature has occurred in India in the twentieth century [38].
It is these negative trends in adult size that are of especial importance for the coordination of maternal and offspring phenotype, as the offspring must necessarily adapt its growth strategy to smaller maternal pelvic dimensions to achieve successful birth. For example, the pelvic dimensions of Indian mothers are substantially reduced compared with those of Europeans [7,89]. Focusing on such secular trends therefore offers new insight into how resolution of the obstetric dilemma must be renegotiated over time.
The possibility that maternal pelvic dimensions might reduce across generations has major implications for the mechanisms regulating late fetal growth [90]. If the predominant influences on neonatal size were genetic, the risk of obstructed labour would be steadily exacerbated as any secular decline in maternal stature progressed. Whilst genetic adaptation could in theory occur through the differential survival of ‘smaller genotypes’ across generations, this would occur at the cost of high burdens of maternal and offspring mortality, and such adaptation might be too slow for the lineage to survive at all. From this, we can make two predictions: first, that the influence of genetic factors on birthweight should be relatively low, and second, that there should be relatively few genes exerting a large effect on birthweight, as their presence would constitute a major risk factor for obstructed labour in the offspring of short mothers. Both of these hypotheses are supported by evidence.
While paternal birthweight, independent of maternal birthweight, is correlated with offspring birthweight [91], indicating a paternal genetic contribution, the total proportion of variability in birthweight and length attributable to genetic factors is only approximately 30% [92,93]. This magnitude of heritability is substantially lower than at later ages, with heritability of adult height approaching 90% [94]. Figure 4 illustrates the changes in heritability in weight and height that occur between birth and 3 years of age, as well as a decline in the heritability of weight during the last trimester of pregnancy—from approximately 50% at 25 weeks gestation to approximately 30% at 42 weeks [95]. This dip in genetic influence during the last trimester allows late fetal growth to be regulated primarily by maternal and uterine factors, before the impact of genotype re-emerges in post-natal life.
Figure 4.
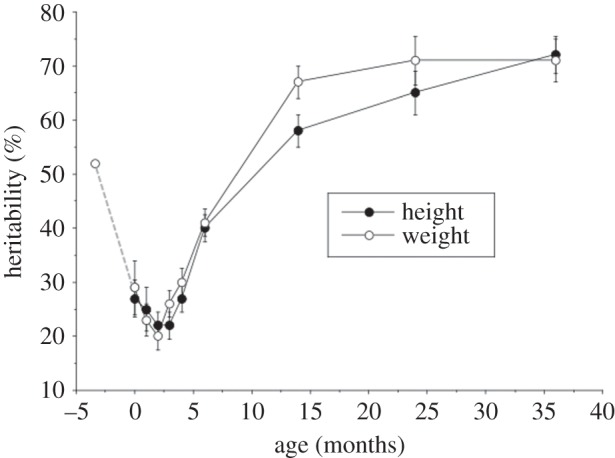
Estimates of heritability in weight and length/height in The Netherlands Twin Register study, with data from another study of late pregnancy added. Heritability of weight declines from approximately 50% at 25 weeks gestation to approximately 30% at birth, then increases to approximately 70% by 36 months. The post-natal pattern for length is very similar. Adapted from [93,95].
Similarly, although a very small number of alleles have been reported to increase birthweight by up to 90 g, or 155 g for those with two such alleles [96–98], such large effects are extremely rare, and the more common magnitude of effect of such alleles is 20–30 g [99]. Since genome-wide association studies have greatest power to find large effects, it is unlikely that this conclusion is an artefact of the limited data available to date; rather it is likely to represent a relatively accurate summary of the polygenic basis of birthweight variability. Finally, the reduced expression of genes that promote fetal growth through genomic imprinting may be a further mechanism for reducing the risk of neonatal proportions exceeding maternal pelvic dimensions [90].
Nevertheless, although environmental factors account for much of variability in size at birth, it is important to remember that these effects reflect ecological stresses accumulated across multiple generations, which may potentially prevent full resolution of the obstetric dilemma.
8. Integrating ecological signals
As described by Haig [58], the fetus manipulates maternal metabolism during pregnancy to increase the supply of nutrients passing through the placenta, while maternal physiology counteracts to suppress these effects. Within this tug-of-war [57], the influence of maternal phenotype on fetal growth is well established. For example, reduced rates of fetal growth are typical of first-borns, owing to incomplete penetration of the spiral arteries [100] of the offspring of mothers who smoke or are anemic [101,102] and of the offspring of mothers with lower body mass index [103,104].
The tug-of-war brings the offspring into contact with a composite maternal phenotype. Influences on maternal metabolism range from immediate (e.g. maternal malaria during pregnancy), recent (maternal nutritional status at the time of conception) and developmental (childhood nutrition) to trans-generational (the mother's own fetal experience). Moreover, some nutritional influences during pregnancy propagate across two generations [105], while the father may also generate epigenetic effects in the offspring through imprinting of the sperm [106]. This highlights the complexity of accumulated nutritional influences on growth and development of each generation.
The niche of pregnancy thus represents a stabilized metabolic environment in which maternal phenotype integrates a wide variety of short- and long-term ecological signals [51]. Kaplan and co-workers [107,108] have described somatic tissues as ‘embodied capital’, representing the accumulation during development of physical resources for investment in reproduction. Building on this approach, I have referred to the overall signal shaping the developing offspring as maternal capital, defined as ‘any aspect of maternal phenotype, whether somatic or behavioral, which enables differential investment in offspring’ [38]. Through stable physical traits such as uterine volume, along with homeostatic systems regulating metabolism, maternal capital allows ‘short-term fluctuations [to be] smoothed out to provide a more reliable rating of environmental quality’, thus damping out short-term ecological stresses [37]. Such maternal buffering is critical, first because humans appear to have evolved in a volatile ecological niche [3,109], and second because the early hyperplasic stages of growth are most sensitive to environmental effects, and therefore benefit most from such maternal buffering [51]. As we saw in §5, it seems that the human brain in particular benefits from such protection during the first 40 weeks of life after conception.
Since many aspects of maternal capital reflect the mother's own development, and hence grandmaternal effects, there are limits to the extent to which the obstetric dilemma can be renegotiated rapidly, even though plasticity. As discussed by Haig [58], the tug-of-war over maternal investment is also mediated by paternal factors which influence the hormonal signals of nutritional demand emitted by the offspring. To the extent that the paternally derived component of the offspring's growth strategy adapts to ecological stresses, it too must do so through the transducing effect of maternal phenotype. The capacity of lineages to evolve contrasting fetal growth trajectories, incorporating alternative resolutions of the obstetric dilemma, is illustrated by the consequences of inter-ethnic unions.
9. When two worlds collide
Investigating the phenotype of offspring produced by two parents from lineages of contrasting body size offers unique insight into the extent to which the obstetric dilemma can be resolved. Studies of the independent maternal and paternal influences on offspring growth were initially undertaken in horses and cattle [110–112], but a similar approach has recently been applied to humans through the analysis of the offspring of inter-ethnic unions. Large ethnic differences in adult body size can be assumed to have emerged over multiple generations and to represent the adaptation of growth to contrasting ecological conditions, potentially including genetic effects.
Among contemporary populations, a particularly notable contrast is between South Asians and Europeans. Not only is there a substantial difference in average adult height [38,113], but South Asians are also characterized by lower levels of lean mass relative to height, and by smaller pelvic dimensions in the mother [38,89,114]. A comparison of offspring of Indian and European parents in the UK showed that offspring with two Indian parents weighed approximately 400 g less than offspring with two European parents. Some of this effect could be attributed to maternal phenotype: the offspring of Indian mothers weighed approximately 150 g less than the offspring of European mothers if the father in each case was European, and 250 g less if the father was Indian [115]. Thus, Indian mothers in the UK clearly produce smaller offspring than do European mothers, and their reduced body size is likely to be one of the most important underlying factors.
However, compared to the offspring of two Indian parents, those of Indian mother and European father weighed approximately 240 g more, indicating that any constraint of the Indian mother is not absolute, but can rather by mediated by paternal effects. Similarly, compared to the offspring of two European parents, those of European mother and Indian father weighed approximately 100 g less, indicating that the effect of the Indian father is to reduce the birthweight of his offspring even when maternal nutrition was apparently sufficient to produce a larger neonate [115].
This study indicates that the paternal component of the ‘Indian growth strategy’ has adapted to the constraints of the Indian mother, and given the smaller pelvic dimensions of Indian females [89], the obstetric dilemma may represent one of the relevant selective pressures. More detailed studies of Indian and European infants have shown that head size is smaller in Indian neonates relative to European neonates, but that the primary differences are in indices of lean mass [72,116]. It remains unclear as yet whether the paternal contribution to the offspring's growth strategy represents a genetic adaptation or an epigenetic effect, but its implications for the obstetric dilemma are clear. In a similar inter-ethnic analysis, comparing Europeans and Asians in the USA, a higher rate of caesarean delivery was apparent in offspring of Asian mothers and European fathers compared with two European parents, whereas no such elevated risk was apparent in the offspring of European mothers and Asian fathers [117].
These studies provide compelling evidence that contrasting fetal growth strategies can emerge in different populations, representing locally adapted resolution of the obstetric dilemma. Because these strategies represent trans-generational processes, always separated by a one-generation time-lag, they cannot immediately achieve optimal co-adaptation to new nutritional signals, including those deriving from maternal capital.
10. Antagonistic effects of nutritional stresses
The studies of inter-ethnic unions have offered new insight into the typical tight fit between the dimensions of the neonate and the maternal pelvis, and the potential for nutritional stresses to elevate the risk of obstructed labour. Nutritional factors can change over the short-term, potentially generating disparity between the dimensions of the pelvis and the magnitude of fetal growth. For example, nutritional constraint during maternal development can perturb shape as well as size of the pelvis. Poor diet and limited exposure to sunlight caused rickets in many women during the industrial revolution, leading to a rise in the need for caesarean deliveries at the start of the twentieth century [7]. Placental dysfunction, maternal metabolic disease and lipogenic diets during pregnancy can similarly perturb fetal growth [118]. However, because of the one-generation time-lag in exposure between the two parties, nutritional stresses impacting the development of the maternal pelvis may be very different from those impacting development of the fetus, potentially generating antagonistic effects on the two traits.
On a broader timescale, such exacerbation of the obstetric dilemma may have occurred around the time of the emergence of agriculture. Data from many populations show that stature tended to decrease during this period [119]. The shift to high-cereal diets may have increased maternal dietary glycemic load, potentially increasing glucose availability to the offspring, while the associated increase in the burden of infectious disease may have favoured higher levels of fetal fat accretion in the last trimester of pregnancy [7]. These metabolic effects cannot yet be reconstructed with confidence, but the skeletal record shows that pelvic dimensions as well as stature declined in Mediterranean populations from 9000 BC, before recovering, and there are some indications in the archaeological record that the level of perinatal mortality was greater in early agricultural populations than in Holocene foragers, which may indicate an exacerbated obstetric dilemma [7].
A more detailed picture is emerging for a remarkably similar scenario in contemporary populations, driven by economic cycles that have generated secular trends in both weight and height. During the second half of the twentieth century, maternal height initially increased in Africa, only to decline from the 1970s in association with falling per capita income, as a consequence of economic structural adjustment policies [120]. In the opposite direction, exposure to international food markets is associated with higher rates of obesity in urban African populations [121], with knock-on effects on maternal metabolism. Crucially, short maternal stature and maternal obesity are both risk factors for gestational diabetes [122,123], which can lead to abnormally large ‘macrosomic’ neonates. Macrosomia is associated with all the complications of the obstetric dilemma: haemorrhage, prolonged labour and perineal trauma in the mother, and shoulder dystocia, asphyxia, birth trauma and death in the offspring [124]. These contrasting nutritional trends—downward for maternal stature, upward for maternal weight—are therefore predicted to increase the magnitude of the obstetric dilemma.
More generally, macrosomia is now a significant public health problem in many low- and middle-income populations. For inter-population comparisons, a fixed birthweight threshold for categorizing macrosomia is inappropriate owing to ethnic differences in the normal range of birthweight. Using a population-specific 90th centile categorization, Koyanagi et al. [124] examined the prevalence and correlates of macrosomia across 23 low- and middle-income countries. Consistently in African, Asian and Latin American countries, risk factors were older mothers, tall stature, male offspring, maternal obesity and maternal diabetes (figure 5a). Producing a macrosomic offspring was associated with an increased risk of caesarean delivery, even after adjustment for elective caesareans; however, this risk was also systematically increased in primiparous compared with multiparous mothers. The associations of maternal age and parity and offspring gender with the risk of macrosomia are a crucial reminder that maternal investment strategy varies across the reproductive career in accordance with the maximization of reproductive fitness.
Figure 5.
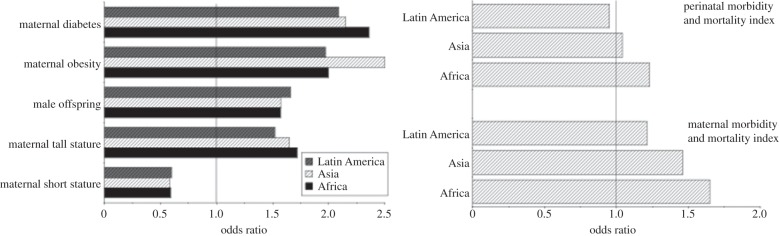
(a) Odds ratio for giving birth to macrosomic infants (defined as above the 90th centile for birthweight in the population) according to maternal stature, obesity and diabetes, along with offspring sex, across 23 countries. Countries are grouped by region (Africa, Asia, Latin America), demonstrating similar risks. The data were adjusted for country, maternal age, size and metabolic status and offspring sex. (b) Odds ratios for maternal or perinatal morbidity and mortality arising from macrosomic offspring, adjusted for country, maternal age, size and metabolic status and offspring sex. Adapted from [124].
These risks for macrosomic offspring translate directly into elevated rates of maternal morbidity and mortality, as illustrated in figure 5b. Intriguingly, however, the odds ratio for perinatal morbidity and mortality only exceeded unity in Africa (OR 1.23, 95% CI 1.08, 1.42) and did not differ significantly from unity in Asia (1.04, 95% CI 0.90, 1.19) or Latin America (0.95, 95% CI 0.82, 1.10). This indicates that the adverse consequences of producing large offspring are born disproportionately by the mother rather than the offspring [124], and this has further implications for how selection may act on the obstetric dilemma. If it is the mother who pays the greatest penalty for large offspring, especially those reproducing for the first time, then selection should act more strongly on maternal rather than offspring factors that constrain fetal growth.
This perspective is supported by experimental studies, which reveal more robustly the effect of short-term changes in maternal energy supply on birth size of the offspring. Nutritional supplementation programmes aiming to reduce the prevalence of low birthweight have tended to achieve relatively modest increases in birthweight. For example, a randomized trial in The Gambia showed that maternal supplementation from 20 weeks gestation increased birthweight on average by 136 g, although this effect was greater in the ‘hungry season’ (201 g), when mothers tended to be lighter, than in the ‘harvest season’ (94 g) [125]. This illustrates that the effect of supplementation is mediated by maternal condition. However, the supplemented mothers also normalized their reproductive function faster than unsupplemented mothers, enabling them to conceive the next offspring sooner [126]. This shows that mothers retain priority control over ‘energy windfalls’ during pregnancy, and convert them primarily into larger family size rather than substantially larger individual offspring.
Indeed, secular trends in birthweight appear to occur much more slowly than those in adult size. Whereas age at maturation and adult height appear able to change at a rate of a standard deviation per five to six generations, with such trends able to continue over many decades, the equivalent rate of change for birthweight is typically 10–30 generations, with slow rates most evident where data from many decades are available [127]. A similar scenario has been observed in a longitudinal study of macaques, where increasing the supply of nutrition immediately increased maternal weight, but took three generations to impact birthweight [128]. It is likely that the obstetric dilemma acts as a natural constraint on secular changes in birthweight, and the health risks generated by macrosomic offspring indicate the consequences of this constraint being over-ridden.
11. Conclusion
In this review, I have argued that the magnitude of the obstetric dilemma is not invariant, rather it reflects different ‘resolutions’ that have emerged through the impact of ecological conditions on growth patterns. I have paid particular attention to the role of contrasting nutritional signals in shaping this resolution.
Since maternal size can vary across generations in response to ecological change, fetal growth strategy must reduce its dependence on genotype and instead respond to signals of maternal phenotype. The tug-of-war over maternal investment enables fetal adaptation, but in response to maternal strategy rather than the environment per se. Like Odysseus sailing between the twin monsters of Scylla and Charybdis in ancient Greek mythology, the fetus must avoid two perils: gaining insufficient nutritional investment (particularly brain growth) to be viable in post-natal life, versus becoming too large for a successful delivery. This dilemma appears to be resolved by the offspring matching its growth trajectory to metabolic signals of maternal phenotype, resulting in a typically tight fit between the dimensions of the maternal pelvis and those of the offspring brain and body.
Because this match represents a ‘trans-generational negotiation’, compounded by a one-generation time-lag between the stresses that shape maternal and offspring growth patterns, short-term nutritional stresses can perturb it, potentially exacerbating the obstetric dilemma. Many women in low- and middle-income countries experienced under-nutrition during their development, but are now exposed to obseogenic environments in adulthood. This scenario exacerbates the obstetric dilemma from each direction, and is contributing to elevated rates of obstructed labour and an epidemic of macrosomic offspring. Public health nutrition therefore has major implications for the global burden of maternal mortality. It should be recognized that this further implicates the global economic order, which is a key factor contributing to both persisting under-nutrition (constraining maternal pelvic growth) and the emerging obesity epidemic (promoting fetal weight gain) [129].
References
- 1.Harcourt-Smith WE, Aiello LC. 2004. Fossils, feet and the evolution of human bipedal locomotion. J. Anat. 204, 403–416. ( 10.1111/j.0021-8782.2004.00296.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnefille R, Potts R, Chalie F, Jolly D, Peyron O. 2004. High-resolution vegetation and climate change associated with Pliocene Australopithecus afarensis. Proc. Natl Acad. Sci. USA 101, 12 125–12 129. ( 10.1073/pnas.0401709101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts R. 1996. Humanity‘s descent: the consequences of ecological instability. New York, NY: William Morrow & Co. [Google Scholar]
- 4.Washburn SL. 1960. Tools and human evolution. Sci. Am. 203, 63–75. ( 10.1038/scientificamerican0960-62) [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg K, Trevathan W. 2002. Birth, obstetrics and human evolution. BJOG 109, 1199–1206. ( 10.1046/j.1471-0528.2002.00010.x) [DOI] [PubMed] [Google Scholar]
- 6.Trevathan WR. 2011. Human birth: an evolutionary perspective. New Brunswick, Canada: AldineTransaction. [Google Scholar]
- 7.Wells JC, DeSilva JM, Stock JT. 2012. The obstetric dilemma: an ancient game of Russian roulette, or a variable dilemma sensitive to ecology? Am. J. Phys. Anthropol. 149(Suppl. 55), 40–71. ( 10.1002/ajpa.22160) [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2006. Neonatal and perinatal mortality: country, regional and global estimates. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 9.World Health Organization. 2005. World Health Report: make every woman and child count. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 10.Arrowsmith S, Hamlin EC, Wall LL. 1996. Obstructed labor injury complex: obstetric fistula formation and the multifaceted morbidity of maternal birth trauma in the developing world. Obstet. Gynecol. Surv. 51, 568–574. ( 10.1097/00006254-199609000-00024) [DOI] [PubMed] [Google Scholar]
- 11.Krogman WM. 1951. The scars of human evolution. Sci. Am. 184, 54–57. [Google Scholar]
- 12.Rosenberg K. 1992. The evolution of modern childbirth. Yearb. Phys. Anthropol. 35, 89–124. ( 10.1002/ajpa.1330350605) [DOI] [Google Scholar]
- 13.Ruff CB, Trinkaus E, Holliday TW. 1997. Body mass and encephalization in Pleistocene Homo. Nature 387, 173–176. ( 10.1038/387173a0) [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ. 2003. The secular trend in human physical growth: a biological view. Econ. Hum. Biol. 1, 161–168. ( 10.1016/S1570-677X(02)00033-3) [DOI] [PubMed] [Google Scholar]
- 15.Malina RM. 1990. Research on secular trends in auxology. Anthropol. Anz. 48, 209–227. [PubMed] [Google Scholar]
- 16.Dunsworth HM, Warrener AG, Deacon T, Ellison PT, Pontzer H. 2012. Metabolic hypothesis for human altriciality. Proc. Natl Acad. Sci. USA 109, 15 212–15 216. ( 10.1073/pnas.1205282109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmann C. 1847. Über die Verhältnisse der wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien 3, 595–708. [Google Scholar]
- 18.Allen JA. 1877. The influence of physical conditions on the genesis of species. Radical Rev. 1, 108–140. [Google Scholar]
- 19.Newman MT. 1953. The application of ecological rules to the racial anthropology of the aboriginal New World. Am. Anthropol. 55, 311–327. ( 10.1525/aa.1953.55.3.02a00020) [DOI] [Google Scholar]
- 20.Roberts DF. 1953. Body weight, race and climate. Am. J. Phys. Anthropol. 11, 533–558. ( 10.1002/ajpa.1330110404) [DOI] [PubMed] [Google Scholar]
- 21.Roberts DF. 1973. Climate and human variability. An Addison-Wesley module in anthropology, No. 34. Reading, MA: Addison-Wesley. [Google Scholar]
- 22.Katzmarzyk PT, Leonard WR. 1998. Climatic influences on human body size and proportions: ecological adaptations and secular trends. Am. J. Phys. Anthropol. 106, 483–503. () [DOI] [PubMed] [Google Scholar]
- 23.Wells JC. 2012. Ecogeographical associations between climate and human body composition: analyses based on anthropometry and skinfolds. Am. J. Phys. Anthropol. 147, 169–186. ( 10.1002/ajpa.21591) [DOI] [PubMed] [Google Scholar]
- 24.Ashton KG. 2002. Patterns of within-species body size variation of birds: strong evidence for Bergmann's rule. Glob. Ecol. Biogeogr. 11, 505–523. ( 10.1046/j.1466-822X.2002.00313.x) [DOI] [Google Scholar]
- 25.Ashton KG, Feldman CR. 2003. Bergmann's rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57, 1151–1163. ( 10.1111/j.0014-3820.2003.tb00324.x) [DOI] [PubMed] [Google Scholar]
- 26.Ashton KG, Tracy MC, deQueiroz A. 2000. Is Bergmann's rule valid for mammals? Am. Nat. 156, 390–415. ( 10.1086/303400) [DOI] [PubMed] [Google Scholar]
- 27.Paterson JD. 1996. Coming to America: acclimation in macaque body structures and Bergmann's Rule. Int. J. Primatol. 17, 585–612. ( 10.1007/BF02735193) [DOI] [Google Scholar]
- 28.Trinkaus E. 1981. Neanderthal limb proportions and cold adaptation. In Aspects of human evolution (ed. Stringer CBS.), pp. 187–224. London, UK: Taylor and Francis. [Google Scholar]
- 29.Ruff C. 2002. Variation in human body size and shape. Annu. Rev. Anthropol. 31, 211–232. ( 10.1146/annurev.anthro.31.040402.085407) [DOI] [Google Scholar]
- 30.Ruff CB. 1994. Morphological adaptation to climate in modern and fossil humans. Yrbk. Phys. Anthropol. 37, 65–107. ( 10.1002/ajpa.1330370605) [DOI] [Google Scholar]
- 31.Dobzhansky T, Boesinger E. 1983. Human culture: a moment in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 32.Fisher RA. 1930. The genetical theory of natural selection. London, UK: Oxford University Press. [Google Scholar]
- 33.Lango Allen H, et al. 2010. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467, 832–838. ( 10.1038/nature09410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taal HR, et al. 2012. Common variants at 12q15 and 12q24 are associated with infant head circumference. Nat. Genet. 44, 532–538. ( 10.1038/ng.2238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grabowski MW. 2013. Human obstetrics and the evolution of constraints. Evol. Biol. 40, 57–75. ( 10.1007/s11692-012-9174-7) [DOI] [Google Scholar]
- 36.Kurki HK. 2013. Skeletal variability in the pelvis and limb skeleton of humans: does stabilizing selection limit female pelvic variation? Am. J. Hum. Biol. 25, 795–802. ( 10.1002/ajhb.22455) [DOI] [PubMed] [Google Scholar]
- 37.Wells JC. 2003. The thrifty phenotype hypothesis: thrifty offspring or thrifty mother? J. Theor. Biol. 221, 143–161. ( 10.1006/jtbi.2003.3183) [DOI] [PubMed] [Google Scholar]
- 38.Wells JC. 2010. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am. J. Hum. Biol. 22, 1–17. ( 10.1002/ajhb.20994) [DOI] [PubMed] [Google Scholar]
- 39.Wells JC, Cole TJ. 2002. Birth weight and environmental heat load: a between-population analysis. Am. J. Phys. Anthropol. 119, 276–282. ( 10.1002/ajpa.10137) [DOI] [PubMed] [Google Scholar]
- 40.Wells JC. 2002. Thermal environment and human birth weight. J. Theor. Biol. 214, 413–425. ( 10.1006/jtbi.2001.2465) [DOI] [PubMed] [Google Scholar]
- 41.Kelly RL. 1995. The foraging spectrum. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 42.Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis—the brain and the digestive-system in human and primate evolution. Curr. Anthropol. 36, 199–221. ( 10.1086/204350) [DOI] [Google Scholar]
- 43.Holliday MA. 1978. Body composition and energy needs during growth. In Human growth, vol. 2 (eds Falkner F, Tanner JM.), pp. 117–139. New York, NY: Plenum. [Google Scholar]
- 44.Sellen DW. 2006. Lactation, complementary feeding, and human life history. In The evolution of human life history (eds Hawkes K, Paine RL.), pp. 155–196. Santa Fe, NM: School for American Research Press. [Google Scholar]
- 45.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 46.McCance RA, Widdowson EM. 1956. The effects of chronic undernutrition and of total starvation on growing and adult rats. Br. J. Nutr. 10, 363–373. ( 10.1079/BJN19560054) [DOI] [PubMed] [Google Scholar]
- 47.Widdowson EM, McCance RA. 1975. A review: new thoughts on growth. Pediatr. Res. 9, 154–156. ( 10.1203/00006450-197503000-00010) [DOI] [PubMed] [Google Scholar]
- 48.Wells JC, Chomtho S, Fewtrell MS. 2007. Programming of body composition by early growth and nutrition. Proc. Nutr. Soc. 66, 423–434. ( 10.1017/S0029665107005691) [DOI] [PubMed] [Google Scholar]
- 49.Dick JL. 1922. Rickets: a study of economic conditions and their effects on the health of the nation. London, UK: William Heinemann. [Google Scholar]
- 50.Kuzawa CW. 2005. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am. J. Hum. Biol. 17, 5–21. ( 10.1002/ajhb.20091) [DOI] [PubMed] [Google Scholar]
- 51.Wells JC. 2014. Adaptive variability in the duration of critical windows of plasticity: implications for the programming of obesity. Evol. Med. Public Health 2014, 109–121. ( 10.1093/emph/eou019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill K. 1993. Life history theory and evolutionary anthropology. Evol. Anthropol. 2, 78–89. ( 10.1002/evan.1360020303) [DOI] [Google Scholar]
- 53.Trivers RL. 1974. Parent–offspring conflict. Am. Zool. 14, 249–264. [Google Scholar]
- 54.Godfray HC, Johnstone RA. 2000. Begging and bleating: the evolution of parent–offspring signalling. Phil. Trans. R. Soc. Lond. B 355, 1581–1591. ( 10.1098/rstb.2000.0719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bateson P. 1994. The dynamics of parent–offspring relationships in mammals. Trends Ecol. Evol. 9, 399–403. ( 10.1016/0169-5347(94)90066-3) [DOI] [PubMed] [Google Scholar]
- 56.Hinde CA, Johnstone RA, Kilner RM. 2010. Parent–offspring conflict and coadaptation. Science 327, 1373–1376. ( 10.1126/science.1186056) [DOI] [PubMed] [Google Scholar]
- 57.Moore T, Haig D. 1991. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 7, 45–49. ( 10.1016/0168-9525(91)90230-N) [DOI] [PubMed] [Google Scholar]
- 58.Haig D. 1993. Genetic conflicts in human pregnancy. Q. Rev. Biol. 68, 495–532. ( 10.1086/418300) [DOI] [PubMed] [Google Scholar]
- 59.Wells JC. 2003. Parent–offspring conflict theory, signaling of need, and weight gain in early life. Q. Rev. Biol. 78, 169–202. ( 10.1086/374952) [DOI] [PubMed] [Google Scholar]
- 60.Haig D. 1999. Genetic conflicts of pregnancy and childhood. In Evolution in health and disease (ed. Stearns SC.), pp. 77–90. Oxford, UK: Oxford University Press. [Google Scholar]
- 61.Epstein HT. 1973. Possible metabolic constraints on human brain weight at birth. Am. J. Phys. Anthropol. 39, 135–136. ( 10.1002/ajpa.1330390114) [DOI] [PubMed] [Google Scholar]
- 62.Barton RA, Capellini I. 2011. Maternal investment, life histories, and the costs of brain growth in mammals. Proc. Natl Acad. Sci. USA 108, 6169–6174. ( 10.1073/pnas.1019140108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacKay DF, Smith GC, Dobbie R, Pell JP. 2010. Gestational age at delivery and special educational need: retrospective cohort study of 407 503 schoolchildren. PLoS Med. 7, e1000289 ( 10.1371/journal.pmed.1000289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coates KW, Galan HL, Shull BL, Kuehl TJ. 1995. The squirrel monkey: an animal model of pelvic relaxation. Am. J. Obstet. Gynecol. 172, 588–593. ( 10.1016/0002-9378(95)90577-4) [DOI] [PubMed] [Google Scholar]
- 65.Coates KW, Gibson S, Williams LE, Brady A, Abee CR, Shull BL, Kuehl TJ. 1995. The squirrel monkey as an animal model of pelvic relaxation: an evaluation of a large breeding colony. Am. J. Obstet. Gynecol. 173, 1664–1669; discussion 1669–70 ( 10.1016/0002-9378(95)90407-7) [DOI] [PubMed] [Google Scholar]
- 66.Frank LG, Glickman SE. 1994. Giving birth through a penile clitoris: parturition and dystocia in the spotted hyaena (Crocuta crocuta). J. Zool. 234, 659–690. ( 10.1111/j.1469-7998.1994.tb04871.x) [DOI] [Google Scholar]
- 67.Leutenegger W. 1982. Encephalization and obstetrics in primates with particular reference to human evolution. In Primate brain evolution: methods and concepts (eds Armstrong E, Falk D.), pp. 85–95. New York, NY: Plenum Press. [Google Scholar]
- 68.Leary S, et al. 2006. Geographical variation in neonatal phenotype. Acta Obstet. Gynecol. Scand. 85, 1080–1089. ( 10.1080/00016340600697447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wells JC. 2013. The thrifty phenotype and the hierarchical preservation of tissues under stress. Int. J. Epidemiol. 42, 1223–1227. ( 10.1093/ije/dyt130) [DOI] [PubMed] [Google Scholar]
- 70.Hogue CJ, Buehler JW, Strauss LT, Smith JC. 1987. Overview of the National Infant Mortality Surveillance (NIMS) project—design, methods, results. Public Health Rep. 102, 126–138. [PMC free article] [PubMed] [Google Scholar]
- 71.Bartz S, et al. 2014. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J. Clin. Endocrinol. Metab. 99, 2128–2137. ( 10.1210/jc.2013-4018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJP, Joglekar C, Kellingray S. 2003. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int. J. Obes. Relat. Metab. Disord. 27, 173–180. ( 10.1038/sj.ijo.802219) [DOI] [PubMed] [Google Scholar]
- 73.Hales CN, Barker DJ. 1992. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35, 595–601. ( 10.1007/BF00400248) [DOI] [PubMed] [Google Scholar]
- 74.Latini G, De Mitri B, Del Vecchio A, Chitano G, Felice C, Zetterström R. 2004. Foetal growth of kidneys, liver and spleen in intrauterine growth restriction: ‘programming’ causing ‘metabolic syndrome’ in adult age. Acta Paediatr. 93, 1635–1639. ( 10.1111/j.1651-2227.2004.tb00855.x) [DOI] [PubMed] [Google Scholar]
- 75.Ruff C. 2010. Body size and body shape in early hominins—implications of the Gona pelvis. J. Hum. Evol. 58, 166–178. ( 10.1016/j.jhevol.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 76.Papiernik E, Alexander GR, Paneth N. 1990. Racial differences in pregnancy duration and its implications for perinatal care. Med. Hypotheses 33, 181–186. ( 10.1016/0306-9877(90)90173-C) [DOI] [PubMed] [Google Scholar]
- 77.Papiernik E, Cohen H, Richard A, de Oca MM, Feingold J. 1986. Ethnic difference in duration of pregnancy. Ann. Hum. Biol. 13, 259–265. ( 10.1080/03014468600008431) [DOI] [PubMed] [Google Scholar]
- 78.Mathai M, Thomas S, Peedicayil A, Regi A, Jasper P, Joseph R. 1995. Growth pattern of the Indian fetus. Int. J. Gynaecol. Obstet. 48, 21–24. ( 10.1016/0020-7292(94)02237-2) [DOI] [PubMed] [Google Scholar]
- 79.Tambyraja RL. 1991. The prematurity paradox of the small Indian baby. Indian J. Pediatr. 58, 415–419. ( 10.1007/BF02750921) [DOI] [PubMed] [Google Scholar]
- 80.Vicedo-Cabrera AM, Iniguez C, Barona C, Ballester F. 2014. Exposure to elevated temperatures and risk of preterm birth in Valencia, Spain. Environ. Res. 134C, 210–217. ( 10.1016/j.envres.2014.07.021) [DOI] [PubMed] [Google Scholar]
- 81.Mahmood TA, Campbell DM, Wilson AW. 1988. Maternal height, shoe size, and outcome of labour in white primigravidas: a prospective anthropometric study. Br. Med. J. 297, 515–517. ( 10.1136/bmj.297.6647.515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sokal D, Sawadogo L, Adjibade A. 1991. Short stature and cephalopelvic disproportion in Burkina Faso, West Africa. Operations Research Team. Int. J. Gynaecol. Obstet. 35, 347–350. ( 10.1016/0020-7292(91)90671-Q) [DOI] [PubMed] [Google Scholar]
- 83.Khunpradit S, Patumanond J, Tawichasri C. 2005. Risk indicators for cesarean section due to cephalopelvic disproportion in Lamphun hospital. J. Med. Assoc. Thai 88(Suppl. 2), S63–S68. [PubMed] [Google Scholar]
- 84.Drukker JW, Tassenaar V. 1997. Paradoxes of modernization and material well-being in The Netherlands during the nineteenth century. In Health and welfare during industrialization (eds Steckel RH, Floud R.), pp. 331–377. Chicago, IL: University of Chicago Press. [Google Scholar]
- 85.Fredriks AM, et al. 1998. Nederlandse groeidiagrammen 1997 in historisch perspectief. In De vierde Landelijke Groeistudie (1997) Presenatie niewe groeidiagrammen (ed. Wit JM.), pp. 1–13. Leiden, The Netherlands: TNO Preventie en Gezondheit. [Google Scholar]
- 86.Harrison KA, Briggs ND, John CT, Memberr MTB, Lolomari DO. 1988. Growth during early teenage pregnancy. Lancet 331, 1226–1227. ( 10.1016/S0140-6736(88)92046-6) [DOI] [PubMed] [Google Scholar]
- 87.Holland EL, Cran GW, Elwood JH, Pinkerton JHM, Thompson W. 1982. Associations between pelvic anatomy, height and year of birth of men and women in Belfast. Ann. Hum. Biol. 9, 113–120. ( 10.1080/03014468200005581) [DOI] [PubMed] [Google Scholar]
- 88.Lukacs JR. 2007. Human biological diversity in ancient India: Dr Irawati Karve and contemporary issues in biological anthropology. In Anthropology for archaeology: proceedings of the Professor Irawati Karve birth centenary seminar (eds Walimbe SR, Joglekar PP, Basa KK.), pp. 193–206. Pune, India: Deccan College Post-Graduate and Research Institute. [Google Scholar]
- 89.Pan N. 1996. Measurements of the pelvis in Hindu females. J. Anat. 63, 263–266. [PMC free article] [PubMed] [Google Scholar]
- 90.Pembrey M. 1996. Imprinting and transgenerational modulation of gene expression; human growth as a model. Acta Genet. Med. Gemellol. (Roma) 45, 111–125. [DOI] [PubMed] [Google Scholar]
- 91.Magnus P, Gjessing HK, Skrondal A, Skjærven R. 2001. Paternal contribution to birth weight. J. Epidemiol. Community Health 55, 873–877. ( 10.1136/jech.55.12.873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. 2007. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent–offspring data. Am. J. Epidemiol. 165, 734–741. ( 10.1093/aje/kwk107) [DOI] [PubMed] [Google Scholar]
- 93.Mook-Kanamori DO, van Beijsterveldt CE, Steegers EA, Aulchenko YS, Raat H, Hofman A, Eilers PH, Boomsma DI, Jaddoe VWV. 2012. Heritability estimates of body size in fetal life and early childhood. PLoS ONE 7, e39901 ( 10.1371/journal.pone.0039901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silventoinen K, et al. 2003. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin. Res. 6, 399–408. ( 10.1375/136905203770326402) [DOI] [PubMed] [Google Scholar]
- 95.Gielen M, Lindsey PJ, Derom C, Smeets HJM, Souren NY, Paulussen ADC, Derom R, Nijhuis JG. 2008. Modeling genetic and environmental factors to increase heritability and ease the identification of candidate genes for birth weight: a twin study. Behav. Genet. 38, 44–54. ( 10.1007/s10519-007-9170-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adkins RM, Somes G, Morrison JC, Hill JB, Watson EM, Magann EF, Krushkal J. 2010. Association of birth weight with polymorphisms in the IGF2, H19, and IGF2R genes. Pediatr. Res. 68, 429–434. ( 10.1203/PDR.0b013e3181f1ca99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Petry CJ, et al. 2005. Common polymorphism in H19 associated with birthweight and cord blood IGF-II levels in humans. BMC Genet. 6, 22 ( 10.1186/1471-2156-6-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishida M, et al. 2012. Maternal inheritance of a promoter variant in the imprinted PHLDA2 gene significantly increases birth weight. Am. J. Hum. Genet. 90, 715–719. ( 10.1016/j.ajhg.2012.02.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horikoshi M, et al. 2013. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat. Genet. 45, 76–82. ( 10.1038/ng.2477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khong TY, Adema ED, Erwich JJ. 2003. On an anatomical basis for the increase in birth weight in second and subsequent born children. Placenta 24, 348–353. ( 10.1053/plac.2002.0922) [DOI] [PubMed] [Google Scholar]
- 101.Williams LA, Evans SF, Newnham JP. 1997. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. Br. Med. J. 314, 1864–1868. ( 10.1136/bmj.314.7098.1864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang N, Tikellis G, Sun C, Pezic A, Wang L, Wells JCK, Cochrane J, Ponsonby A-L, Dwyer T. 2014. The effect of maternal prenatal smoking and alcohol consumption on the placenta-to-birth weight ratio. Placenta 35, 437–441. ( 10.1016/j.placenta.2014.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dharmalingam A, Navaneetham K, Krishnakumar CS. 2010. Nutritional status of mothers and low birth weight in India. Matern. Child Health J. 14, 290–298. ( 10.1007/s10995-009-0451-8) [DOI] [PubMed] [Google Scholar]
- 104.Hypponen E, Power C, Smith GD. 2004. Parental growth at different life stages and offspring birthweight: an intergenerational cohort study. Paediatr. Perinat. Epidemiol. 18, 168–177. ( 10.1111/j.1365-3016.2004.00556.x) [DOI] [PubMed] [Google Scholar]
- 105.Cropley JE, Suter CM, Beckman KB, Martin DIK. 2006. Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc. Natl Acad. Sci. USA 103, 17 308–17 312. ( 10.1073/pnas.0607090103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaati G, Bygren LO, Pembrey M, Sjöström M. 2007. Transgenerational response to nutrition, early life circumstances and longevity. Eur. J. Hum. Genet. 15, 784–790. ( 10.1038/sj.ejhg.5201832) [DOI] [PubMed] [Google Scholar]
- 107.Kaplan H, Lancaster JB, Johnson SE, Bock J. 1995. Does observed fertility maximize fitness among New Mexican men? A test of an optimality model and a new theory of parental investment in the embodied capital of offspring. Hum. Nat. 6, 325–360. ( 10.1007/BF02734205) [DOI] [PubMed] [Google Scholar]
- 108.Hill K, Kaplan H. 1999. Life history traits in humans: theory and empirical studies. Annu. Rev. Anthropol. 28, 387–430. ( 10.1146/annurev.anthro.28.1.397) [DOI] [PubMed] [Google Scholar]
- 109.Wells JC. 2012. Ecological volatility and human evolution: a novel perspective on life history and reproductive strategy. Evol. Anthropol. 21, 277–288. ( 10.1002/evan.21334) [DOI] [PubMed] [Google Scholar]
- 110.Walton A, Hammond J. 1938. The maternal effects on growth and conformation in Shire horse-Shetland pony crosses. Proc. R. Soc. Lond. B 121, 311–335. ( 10.1098/rspb.1938.0029) [DOI] [Google Scholar]
- 111.Batra TR, McAllister AJ, Lee AJ, Lin CY, Roy GL, Vesely JA, Wauthy J, Winter KA. 1983. Body weights and dimensions of pureline and crossline heifers of the Canadian dairy cattle breeding project. Can. J. Anim. Dairy Sci. 63, 511–522. ( 10.4141/cjas83-060) [DOI] [Google Scholar]
- 112.Joubert DM, Hammond JA. 1958. A crossbreeding experiment with cattle, with special reference to the maternal effect in South Devon–Dexter crosses. J. Agr. Sci. 51, 325–341. ( 10.1017/S0021859600035140) [DOI] [Google Scholar]
- 113.Baten J, Blum M. 2012. Growing tall but unequal: new findings and new background evidence on anthropometric welfare in 156 countries, 1810–1989. Econ. Hist. Dev. Regions 27(Suppl. 1), S66–S85. ( 10.1080/20780389.2012.657489) [DOI] [Google Scholar]
- 114.Deurenberg P, Deurenberg-Yap M, Guricci S. 2002. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes. Rev. 3, 141–146. ( 10.1046/j.1467-789X.2002.00065.x) [DOI] [PubMed] [Google Scholar]
- 115.Wells JC, Sharp G, Steer PJ, Leon DA. 2013. Paternal and maternal influences on differences in birth weight between Europeans and Indians born in the UK. PLoS ONE 8, e61116 ( 10.1371/journal.pone.0061116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stanfield KM, Wells JC, Fewtrell MS, Frost C, Leon DA. 2012. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study. Int. J. Epidemiol. 41, 1409–1418. ( 10.1093/ije/dys139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nystrom MJ, Caughey AB, Lyell DJ, Druzin ML, El-Sayed YY. 2008. Perinatal outcomes among Asian-white interracial couples. Am. J. Obstet. Gynecol. 199, 385.e1–385.e5. ( 10.1016/j.ajog.2008.06.065) [DOI] [PubMed] [Google Scholar]
- 118.Wells JC. 2007. The thrifty phenotype as an adaptive maternal effect. Biol. Rev. Camb. Philos. Soc. 82, 143–172. ( 10.1111/j.1469-185X.2006.00007.x) [DOI] [PubMed] [Google Scholar]
- 119.Cohen MN, Armelagos GJ. 1984. Palaeopathology and the origins of agriculture. Orlando, FL: Academic Press. [Google Scholar]
- 120.Garenne M. 2011. Trends in nutritional status in adult women in sub-Saharan Africa. DHS Comparative Reports 27 Calverton, MD: ICF Macro. [Google Scholar]
- 121.Wells JC. 2013. Obesity as malnutrition: the dimensions beyond energy balance. Eur. J. Clin. Nutr. 67, 507–512. ( 10.1038/ejcn.2013.31) [DOI] [PubMed] [Google Scholar]
- 122.Jang HC, Min HK, Lee HK, Cho NH, Metzger BE. 1998. Short stature in Korean women: a contribution to the multifactorial predisposition to gestational diabetes mellitus. Diabetologia 41, 778–783. ( 10.1007/s001250050987) [DOI] [PubMed] [Google Scholar]
- 123.Moses RG, Mackay MT. 2004. Gestational diabetes: is there a relationship between leg length and glucose tolerance? Diabetes Care 27, 1033–1035. ( 10.2337/diacare.27.5.1033) [DOI] [PubMed] [Google Scholar]
- 124.Koyanagi A, Zhang J, Dagvadorj A, Hirayama F, Shibuya K, Souza JP, Gülmezoglu AM. 2013. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet 381, 476–483. ( 10.1016/S0140-6736(12)61605-5) [DOI] [PubMed] [Google Scholar]
- 125.Ceesay SM, Prentice AM, Cole TJ, Foord F, Poskitt EME, Weaver LT, Whitehead RG. 1997. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. Br. Med. J. 315, 786–790. ( 10.1136/bmj.315.7111.786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lunn PG, Austin S, Prentice AM, Whitehead RG. 1984. The effect of improved nutrition on plasma prolactin concentrations and postpartum infertility in lactating Gambian women. Am. J. Clin. Nutr. 39, 227–235. [DOI] [PubMed] [Google Scholar]
- 127.Wells JC, Stock JT. 2011. Re-examining heritability: genetics, life history and plasticity. Trends Endocrinol. Metab. 22, 421–428. ( 10.1016/j.tem.2011.05.006) [DOI] [PubMed] [Google Scholar]
- 128.Price KC, Hyde JS, Coe CL. 1999. Matrilineal transmission of birth weight in the rhesus monkey (Macaca mulatta) across several generations. Obstet. Gynecol. 94, 128–134. ( 10.1016/S0029-7844(99)00269-0) [DOI] [PubMed] [Google Scholar]
- 129.Wells JC. 2012. Obesity as malnutrition: the role of capitalism in the obesity global epidemic. Am. J. Hum. Biol. 24, 261–276. ( 10.1002/ajhb.22253) [DOI] [PubMed] [Google Scholar]