Abstract
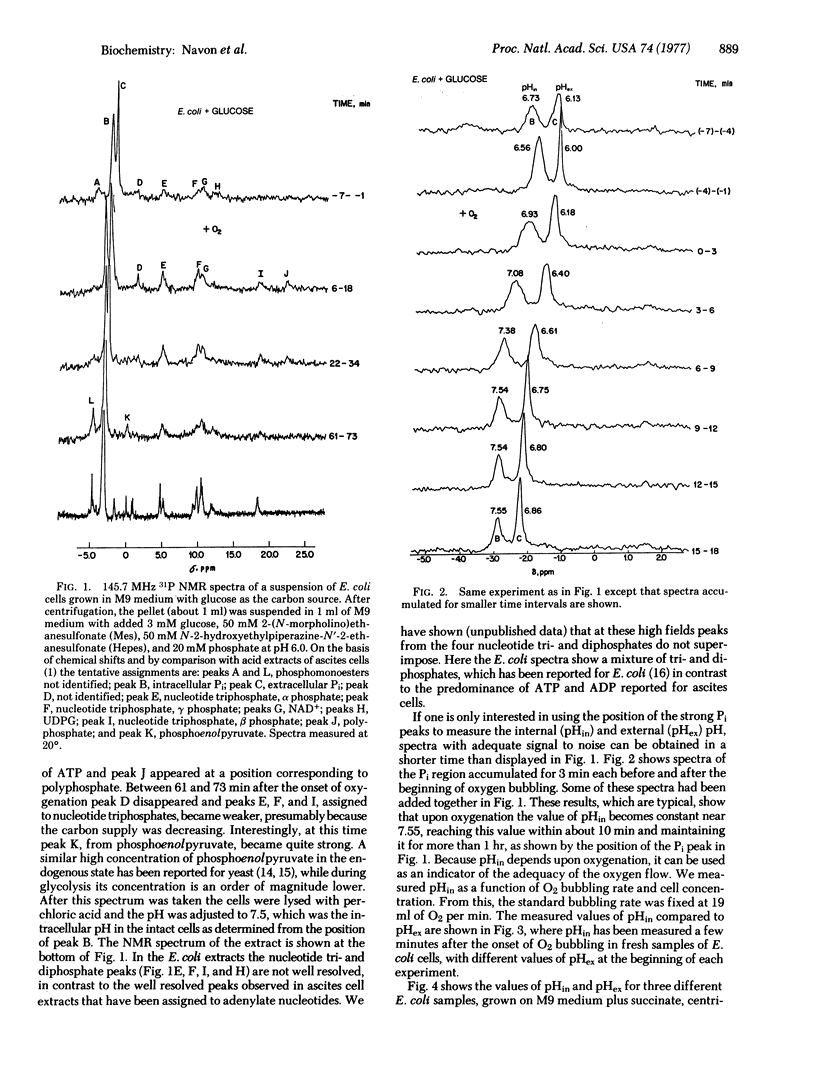
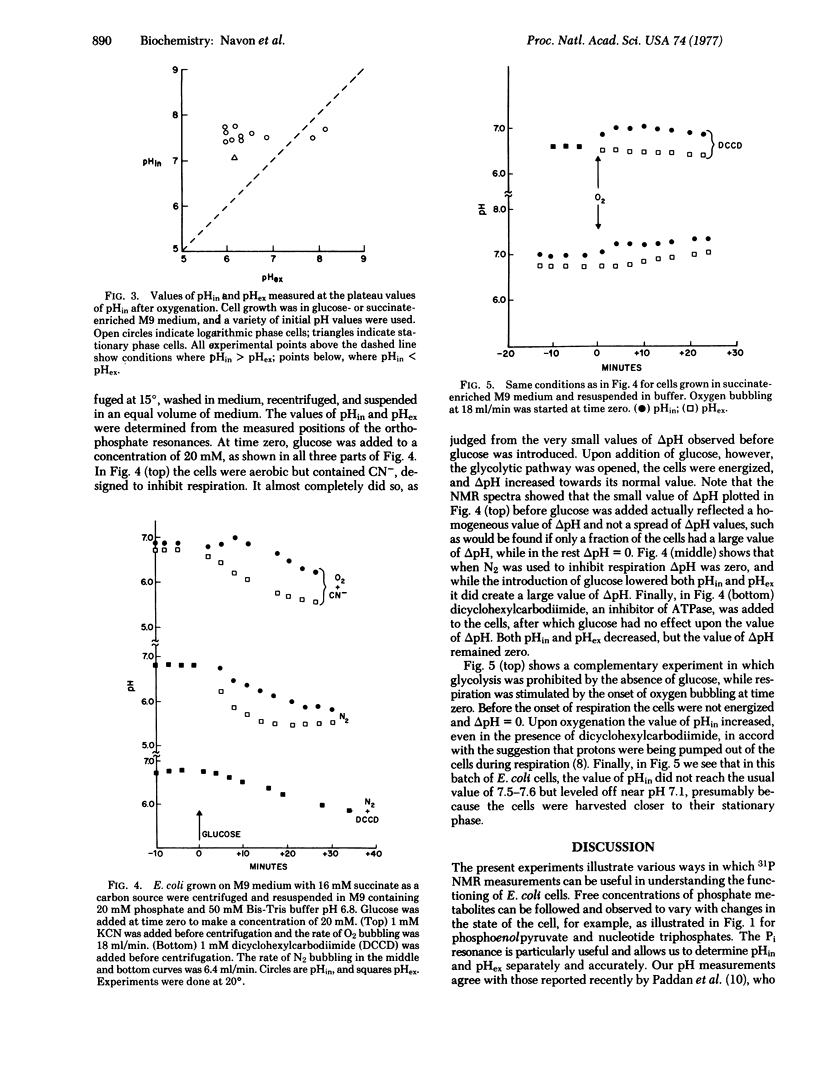
31P nuclear magnetic resonance spectra at 145.7 MHZ were obtained of concentrated suspensions of E. coli cells. The position of the Pi resonance was used to determine the pH, and in most experiments it was possible to distinguish the intracellular (pHin) and extracellular (pHex) values. During respiration pHin approached 7.55, while pHex varied from 6.0 to 8.0. With succinate as a carbon source and in a N2 environment, pHin - pHex. Upon addition of glucose, pHin greater than pHex. In the presence of an ATPase (adenosinetriphosphatase; ATP phosphohydrolase; EC 3.6.1.3) inhibitor dicyclohexylcarbodiimide, pHin remained equal to pHex even in the presence of glucose. In other experiments, oxygenation brought pHin above pHex even in the presence of dicyclohexylcarbodiimide. These experiments are consistent with Mitchell's hypothesis that, first, delta pH can be created by the reversal of the ATPase reaction and, second, that protons are pumped outward during respiration. In addition to Pi, about 10 more resonances were resolved, several of which were assigned to different phosphate metabolites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barwell C. J., Hess B. Regulation of pyruvate kinase during glyconeogenesis in Saccharomyces cerevisiae. FEBS Lett. 1971 Nov 15;19(1):1–4. doi: 10.1016/0014-5793(71)80591-4. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson T. O., Costello A. J., Omachi A. Phosphate metabolism in intact human erythrocytes: determination by phosphorus-31 nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2487–2490. doi: 10.1073/pnas.71.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wong P. T. The intracellular pH of Escherichia coli. Biochim Biophys Acta. 1969 Oct 14;193(1):212–214. doi: 10.1016/0005-2736(69)90074-1. [DOI] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. 31P nuclear magnetic resonance studies of Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):87–91. doi: 10.1073/pnas.74.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T. Observations on yeast pyruvate kinase activity in vivo. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1076–1083. doi: 10.1016/0006-291x(70)90904-6. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. The proton-translocating ATPase of Escherichia coli. FEBS Lett. 1974 Mar 15;40(1):1–4. doi: 10.1016/0014-5793(74)80880-x. [DOI] [PubMed] [Google Scholar]
- White S. H., O'Brien W. M. The buffer value and transmembrane potential of Escherichia coli. Biochim Biophys Acta. 1972 Mar 17;255(3):780–785. doi: 10.1016/0005-2736(72)90390-2. [DOI] [PubMed] [Google Scholar]


