Abstract
Human birthweight is subject to stabilizing selection. Large babies are at risk of obstetric complications such as obstructed labour, which endangers both mother and child. Small babies are also at risk with reduced survival. Fetal growth requires remodelling of maternal spiral arteries to provide an adequate maternal blood supply to the placenta. This arterial transformation is achieved by placental trophoblast cells, which invade into the uterine wall. Under-invasion is associated with fetal growth restriction; but if invasion is excessive large babies can result. A growing body of evidence suggests that this process is controlled by interactions between killer-cell immunoglobulin-like receptors (KIRs) expressed on maternal uterine natural killer cells (uNK) and their corresponding human leukocyte antigen-C (HLA-C) ligands on invading trophoblast. Mothers with the KIR AA genotype and a fetus with a paternal HLA-C2 allele tend to have small babies, because this combination inhibits cytokine secretion by uNK. Mothers with the activating KIR2DS1 gene and an HLA-C2 fetus are more likely to have large babies. When KIR2DS1 binds to HLA-C2 this increases secretion of cytokines that enhance trophoblast invasion. We conclude that specific combinations of the highly polymorphic gene systems, KIR and HLA-C, contribute to successful reproduction by maintaining birthweight between two extremes.
Keywords: birthweight, natural killer cells, immunology, pre-eclampsia, fetal growth restriction, placental development
1. Introduction
The role of the immune system in the development of both the brain and the placenta during the evolution of modern humans may not be immediately obvious. This paper reviews the part played by the immune system at the moment in human life history when brain size and pelvic anatomy interact in a way that is critical to success or failure of pregnancy. Human brains are now so large that it is an extremely tight fit for them to squeeze through the pelvis. Indeed, babies often do get stuck during the passage through the birth canal resulting in obstructed labour. It is not just pregnancies with very large babies where mothers and their babies are at risk of morbidity and mortality. At the opposite end of the spectrum when babies are too small this can have equally damaging consequences. Karn and Penrose first noted this selective pressure at the extremes of the birthweight spectrum in the 1930s, but it was Bodmer who pointed out that human birthweight is a prime example of balancing or stabilizing selection [1,2]. Although death of babies is now less frequent due to advances in neonatal medicine, there is still considerable morbidity. Our recent study in Norway shows babies were most frequently admitted to the neonatal ward when they were born at the extremes of the birthweight spectrum [3]. This suggests there is still strong selective pressure to maintain human birthweight between these two extremes. This situation, encapsulated in the notion of the Obstetric Dilemma, is a particular problem for humans.
2. Placentation and fetal growth
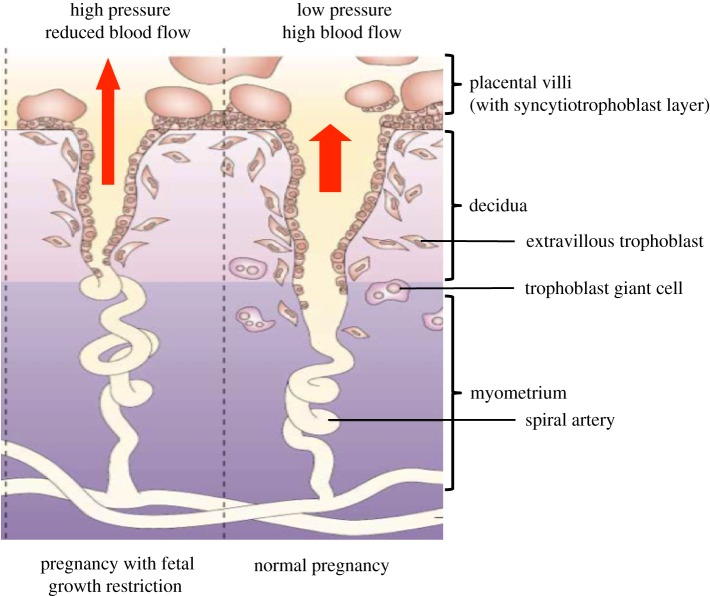
Growth of the fetus and size at birth must depend on the delivery of sufficient nutrients and oxygen to the placenta. The supply line in the uterus is via the maternal arteries supplying blood to the placenta—the so-called spiral arteries. In humans, these are structurally transformed during early pregnancy to allow blood flow to increase about 100-fold [4]. Arterial transformation depends on an unusual process during placentation, whereby fetal trophoblast cells from the placenta infiltrate into the uterine wall, home to the arteries, and destroy the smooth muscle media. As a result of this trophoblast modification, the arteries become conduits capable of high conductance at low pressure with a reduction in velocity of the blood entering the placenta. Terminal dilatation of the arteries results in a further reduction in the flow rate into the intervillous space. This permits adequate time for gas exchange, especially towards the end of the pregnancy when fetal demands are highest. When there is failure of arterial conversion by trophoblast, then arterial blood will jet into the intervillous space from the non-transformed arteries causing damage to the villous tree (figure 1). Transport of oxygen and nutrients to the fetus is reduced and this manifests as fetal growth restriction (FGR). In more severe cases, the mother may develop pre-eclampsia, a systemic syndrome that occurs when the placenta becomes stressed as a result of the reduced blood flow [5]. Soluble factors released from the stressed placenta can trigger an inflammatory condition with diverse clinical manifestations. These include oedema, proteinuria and high blood pressure and can progress to eclampsia and maternal death. This is one of the principal reasons for clinical problems in both mothers and babies in pregnancies with very low birthweights. There are other clinical conditions besides pre-eclampsia and FGR resulting from defective placentation. Known collectively as the Great Obstetric Syndromes (GOS), they include unexplained stillbirth, placental abruption and preterm labour [6].
Figure 1.
Fetal growth restriction is associated with reduced remodelling of maternal spiral arteries by trophoblast cells. In normal pregnancy (right-hand panel), extravillous trophoblast cells migrate as far as the myometrium and also infiltrate into the arterial media and endothelium of maternal spiral arteries. This results in dilatation and increased flow of maternal blood at low pressure into the intervillous space (red arrow). In pregnancies affected by pre-eclampsia or FGR (left-hand panel), the depth of trophoblast invasion is reduced with less spiral artery remodelling. Blood flows at higher pressure and is more pulsatile, resulting in placental stress, reduced placental development and poor fetal growth. (Figure adapted from Moffett-King [5], with permission.)
3. The Obstetric Dilemma
Very large babies (approx. more than 4 kg) represent the other extreme of the birthweight spectrum, and these pregnancies are also at risk of clinical problems due to the difficulty of the passage of their head through the pelvis. Fetal obstruction results in prolonged labour, fetal death from asphyxia, soft tissue damage to pelvic organs and post-partum haemorrhage [7]. Estimation of placental size by sonography shows that, similar to the GOS, the origins of macrosomia are present early in gestation [8]. The risk of cephalopelvic disproportion has arisen because of the anatomical adaptations necessary for efficient bipedalism. These resulted in narrowing of the birth canal, imposing considerable constraints during parturition that are characteristic of modern humans even in comparison with Neanderthals [9]. The human head followed by the shoulders needs to rotate to fit the three planes of the pelvis that all have different shapes and orientations unlike other primates [10].
The combination of a narrow birth canal and the enlarged brain means that the Obstetric Dilemma is a particular problem for humans. Fetal growth in utero depends on the development of a good maternal blood supply to the placenta that requires modification of the uterine spiral arteries. Trophoblast cells from the placenta invade deeply into the stroma to effect arterial conversion. The extravillous trophoblast cells (EVT) encircle the arteries, and then cause direct destruction of the smooth muscle of the arterial wall with complete loss of vasoconstriction. By contrast, trophoblast invasion is much more limited in extent in Old World monkeys, where trophoblast cells only move down the inside of the arteries to replace the endothelium and functionally modify the media [11,12]. Among primates, deep trophoblast invasion through the decidua and into the inner myometrium is characteristic of human placentation, probably as a consequence of the increased blood supply needed to support the in utero development of the large brain. Indeed, brain development continues for some years after birth in humans [13]. Thus, the extent of trophoblast invasion has a critical effect on fetal access to maternal oxygen and nutrients. Under-invasion is associated with GOS such as FGR and pre-eclampsia, a condition only occurring in humans; over-invasion can result in obstructed labour due to the large fetus. A consequence of the balancing selection operating to prevent obstructed labour and the birthweight from being too large is the persistence of pregnancies with pre-eclampsia and FGR in all populations. The Obstetric Dilemma means that the birthweight must therefore be kept closely between the two dangerous extremes.
4. Regulation of placentation by the decidual immune system
Because trophoblast transformation of the arteries affects the growth and development of the fetus and placenta, there is much interest in how trophoblast invasion is controlled and thus how the supply line to the fetus is regulated. In vivo and in vitro observations show that human trophoblast cells are inherently highly invasive and can penetrate right through the wall of the uterus—unless they are controlled. This is seen, for example, after a caesarian section if the placenta implants on the scar in a subsequent pregnancy. Because the mucosal lining of the uterus (decidua) is missing at the site of the scar, trophoblast invasion proceeds unchecked, a condition known as placenta percreta, which can lead to uterine rupture [14]. The same behaviour occurs in ectopic pregnancy where implantation occurs in the fallopian tube, which also lacks decidua. This suggests decidual tissue must be essential for regulation of trophoblast invasion.
To understand how decidua regulates placentation, we have focused on the decidual immune system [15,16]. Hints that the immune system was involved came initially from studying the epidemiology of pre-eclampsia [17]. Classically, this is a disease of first pregnancies, giving rise to the idea that women became ‘immune’ afterwards. Change of male partners after a normal first pregnancy can trigger the condition; conversely, women who have already had pre-eclampsia may then be protected when they have a baby with a new partner [18]. These features, memory and specificity, are characteristic of the immune system. The familial and genetic contribution from the mother is well established, but, importantly, several studies have also shown that there is a paternal contribution to both pre-eclampsia and birthweight [19–21]. There is therefore circumstantial evidence that interactions between maternal and fetal immune system genes may determine successful pregnancy outcome.
There are two sites where fetal cells are in direct contact with maternal immune cells. Placental villi are covered by syncytiotrophoblast and present a large surface area in contact with maternal blood. Intermingling of maternal and fetal cells also occurs in the uterus at the site of placental attachment where EVT invade into decidua (figure 1). A territorial boundary must be defined here between two individuals. Because of the need to regulate the blood flow to the placenta and thus the birthweight, this boundary needs to be established in the right place. If the placenta (fetus) is too intrusive, the mother is at risk of uterine rupture and death. If trophoblast invasion is not far enough, spiral arteries are not sufficiently transformed, the blood flow to placenta is reduced, fetal growth is compromised and the mother is at risk of pre-eclampsia. There is growing evidence for the role of the decidual immune system in the subtle delineation of this uterine maternal/fetal interface. Because these two individuals are genetically different, the invading trophoblast cells will express molecules encoded by paternal genes meaning that, in immunological terms, the fetus is ‘non-self’ to the mother. How do maternal immune cells recognize and respond to the fetal trophoblast cells in the decidua, and do these interactions regulate trophoblast invasion?
5. T cells and allorecognition in pregnancy
T cells recognize antigenic peptides presented by major histocompatibility complex (MHC) molecules. These genes were originally discovered by Peter Medawar as the gene system whose products determine rejection or acceptance of transplants [22]. Unlike somatic cells, syncytiotrophoblast lacks expression of both MHC class I and II molecules, so cannot be recognized by T cells. However, maternal T-cell responses against fetal alloantigens do occur during pregnancy, generating fetal-specific T cells and humoral responses [23]. During pregnancy, temporary breaks in the synciotrophoblast layer can permit fetal cells to enter the maternal circulation. It is likely that these deported cells give rise to most of the fetal antigen-specific responses that have been described in T cells in maternal blood such as that to male HY minor histocompatibility antigen [24]. There is no evidence that any of these responses can directly affect syncytiotrophoblast.
EVT in the decidua also express an unusual MHC profile: they lack class II expression and do not express the human leukocyte antigen-A (HLA-A) or -B molecules normally found on all somatic cells [25]. Instead EVT express classical HLA-C as well as non-classical molecules, HLA-E and -G. The latter two are essentially monomorphic in human populations so will not vary from pregnancy to pregnancy [25]. Since most T cells are restricted to recognize self-HLA-A or -B molecules in association with a non-self-peptide, the unusual MHC profile of EVT means that only HLA-C-restricted T cells are capable of directly recognizing EVT in the decidua. A recent study has shown that in HLA-C mismatched pregnancies there are decidual T cells present that can recognize fetal HLA-C when stimulated with fetal cord blood. These are not present in HLA-C matched pregnancies. HLA-C mismatched pregnancies were also associated with an increase in CD4+CD25bright regulatory T cells (Tregs) in the decidua [26]. Tregs represent a subset of CD4+ T cells that act to suppress immune responses against self-antigens. They are characterized by expression of the transcription factor Foxp3 [27]. However since EVT does not express MHC class II, these CD4+ Tregs presumably recognize fetal HLA-C through indirect allorecognition via presentation of HLA-C peptides on maternal antigen presenting cells (APC). To date there is no evidence that T cells are present in human decidua that can directly recognize fetal alloantigens presented by HLA-C on trophoblast. However, decidual Tregs are generated in HLA-C mismatched pregnancies, and these may contribute to generation of a tolerogenic environment in the decidua.
Although there are significant differences in placental anatomy and trophoblast MHC expression compared to humans, elegant experiments possible in mice have given broadly similar results to those seen in humans. T cells specific for paternal antigens are generated in allogeneic matings but do not lead to pregnancy failure in normal pregnancies [28]. Adoptive transfer of CD8+ T cells specific for H2-Kb, to H2-Kb-negative females carrying H2-Kb-positive pups, did not result in pregnancy failure even though H2-Kb is expressed on trophoblast [29,30]. However, expansion of Tregs is seen during both syngeneic and allogeneic pregnancies and depletion of Tregs during pregnancy results in fetal resorption only in allogeneic matings [31]. Tregs appear to play an important role in preventing rejection of allogeneic pregnancies but the exact mechanism is unclear. In both mouse and humans, multiple other mechanisms have been described that contribute to T-cell tolerance. These include: ‘trapping’ of APCs within the decidua which decreases migration to regional lymph nodes, reduced T-cell entry into decidua by silencing of chemokine expression, stimulation of APCs by HLA-G to induce tolerogenic cytokine secretion and many others (reviewed in [16,32]). However, in humans there is no evidence that T-cell-dependent adaptive responses to fetal antigens, either systemically or in decidua, are involved in damaging placental cells or causing pregnancy failure.
6. Uterine natural killer cells and recognition of trophoblast HLA class I molecules
T cells comprise only 5–20% of decidual leukocytes. The overwhelming majority of leukocytes in the decidua are uterine natural killer cells (uNK). These are uniquely found in the endometrium during the luteal phase of the menstrual cycle when implantation occurs, and in decidua during early pregnancy [5]. Uterine NK cells are phenotypically and functionally quite distinct from NK cells circulating in peripheral blood and do express a range of receptors that can bind molecules expressed by placental cells [33,34]. Of these, our focus has been on those receptors that could be involved in specific recognition of paternal difference [5,16]. We have therefore studied NK receptors that can bind the MHC molecules expressed by EVT: HLA-C, -E and -G. The latter two are essentially monomorphic in human populations so will not vary from pregnancy to pregnancy [25]. Uterine NK cells express killer-cell immunoglobulin-like receptors (KIRs) that can recognize HLA-C, and invading trophoblast cells abundantly express both maternal and paternal HLA-C molecules [35]. However, the usual simple relationship between receptor and ligand is complicated by extreme polymorphism of both the KIR expressed by uNK and their trophoblast HLA-C ligands, with many different variants found within all populations. This means that there is a range of possible interactions between variants of maternal KIR and alleles of fetal HLA-C ligands to which uNK cells bind. Each pregnancy will thus bring together different combinations of maternal KIR and fetal HLA-C, with potentially different outcomes.
7. Killer immunoglobulin-like receptors expressed by natural killer cells
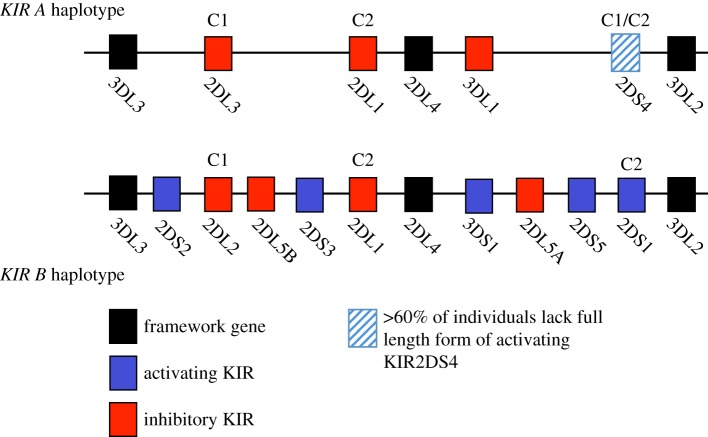
The KIR gene family is complex with a wide range in both the number of KIR genes inherited by the mother as well as allelic variation of individual KIR genes [36]. Up to 14 different KIR genes are present in a linear array in the Leucocyte Receptor Complex (LRC) on chromosome 19q13.4. KIRs are major regulators of NK cell function with different KIR conferring either an inhibitory or activating signal to the NK cell. Their most important known ligands are HLA-C molecules. KIRs are named based on the number of extracellular Ig-like domains (two or three domains) and whether they have a long or short cytoplasmic tail (e.g. KIR2DL1 or KIR2DS1). Binding of KIR with a long cytoplasmic tail to their cognate ligands results in transduction of an inhibitory signal, whereas ligation of KIR with a short tail results in activation of NK cells. This complexity has been simplified by the clear distinction between two KIR haplotypes, A and B. The KIR A haplotype is the most common and contains six inhibitory genes in most individuals. The KIR B haplotype is much more variable in gene content and many of the additional KIR are activating. An individual's KIR genotype can thus be designated ‘AA’, ‘AB’ or ‘BB’ (figure 2). Although there are over 1000 alleles of HLA-C, these can be distinguished by KIR as two distinct groups, HLA-C1 and -C2, based on a dimorphism at position 80 of the α1 domain of the HLA-C molecule [36]. To date, there is evidence that five members of the KIR family are capable of binding to HLA-C allotypes. The known KIR specificities for HLA-C are shown in figure 2. The strongest and most specific binding is of KIR2DL1 to HLA-C2 allotypes resulting in a strong inhibitory signal to NK cells.
Figure 2.
Representative KIR A and B haplotypes of the KIR gene family, together with their known HLA-C binding specificities (C1 and C2) depicted above their cognate KIR receptors. KIR2DS4 binds a limited number of C1 and C2 allotypes. Activating KIR are shown as blue boxes, inhibitory KIR are shown in red. Framework KIR genes that are present in all haplotypes are shown in black. Note that many of the KIR shown on the B haplotype are not present in all individuals.
8. Combinations of maternal KIR and paternal HLA-C genotypes regulate birthweight
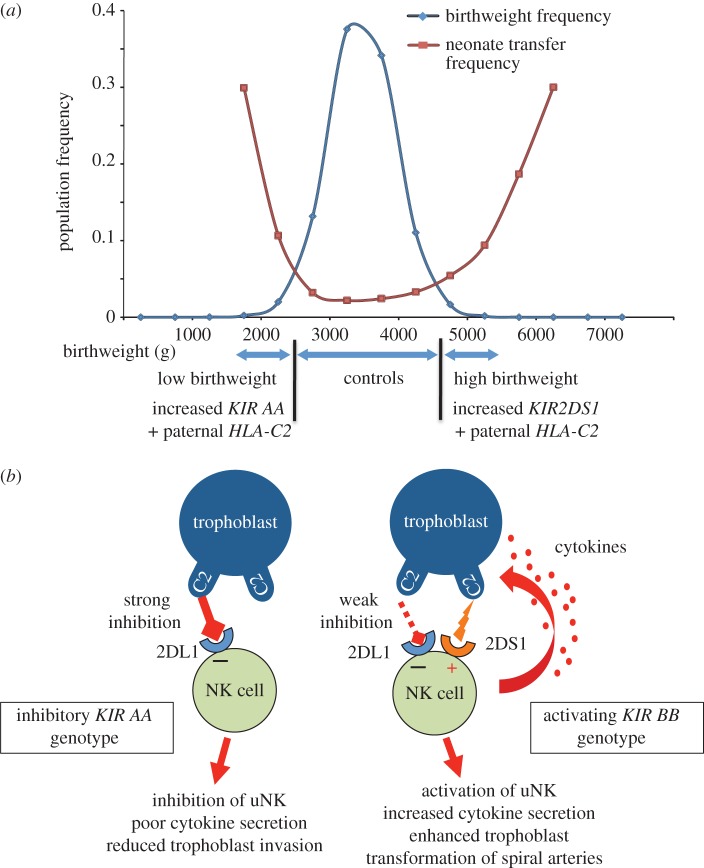
To discover how this great polymorphism of maternal KIR and fetal HLA-C genes affects human reproductive success, we have studied pregnancies where the KIR genotype of the mother and HLA-C group of her fetus is known. Our results show that there is a consistent relationship between particular combinations of maternal KIR and fetal HLA-C ligands and birthweight [3,35,37,38]. We compared maternal KIR and fetal HLA-C genotypes from a large number of first pregnancies selected from the Norwegian Mother–Baby cohort (MoBa) with known birthweight corrected for gestational age and fetal sex. A HLA-C2 group in the fetus is associated with pregnancies at the two extremes of birthweight especially when the HLA-C2 allele is inherited from the father rather than the mother [3]. If the mother has two KIR A haplotypes (KIR AA genotype), then she will have inherited two copies of the inhibitory KIR for HLA-C2 allotypes, namely KIR2DL1. Functionally, when uNK cells from a KIR AA woman bind trophoblast expressing a paternal HLA-C2 allotype this results in a strong inhibitory signal. By contrast, high birthweight pregnancies are associated with a paternally derived HLA-C2 allele, when the mother has inherited the activating KIR for HLA-C2, KIR2DS1 that is located on the KIR B haplotype [3]. This effect is quite significant; the average increase in birthweight in pregnancies with a fetus with paternal HLA-C2 and KIR2DS1 in the mother is approximately 200 g, more than the differences found with sex of the baby (approx. 50 g) and high altitude (100 g decrease per 1000 m increase in altitude).
This immune interaction is not comparable to other situations such as organ transplantation where there is clear self/non-self discrimination, because it is only HLA-C alleles carrying the C2 epitope that confer any effect. To date, in pregnancies where the fetus is homozygous for HLA-C1 there is no influence of any KIR genotype on any clinical outcome in pregnancy that we have studied. This may be due to the tight specificity and strong inhibition conferred by KIR2DL1/HLA-C2 interactions compared with the weaker and more promiscuous KIR2DL2/3 interactions with HLA-C molecules [39]. To summarize, at the site of placentation all the influences of HLA-C in pregnancy are mediated by paternal HLA-C2 with the maternal KIR genotype determining the outcome (high or low birthweight). In pregnancies where the fetus is homozygous for HLA-C1, then the effect of the maternal KIR is neutral and no associations are seen [3,35].
9. Functions of uterine natural killer cells during placentation
The functional effects mediated by uNK cells at the uterine/placental interface relating to these different KIR/HLA-C combinations are hard to determine given the ethical and logistical problems of studying the site of placentation in early pregnancy in humans. The great disparity in placental strategies in different species also means animal models are generally uninformative. Furthermore, KIR genes are only a feature of simian primates, but even in these species there are fundamental differences in placentation with interstitial trophoblast invasion only a feature of the great apes [11]. A further major difficulty has been the lack of any reproducible and reliable in vitro model systems to study human trophoblast function in vitro. Nonetheless, uNK cells can be isolated and, despite their name, are poorly cytotoxic [40,41]. The input NK cells receive from KIR is either activating or inhibitory, and NK function is a result of integrating this information and responding accordingly. When uNK cells receive an overall activating signal in a woman with a KIR B haplotype containing KIR2DS1, the output of soluble mediators like chemokines and cytokines (e.g. GM-CSF) is increased after co-culture with target cells bearing HLA-C2 molecules. This supernatant containing GM-CSF stimulates trophoblast invasion and migration in vitro [42]. By contrast, when uNK cells from KIR AA women who only have the inhibitory KIR2DL1 for HLA-C2 were ligated with HLA-C2, trophoblast migration was not stimulated. This suggests that there is reduced cytokine output by uNK cells in a woman with a KIR AA genotype when the overall signal is inhibitory. Thus, women with too much uNK cell inhibition (KIR AA) or too much activation (KIR B haplotype with KIR2DS1 present) are more likely to have babies with low or high birthweight, respectively, when their uNK cells encounter trophoblast cells displaying a paternal HLA-C2 allotype. The interpretation we have made based on the genetic and functional evidence is that the overall uNK response determines how far the trophoblast moves into the uterus and modifies the arteries (figure 3). Thus, there is a direct link between the function of the uterine immune system and the birthweight of the baby. This specialized immune interaction may be essential to maintain the birthweight within the strict limits dictated by the Obstetric Dilemma.
Figure 3.
Presence of maternal KIR2DS1 is associated with increased birthweight. (a) Distribution of birthweights in the Norwegian MoBa cohort correlates with increased neonatal morbidity (frequency of transfer to special care baby unit) at the extremes of birthweight [3]. The cohort was divided into low (less than 5th centile) normal (6–89th centile) and high (more than 90th centile) birthweight babies. The frequency of maternal KIR AA genotype + fetal HLA-C2 is increased in the small babies. Presence of maternal KIR2DS1 and fetal HLA-C2 is associated with increased birthweight. (b) Proposed model for maternal KIR/fetal HLA-C interactions at the site of placentation. In this model, the fetus has inherited a paternal HLA-C2 allele and is homozygous for HLA-C2. If the mother has a KIR AA genotype, then KIR2DL1 binds strongly to trophoblast HLA-C2 molecules resulting in strong inhibition of uNK cells. This is associated with defective placentation. In contrast, when the mother has a KIR AB or BB genotype, the KIR2DL1 alleles on the B haplotype tend to inhibit uNK function more weakly when they bind C2. The KIR B haplotypes also contain the activating KIR2DS1. In this situation, uNK cells are stimulated to produce increased levels of cytokines such as GM-CSF that can enhance placentation.
Uterine NK cells also occur in the decidua in mice and appear to play an important role in vascular remodelling during placentation. Murine trophoblast invades into the decidua to a lesser degree than in humans and does not express non-classical MHC, so there are significant differences compared to human trophoblast [30]. Although the major murine NK receptors that recognize MHC are the Ly49 family of receptors, they appear to function analogously to the human KIR. Mouse studies in which a single additional MHC molecule H2-Dd was introduced, showed impaired vascular remodelling and reduced fetal growth compared with identical mice lacking only H2-Dd [43]. This MHC molecule binds the inhibitory receptor Ly49A and can inhibit additional uNK subsets when present. Particularly notable was the fact that reduced fetal growth was seen regardless of the parental origin of the H2-Dd molecule. These results confirm the idea that certain combinations of maternal NK receptors and paternal (or maternal) MHC molecules can influence trophoblast invasion and vascular remodelling. In both human and murine pregnancies, excessive inhibition of NK cell function is associated with reduced fetal growth.
10. Variation of KIR and HLA-C genes affects other diseases of pregnancy
There is other independent evidence that will substantiate this model in humans. In common with low birthweight, obstetric syndromes such as pre-eclampsia and recurrent miscarriage are associated with reduced uterine invasion by trophoblast [44]. Mothers at highest risk of these diseases are homozygous for the KIR A haplotype, and carry a fetus that has inherited HLA-C2 from the father [35,37,38]. Women with a HLA-C2 fetus are protected from these disorders by the KIR B haplotype that contributes to protection in two possible ways. Firstly, many KIR B haplotypes include the activating KIR2DS1 that enhances trophoblast migration through enhanced secretion of ‘pro-invasion’ cytokines as described above. Secondly, the commonest KIR2DL1 allele on the B haplotype is KIR2DL1*004. This binds more weakly to HLA-C2 than KIR2DL1*003, the most common allele on the A haplotype [45]. We predict that there would be reduced inhibition of uNK cells by KIR2DL1*004 on the KIR B haplotype. The fact that diverse GOS with a common underlying pathology of reduced trophoblast invasion also exhibit a similar KIR/HLA-C risk profile, supports the idea that uNK/trophoblast interactions play an important role in all these pregnancy disorders.
If KIR AA and HLA-C2 combinations are significantly disadvantageous in pregnancy, this raises the question as to why they have not been eliminated from human populations by natural selection. All populations have both KIR A and KIR B haplotypes and HLA-C alleles with both C1 and C2 epitopes, suggesting that a balance of KIR A and B haplotypes and their ligands is essential for the survival of a community [46]. However, different populations across the world have different frequencies of KIR AA genotypes and HLA-C2 alleles. Evidence that there is strong selective pressure from reproduction comes from the inverse correlation between KIR AA and HLA-C2 frequencies [37,47].
11. Pregnancy in African women
There are a few populations that do not fit this picture as they have high frequencies of both KIR AA genotypes and HLA-C2 alleles. Notably, these are all found in sub-Saharan Africa where high HLA-C2 frequencies are characteristic. Sub-Saharan Africa is especially important in consideration of reproduction and birth, as maternal mortality rates are the highest in the world. Figures of 600/100 000 births compared with only approximately 15 in high-income countries mean this is one of the most extreme disparities in any health outcome [48,49]. Obviously, poverty and lack of access to medical care are major problems but there are several indications that pregnancy and parturition in women from sub-Saharan Africa have many different features compared with women in other parts of the world [50]. Indeed, it appears that the Obstetric Dilemma is more of a problem for African women. Although there are no reliable records from Africa itself, studies of women of recent African ancestry in Europe and the Americas all indicate that all the GOS (pre-eclampsia, stillbirth, FGR and prematurity) occur more commonly as would be predicted by the high prevalence of HLA-C2 combined with KIR AA genotypes in these African populations [51]. One explanation for maintenance of these ‘reproductively risky’ genotypes is the observation that an individual's resistance to infections such as hepatitis C virus is correlated with a KIR AA genotype and HLA-C1 [52]. A simple model would be that human populations must maintain the KIR A haplotype and HLA-C1 in order to ensure resistance to pathogens. By contrast, reproductive success and the development of the larger human brain and more robust progeny are dependent on maintenance of KIR B haplotypes and HLA-C2. In each human population, the relative frequencies of these haplotypes will be subject to natural selection depending on pathogen load, reproductive pressure and of course will change with time [12]. The fact that all human populations maintain both KIR A and B haplotypes as well as HLA-C1 and -C2, suggests that populations that lose one of these components are doomed to extinction when subjected to extreme selection by events such as infection, famine and warfare.
While the factors responsible for the high incidence of HLA-C2 and the KIR A haplotype in current African populations are not yet clear, this undoubtedly contributes to the high frequency of pre-eclampsia, stillbirth and FGR found in sub-Saharan Africa. It is interesting to note that in such populations there is also an increased frequency of obstructed labour [50]. This may be connected to the bony pelvis as measurements obtained by pelvimetry indicate that in African women some aspects of the birth canal are smaller [53]. A high frequency of HLA-C2 and the KIR A haplotype may therefore reflect selection to reduce the birthweight, but this will also result in an increased risk of the GOS. In keeping with this, the development of the fetal organs is accelerated and the gestational age is shorter at 38 compared with 40 weeks [50,54]. This clearly illustrates how the complex selective pressures acting on the evolution of the KIR/HLA-C system could regulate human reproductive fitness, brain size, pathogen resistance and even the rate of fetal development.
12. Conclusion
The adaptations of the pelvis associated with bipedalism coupled with the larger human brain, severely limit human birthweight. Conversely, neonatal survival of smaller babies is greatly reduced. Human birthweight is thus subject to stabilizing selection. Although the control of birthweight is complex, adequate placentation to permit access to nutrients in maternal blood is essential for normal fetal growth. In humans, this is achieved by trophoblast cells, which remodel maternal spiral arteries. Inadequate trophoblast invasion is associated with obstetric syndromes, including pre-eclampsia, recurrent miscarriage and FGR. We have shown that recognition of HLA-C on invading trophoblast by KIR receptors on maternal uNK plays a role in controlling trophoblast invasion and hence birthweight. Because both KIR and HLA-C genes are highly polymorphic, each pregnancy will bring together different combinations of maternal activating or inhibitory KIR that can recognize fetal HLA-C. Our findings suggest that binding of strongly inhibitory KIR to HLA-C2 expressed on trophoblast is detrimental to placentation. This combination is found more frequently in small babies. The presence of maternal activating receptor KIR2DS1 together with fetal HLA-C2 is associated with increased birthweight, suggesting the activation of uNK favours placentation. The association is strongest when a fetal HLA-C2 allele is inherited from the father. These findings suggest that a balance of maternal activating and inhibitory KIR and HLA-C2 genotype frequencies act to maintain birthweight at an optimum for maternal and fetal survival.
Acknowledgements
The authors thank all the clinical staff and donors, without whom the studies described would not have been possible, and in particular our collaborators at the Norwegian Mother and Child Cohort Study (MoBa): Per Magnus, Lill Trogstad and Hakon Gjessing.
Funding statement
This work was supported by funding from the Wellcome Trust [090108/Z/09/Z], [085992/Z/08/Z] and the British Heart Foundation (PG/09/077/27964). This work was also supported by a Frederick National Laboratory for Cancer Research Contract (HHSN261200800001E) and by the Intramural Research Program of National Institutes of Health, Frederick National Laboratory, Center for Cancer Research. The authors also thank the Centre for Trophoblast Research, Cambridge for generous support.
References
- 1.Karn MN, Penrose LS. 1951. Birth weight and gestation time in relation to maternal age, parity and infant survival. Ann. Eugen. 16, 147–164. ( 10.1111/j.1469-1809.1951.tb02469.x) [DOI] [PubMed] [Google Scholar]
- 2.Cavalli-Sforza LL, Bodmer WF. 1971. The genetics of human populations, pp. 612–613. San Francisco, CA: W. H. Freeman and Company. [Google Scholar]
- 3.Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, Gjessing HK, Carrington M, Moffett A. 2014. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J. Immunol. 192, 5069–5073. ( 10.4049/jimmunol.1400577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. 2009. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30, 473–482. ( 10.1016/j.placenta.2009.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moffett-King A. 2002. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2, 656–663. ( 10.1038/nri886) [DOI] [PubMed] [Google Scholar]
- 6.Brosens I, Pijnenborg R, Vercruysse L, Romero R. 2011. The ‘Great Obstetrical Syndromes’ are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 204, 3193–3201. ( 10.1016/j.ajog.2010.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittman AB, Wall LL. 2007. The evolutionary origins of obstructed labor: bipedalism, encephalization, and the human obstetric dilemma. Obstet. Gynecol. Surv. 62, 739–748. ( 10.1097/01.ogx.0000286584.04310.5c) [DOI] [PubMed] [Google Scholar]
- 8.Schwartz N, Quant HS, Sammel MD, Parry S. 2014. Macrosomia has its roots in early placental development. Placenta 35, 684–690. ( 10.1016/j.placenta.2014.06.373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver TD, Hublin JJ. 2009. Neandertal birth canal shape and the evolution of human childbirth. Proc. Natl Acad. Sci. USA 106, 8151–8156. ( 10.1073/pnas.0812554106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abitbol MM. 1996. Birth and human evolution: anatomical and obstetrical mechanics in primates, pp. 8–18. Westport, CT: Bergin & Garvey. [Google Scholar]
- 11.Carter AM, Pijnenborg R. 2011. Evolution of invasive placentation with special reference to non-human primates. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 249–257. ( 10.1016/j.bpobgyn.2010.10.010) [DOI] [PubMed] [Google Scholar]
- 12.Parham P, Moffett A. 2013. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat. Rev. Immunol. 13, 133–144. ( 10.1038/nri3370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coqueugniot H, Hublin JJ. 2012. Age-related changes of digital endocranial volume during human ontogeny: results from an osteological reference collection. Am. J. Phys. Anthropol. 147, 312–318. ( 10.1002/ajpa.21655) [DOI] [PubMed] [Google Scholar]
- 14.Hannon T, Innes BA, Lash GE, Bulmer JN, Robson SC. 2012. Effects of local decidua on trophoblast invasion and spiral artery remodeling in focal placenta creta—an immunohistochemical study. Placenta 33, 998–1004. ( 10.1016/j.placenta.2012.09.004) [DOI] [PubMed] [Google Scholar]
- 15.Colucci F, Boulenouar S, Kieckbusch J, Moffett A. 2011. How does variability of immune system genes affect placentation? Placenta 32, 539–545. ( 10.1016/j.placenta.2011.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffett A, Colucci F. 2014. Uterine NK cells: active regulators at the maternal–fetal interface. J. Clin. Invest. 124, 1872–1879. ( 10.1172/JCI68107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, Souza JP. 2014. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 121(Suppl. 1), 14–24. ( 10.1111/1471-0528.12629) [DOI] [PubMed] [Google Scholar]
- 18.Li DK, Wi S. 2000. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am. J. Epidemiol. 151, 57–62. ( 10.1093/oxfordjournals.aje.a010122) [DOI] [PubMed] [Google Scholar]
- 19.Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, Varner MW. 2001. Paternal and maternal components of the predisposition to preeclampsia. N. Engl. J. Med. 344, 867–872. ( 10.1056/NEJM200103223441201) [DOI] [PubMed] [Google Scholar]
- 20.Magnus P, Gjessing HK, Skrondal A, Skjaerven R. 2001. Paternal contribution to birth weight. J. Epidemiol. Community Health 55, 873–877. ( 10.1136/jech.55.12.873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice F, Thapar A. 2010. Estimating the relative contributions of maternal genetic, paternal genetic and intrauterine factors to offspring birth weight and head circumference. Early Hum. Dev. 86, 425–432. ( 10.1016/j.earlhumdev.2010.05.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billingham RE, Brent L, Medawar PB. 1953. Actively acquired tolerance of foreign cells. Nature 172, 603–606. ( 10.1038/172603a0) [DOI] [PubMed] [Google Scholar]
- 23.van Kampen CA, Versteeg-vd Voort Maarschalk MF, Langerak-Langerak J, Roelen DL, Claas FH. 2002. Kinetics of the pregnancy-induced humoral and cellular immune response against the paternal HLA class I antigens of the child. Hum. Immunol. 63, 452–458. ( 10.1016/S0198-8859(02)00396-8) [DOI] [PubMed] [Google Scholar]
- 24.Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. 2012. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 189, 1072–1080. ( 10.4049/jimmunol.1200544) [DOI] [PubMed] [Google Scholar]
- 25.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. 2009. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127, 26–39. ( 10.1111/j.1365-2567.2008.03019.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg-vd Voort-Maarschalk M, Roelen DL, van Rood JJ, Claas FH. 2009. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 82, 148–157. ( 10.1016/j.jri.2009.05.003) [DOI] [PubMed] [Google Scholar]
- 27.Littman DR, Rudensky AY. 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858. ( 10.1016/j.cell.2010.02.021) [DOI] [PubMed] [Google Scholar]
- 28.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. 1995. T cell awareness of paternal alloantigens during pregnancy. Science 270, 630–633. ( 10.1126/science.270.5236.630) [DOI] [PubMed] [Google Scholar]
- 29.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. 2007. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 117, 1399–1411. ( 10.1172/JCI28214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, Moffett A, Colucci F, Hemberger M. 2011. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl Acad. Sci. USA 108, 4012–4017. ( 10.1073/pnas.1005342108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aluvihare VR, Kallikourdis M, Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271. ( 10.1038/ni1037) [DOI] [PubMed] [Google Scholar]
- 32.Nancy P, Erlebacher A. 2014. T cell behavior at the maternal–fetal interface. Int. J. Dev. Biol. 58, 189–198. ( 10.1387/ijdb.140054ae) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koopman LA, et al. 2003. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 198, 1201–1212. ( 10.1084/jem.20030305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna J, et al. 2006. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat. Med. 12, 1065–1074. ( 10.1038/nm1452) [DOI] [PubMed] [Google Scholar]
- 35.Hiby SE, et al. 2010. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120, 4102–4110. ( 10.1172/JCI43998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parham P. 2005. MHC class I molecules and KIRs in human history, health and survival. Nat. Rev. Immunol. 5, 201–214. ( 10.1038/nri1570) [DOI] [PubMed] [Google Scholar]
- 37.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. 2004. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965. ( 10.1084/jem.20041214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. 2008. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 23, 972–976. ( 10.1093/humrep/den011) [DOI] [PubMed] [Google Scholar]
- 39.Hilton HG, et al. 2012. Mutation at positively selected positions in the binding site for HLA-C shows that KIR2DL1 is a more refined but less adaptable NK cell receptor than KIR2DL3. J. Immunol. 189, 1418–1430. ( 10.4049/jimmunol.1100431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King A, et al. 2000. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 30, 1623–1631. () [DOI] [PubMed] [Google Scholar]
- 41.Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. 2005. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc. Natl Acad. Sci. USA 102, 15 563–15 568. ( 10.1073/pnas.0507835102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong S, et al. 2013. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J. Clin. Invest. 123, 4264–4272. ( 10.1172/JCI68991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kieckbusch J, Gaynor LM, Moffett A, Colucci F. 2014. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodelling. Nat. Commun. 5, 3359 ( 10.1038/ncomms4359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pijnenborg R, Vercruysse L, Brosens I. 2011. Deep placentation. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 273–285. ( 10.1016/j.bpobgyn.2010.10.009) [DOI] [PubMed] [Google Scholar]
- 45.Bari R, Bell T, Leung WH, Vong QP, Chan WK, Das Gupta N, Holladay M, Rooney B, Leung W. 2009. Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood 114, 5182–5190. ( 10.1182/blood-2009-07-231977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gendzekhadze K, Norman PJ, Abi-Rached L, Graef T, Moesta AK, Layrisse Z, Parham P. 2009. Co-evolution of KIR2DL3 with HLA-C in a human population retaining minimal essential diversity of KIR and HLA class I ligands. Proc. Natl Acad. Sci. USA 106, 18 692–18 697. ( 10.1073/pnas.0906051106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR, Kidd KK, Carrington M. 2007. Global diversity and evidence for coevolution of KIR and HLA. Nat. Genet. 39, 1114–1119. ( 10.1038/ng2077) [DOI] [PubMed] [Google Scholar]
- 48.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. 2006. WHO analysis of causes of maternal death: a systematic review. Lancet 367, 1066–1074. ( 10.1016/S0140-6736(06)68397-9) [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 2010. World Health maternal mortality: 1990 to 2008. Geneva, Switzerland: WHO. [Google Scholar]
- 50.Nakimuli A, Chazara O, Byamugisha J, Elliott AM, Kaleebu P, Mirembe F, Moffett A. 2014. Pregnancy, parturition and preeclampsia in women of African ancestry. Am. J. Obstet. Gynecol. 210, 510–520.e1. ( 10.1016/j.ajog.2013.10.879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakimuli A, et al. 2013. Killer cell immunoglobulin-like receptor (KIR) genes and their HLA-C ligands in a Ugandan population. Immunogenetics 65, 765–775. ( 10.1007/s00251-013-0724-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khakoo SI, et al. 2004. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874. ( 10.1126/science.1097670) [DOI] [PubMed] [Google Scholar]
- 53.Handa VL, Lockhart ME, Fielding JR, Bradley CS, Brubaker L, Cundiff GW, Ye W, Richter HE, Pelvic Floor Disorders Network. 2008. Racial differences in pelvic anatomy by magnetic resonance imaging. Obstet. Gynecol. 111, 914–920. ( 10.1097/AOG.0b013e318169ce03) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel RR, Steer P, Doyle P, Little MP, Elliott P. 2004. Does gestation vary by ethnic group? A London-based study of over 122 000 pregnancies with spontaneous onset of labour. Int. J. Epidemiol. 33, 107–113. ( 10.1093/ije/dyg238) [DOI] [PubMed] [Google Scholar]