Abstract
Epithelial barrier function is maintained by tight junction proteins that control paracellular fluid flux. Among these proteins is junctional adhesion molecule A (JAM-A), an Ig fold transmembrane protein. To assess JAM-A function in the lung, we depleted JAM-A in primary alveolar epithelial cells using shRNA. In cultured cells, loss of JAM-A caused an approximately 30% decrease in transepithelial resistance, decreased expression of the tight junction scaffold protein zonula occludens 1, and disrupted junctional localization of the structural transmembrane protein claudin-18. Consistent with findings in other organs, loss of JAM-A decreased β1 integrin expression and impaired filamentous actin formation. Using a model of mild systemic endoxotemia induced by i.p. injection of lipopolysaccharide, we report that JAM-A−/− mice showed increased susceptibility to pulmonary edema. On injury, the enhanced susceptibility of JAM-A−/− mice to edema correlated with increased, transient disruption of claudin-18, zonula occludens 1, and zonula occludens 2 localization to lung tight junctions in situ along with a delay in up-regulation of claudin-4. In contrast, wild-type mice showed no change in lung tight junction morphologic features in response to mild systemic endotoxemia. These findings support a key role of JAM-A in promoting tight junction homeostasis and lung barrier function by coordinating interactions among claudins, the tight junction scaffold, and the cytoskeleton.
To support efficient gas exchange, the lung must maintain a barrier between the atmosphere and fluid-filled tissues. Without this crucial barrier, the air spaces would flood, and gas exchange would be severely limited.1, 2 In acute lung injury and acute respiratory distress syndrome, fluid leakage into the lung air space is associated with increased patient mortality and morbidity.3, 4 Lung fluid clearance is maintained, in part, by tight junctions that regulate paracellular flux between cells.5, 6, 7
Tight junctions are multiprotein complexes located at sites of cell-cell contact and are composed of transmembrane, cytosolic, and cytoskeletal proteins that together produce a selective barrier to water, ions, and soluble molecules. Among the transmembrane proteins required for epithelial barrier function is the Ig superfamily protein junctional adhesion molecule A (JAM-A).8, 9, 10, 11 JAM-A is ubiquitously expressed and regulates several processes related to cell-cell and cell-matrix interactions, including cell migration and proliferation in addition to barrier function regulation. Specific mechanistic roles for JAM-A in regulating tight junctions continue to be elucidated.
JAM-A signaling is stimulated by cis-dimerization, which provides a platform for multiple proteins to cluster in close apposition.12 In particular, JAM-A has been shown to recruit scaffold proteins, such as zonula occludens 1 (ZO-1), ZO-2, and Par3, to tight junctions, where these proteins enhance the assembly of multiprotein junctional complexes.13, 14 More recently, it was demonstrated that JAM-A directly interacts with ZO-2, which then recruits other scaffold proteins, including ZO-1.15 This nucleates a core complex that includes afadin, PDZ-GEF1, and Rap2c and that stabilizes filamentous actin by repressing rhoA.15 Together, all of these activities of JAM-A promote tight junction formation and barrier function.
Although JAM-A is part of the tight junction complex, the main structural determinants of the paracellular barrier are proteins known as claudins. Claudins are a family of transmembrane proteins that interact to form paracellular channels that either promote or limit paracellular ion and water flux.16, 17, 18 Claudins that promote flux are known collectively as pore-forming claudins, whereas claudins that limit flux are known as sealing claudins.19 In fact, there is a link between JAM-A and claudin expression because it was demonstrated that JAM-A–deficient intestinal epithelium has increased expression of two pore-forming claudins, claudin-10 and claudin-15.20 Critically, increased claudin-10 and claudin-15 leads to a compromised intestinal barrier, as demonstrated by an enhanced susceptibility of JAM-A−/− mice to dextran sulfate sodium–induced colitis.20 However, it is not known whether this relationship between JAM-A and claudin expression occurs in other classes of epithelia.
Several claudins are expressed by the alveolar epithelium. The most prominent alveolar claudins are claudin-3, claudin-4, and claudin-18; several additional claudins are expressed by alveolar epithelium and throughout the lung as well.21, 22 A central role for claudin-18 in regulating lung barrier function was demonstrated in two independently derived strains of claudin-18–deficient mice that showed altered alveolar tight junction morphologic features and increased paracellular permeability.23, 24 Claudin-4 also is an important part of the lung response to acute lung injury because it improves barrier function by limiting alveolar epithelial permeability and promoting lung fluid clearance.25, 26 Although claudin-4–deficient mice show a relatively mild baseline phenotype, these mice have impaired fluid clearance in response to ventilator-induced lung injury.27 An analysis of ex vivo perfused human donor lungs revealed that increased claudin-4 was linked to increased rates of alveolar fluid clearance and decreased physiologic respiratory impairment,28 further underscoring the importance of claudin regulation in promoting efficient barrier function in response to injury.
Although JAM-A has a clear role in regulating gut permeability,20 a recent report that wild-type and JAM-A−/− mice show comparable levels of pulmonary edema in response to intratracheal endotoxin challenge29 raises questions about potential roles for JAM-A in lung barrier function. Herein we used a combination of in vivo and in vitro approaches to assess the contributions of JAM-A to alveolar barrier function. Using a model of mild systemic endotoxemia induced by i.p. injection of Escherichia coli–derived lipopolysaccharide (LPS), we found that JAM-A−/− mice showed greater lung edema than comparably treated wild-type mice. Greater sensitivity to injury was due to aberrant regulation of tight junction protein expression, which was recapitulated by JAM-A–depleted alveolar epithelial cells. JAM-A depletion also resulted in decreased β1 integrin protein levels and disrupted cytoskeletal assembly. Together, these effects indicated that the loss of JAM-A impaired tight junction formation, thus rendering the lung more susceptible to edema and injury.
Materials and Methods
Materials
Unless otherwise specified, materials were from Sigma-Aldrich (St. Louis, MO). Anti–JAM-A, claudin-3, claudin-4, claudin-5, claudin-7, claudin-10, claudin-15, claudin-18, occludin, ZO-1, ZO-1 actin, and β1 integrin were from Life Technologies (Grand Island, NY). E-cadherin antibodies were from Cell Signaling Technology Inc. (Danvers, MA). Minimally cross-reactive fluorescent and horseradish peroxidase secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Isolation and Culture of Rat Type II Alveolar Epithelial Cells
The animal protocols were reviewed and authorized by the Institutional Animal Care and Use Committee of Emory University (Atlanta, GA). Sprague-Dawley rat type II alveolar epithelial cells were isolated from lungs lavaged and perfused with elastase as described elsewhere,30 with modifications.31 Preparations routinely contained >90% to 95% type II alveolar epithelial cells. Freshly isolated cells were cultured in Eagle's minimal essential medium (Life Technologies, Rockville, MD) containing 10% fetal bovine serum, 25 μg/mL gentamicin, and 0.25 μg/mL amphotericin B (Life Technologies) in Transwells coated with rat tail type I collagen (Roche Diagnostics GmbH, Mannheim, Germany) at 7.5 × 105 cells/mL, as described elsewhere.32 Transepithelial resistance of cells in medium cultured on permeable supports was measured using an ohmmeter (World Precision Instruments, Sarasota, FL), as previously described.33, 34
Lentiviral shRNA shuttle vector pFH1+U6-UG-W was modified from pFH1UGW35 using standard molecular techniques to include the H1 and U6 promoters to drive shRNA production (Table 1). Lentiviral particles were produced by the Emory Neuroscience National Institute of Neurological Disorders and Stroke Viral Vector Core Facility. For shRNA knockdown, freshly isolated rat alveolar epithelial cells were cultured, with lentiviral vector constructs added to the apical and basal sides, and were incubated for 24 hours, followed by a medium change. Lentiviral titers were adjusted to maximize JAM-A repression and cell viability.
Table 1.
shRNA Constructs Used in This Study
| JAM-A shRNA1 | 616–624 |
| Sense | 5′-(NheI)CCCCGCCTTCATCAATTCTTCATTTCAAGAGAATGAAGAATTGATGAAGGC TTTTTGG(PacI)-3′ |
| Antisense | 5′-(PacI)CCAAAAAGCCTTCATCAATTCTTCATTCTCTTGAAATGAAGAATTGATGAAGGC GGGG(NheI)-3′ |
| JAM-A shRNA2 | 1594–1612 |
| Sense | 5′-(NheI)CCCCGTGGCTGTTAGTCACTTCATTCAAGAGATGAAGTGACTAACAGCCACTTTTTGG(PacI) -3′ |
| Antisense | 5′-(PacI)CCAAAAAGTGGCTGTTAGTCACTTCATCTCTTGAATGAAGTGACTAACAGCCACGGGG(NheI)-3′ |
| Scrambled | |
| Sense | 5′-(NheI)CCCCAGTCATTGACGACAGCGTATTCAAGAGATACGCTGTCGTCAATGACTTTTTTGG(PacI)-3′ |
| Antisense | 5′-(PacI)CCAAAAAAGTCATTGACGACAGCGTATCTCTTGAATACGCTGTCGTCAATGACTGGGG(NheI)-3′ |
Biochemical Analysis
After 6 days in culture, cells on permeable supports were harvested and lyzed in 2× sample buffer containing 50 mmol/L dithiothreitol, resolved by SDS-PAGE, transferred to Immobilon membranes (Millipore, Billerica, MA), and blotted using primary antibodies and horseradish peroxidase–conjugated goat anti-rabbit IgG or goat anti-mouse IgG. Specific signals corresponding to a given protein were detected by immunoblot analysis using enhanced chemiluminescence reagent (GE Healthcare, Pittsburgh, PA) and were quantified using Image Lab software version 2.0.1, build 18 (Bio-Rad Laboratories, Hercules, CA). Normalization for protein content was performed using parallel samples analyzed for actin. Statistical significance was determined by Student's t-test or analysis of variance as appropriate.
Immunofluorescence Staining
Immunofluorescence staining was performed as described elsewhere.33, 34 After 6 days in culture, the cells were washed with phosphate-buffered saline (PBS) three times, fixed in methanol/acetone 1:1 for 2 minutes at room temperature, and washed three times with PBS, once with PBS + 0.5% Triton X-100 (Roche Diagnostics GmbH), and then once with PBS + 0.5% Triton X-100 + 2% normal goat serum. Cells were incubated with primary anti-rabbit and/or anti-mouse antibodies in PBS + 2% normal goat serum for 1 hour, washed, incubated with Cy2-conjugated goat anti-rabbit IgG and Cy3-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) in PBS + 2% normal goat serum, washed, and then mounted in Mowiol medium (Kuraray America Inc., Houston, TX) under a glass coverslip. Cells were imaged by phase-contrast and fluorescence microscopy using an Olympus IX70 microscope with a U-MWIBA filter pack (BP460–490, DM505, BA515–550) or a U-MNG filter pack (BP530–550, DM570, BA590–800+) or by confocal immunofluorescence microscopy using an Olympus Fluoview FV1000 system (Olympus America Inc., Center Valley, PA). Minimum and maximum intensity were adjusted for images in parallel so that the intensity scale remained linear to maximize dynamic range.
Mice
The mice used consisted of C57BL/6 (wild-type) mice (The Jackson Laboratory, Bar Harbor, ME) or JAM-A−/− mice36 backcrossed onto C57BL/6 for seven generations as described elsewhere.20
LPS Administration
C57BL/6 or JAM-A−/− mice at 6 to 8 weeks of age were either untreated or i.p. injected with either control saline (PBS) or PBS containing 1 mg/kg LPS from E. coli strain O111:B6 (Sigma-Aldrich). Lungs were collected 0, 6, 24, and 48 hours after treatment and were processed for total lung protein analysis, histologic analysis, or determination of the wet/dry ratio.
Total Lung Protein Analysis
Lungs from mice were removed and snap frozen using liquid nitrogen. The samples were homogenized in 1 mL of radioimmunoprecipitation assay buffer (20 mmol/L Tris, pH 7.4, 2 mmol/L EDTA, 2 mmol/L EGTA, 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) containing 1 tablet per 10 mL complete protease inhibitors.25 The samples were microfuged at 5000 × g for 5 minutes, and the supernatant was analyzed by immunoblot as described in Biochemical Analysis.
Histologic Analysis
Lung sections (6 μm) were fixed with 4% paraformaldehyde for 10 minutes, processed for frozen thin sections, and then stained with hematoxylin and eosin or immunostained as described elsewhere.33, 37, 38 For determination of pulmonary edema, lungs were removed, placed on a preweighed piece of aluminum foil, and weighed. Lungs were then heated to 65°C for 24 hours and weighed again. The ratio of wet lung mass/dry lung mass was calculated to give a measure of the water content of the tissue.39
Results
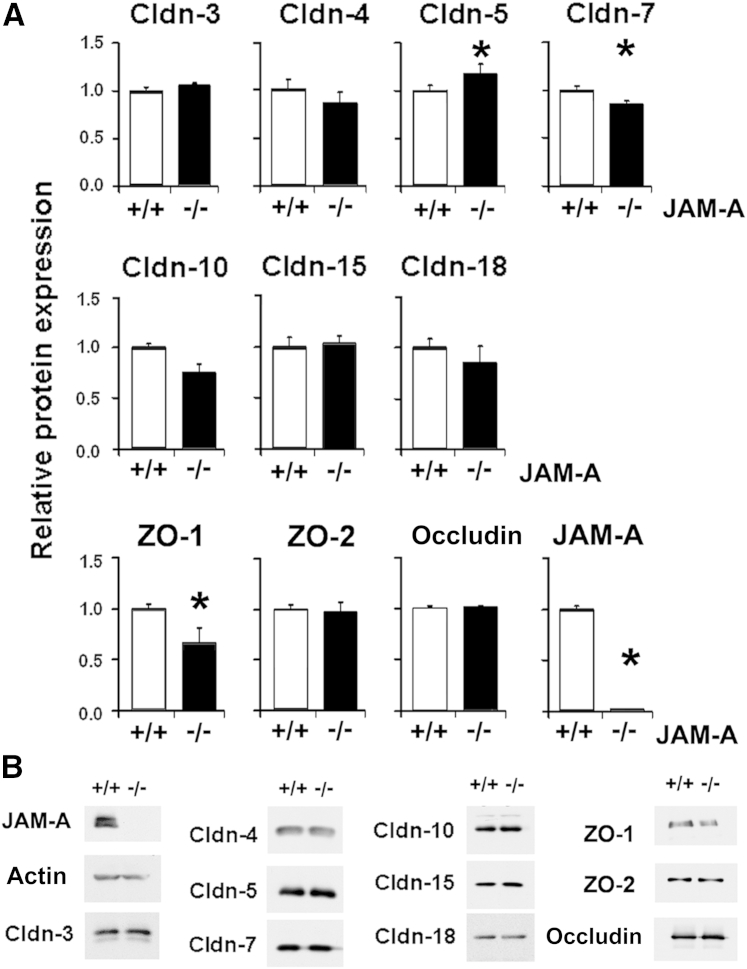
JAM-A−/− mice have previously been shown to have a defect in intestinal permeability linked to changes in tight junction protein expression.15, 20 Therefore, we characterized tight junction protein content of whole lungs of JAM-A−/− mice (Figure 1). We examined two PDZ scaffold proteins, ZO-1 and ZO-2, which are regulated by JAM-A.15 Only ZO-1 showed a significant decrease in lung expression compared with that observed in wild-type mice (Figure 1). There was a mean ± SD 34% ± 15% decrease (n = 4) in total lung ZO-1 expression in JAM-A−/− mice compared with controls. In contrast, levels of total lung ZO-2 were unchanged in JAM-A−/− mice. Occludin expression was also unchanged in lungs of JAM-A−/− mice.
Figure 1.
JAM-A−/− mice have decreased lung zonula occludens 1 (ZO-1) expression. A: Lungs were harvested from littermate control mice (+/+) or JAM-A−/− mice (−/−) and homogenized in a protease inhibitor cocktail, and proteins were resolved by SDS-PAGE, analyzed by immunoblot, and quantified as described in Materials and Methods. Of the tight junction proteins examined, claudin-5 (Cldn-5), Cldn-7, and ZO-1 levels were significantly different in lungs from JAM-A−/− mice compared with those from wild-type mice. JAM-A is undetectable in JAM-A−/− mice. B: Representative immunoblots. Data are expressed as means ± SD. n = 4. ∗P < 0.05.
We then focused on claudin expression. Of the claudins examined, only claudin-5 and claudin-7 showed small, but statistically significant, changes when comparing total lysates of lungs from JAM-A−/− mice with those from littermate controls. Claudin-5 levels increased by a mean ± SD of approximately 17% ± 10% (n = 4), whereas claudin-7 levels decreased by 15% ± 3% (n = 4). Claudin-4, claudin-10, and claudin-18 levels trended downward in response to loss of JAM-A, and the remaining claudins examined (claudin-1, claudin-2, claudin-3, claudin-4, claudin-8, claudin-10, claudin-15, claudin-18, and claudin-23) did not show significant differences between JAM-A–deficient lungs and lungs from littermate controls. Levels of pore-forming claudin-10 and claudin-15), which are up-regulated in the intestine of JAM-A−/− mice and contribute to an increase in gut permeability,20 were not increased in the lungs of JAM-A−/− mice. These results suggest that JAM-A deficiency has different effects on claudin expression in the lungs than in the gut.
Consistent with small changes to total tight junction protein expression at baseline, lungs of wild-type and JAM-A−/− mice had comparable mean ± SD fluid content as determined by lung wet/dry weight ratios [wild type: 3.6 ± 0.1 (n = 3) versus JAM-A−/−: 3.6 ± 0.2 (n = 5)].
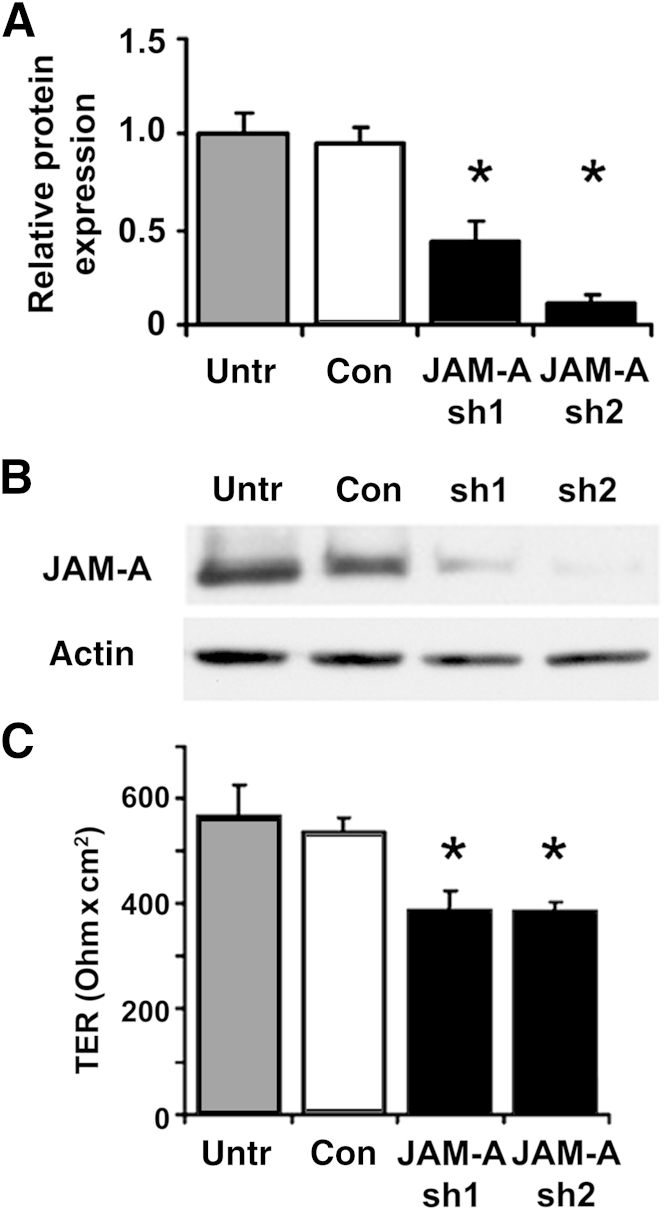
Because the lung is composed of many types of cells, we sought to characterize the effect of depleting JAM-A specifically in alveolar epithelial cells. We chose to analyze rat alveolar epithelial cells because this is a well-established system that we have used to characterize several different aspects of alveolar barrier function at a molecular level.25, 31, 32 Freshly isolated rat type II cells were transduced with lentiviral constructs encoding for shRNAs targeting JAM-A or scrambled controls and then further cultured 6 days to differentiate into model type I cells. Transduction with two different specific shRNAs decreased mean ± SD JAM-A expression to 44% ± 10% and 12% ± 3% of normal levels (n = 3), whereas JAM-A expression by cells transduced with control shRNA was indistinguishable from that by untreated cells (Figure 2, A and B). Using transepithelial resistance as a measure of barrier function, we found that JAM-A depletion in primary rat alveolar epithelial cells reduced transepithelial resistance by approximately 30% compared with control infected cells (Figure 2C). These findings demonstrate that JAM-A expression regulates barrier function of alveolar epithelial cells.
Figure 2.
Depletion of JAM-A protein impairs alveolar epithelial barrier function in vitro. A: Freshly isolated type II cells were either untreated (Untr) or transduced with lentiviral vectors encoding for control scrambled shRNA (Con) or were one of two different JAM-A–specific shRNAs (sh1, sh2), were plated on Transwell permeable supports, and were cultured for 6 days to produce model type I cells. The cells were then harvested and lyzed, and proteins were resolved by SDS-PAGE for immunoblot analysis with an antibody against JAM-A. JAM-A protein was measured by immunoblot, quantified, and normalized to actin. Both JAM-A–specific shRNAs significantly decreased JAM-A protein expression. B: Representative immunoblots. C: Transepithelial resistance (TER) of model type I cells as treated in A was measured using an EVOM voltmeter. JAM-A depletion resulted in a significant decrease in TER. Data are expressed as means ± SD. n = 3. ∗P < 0.05.
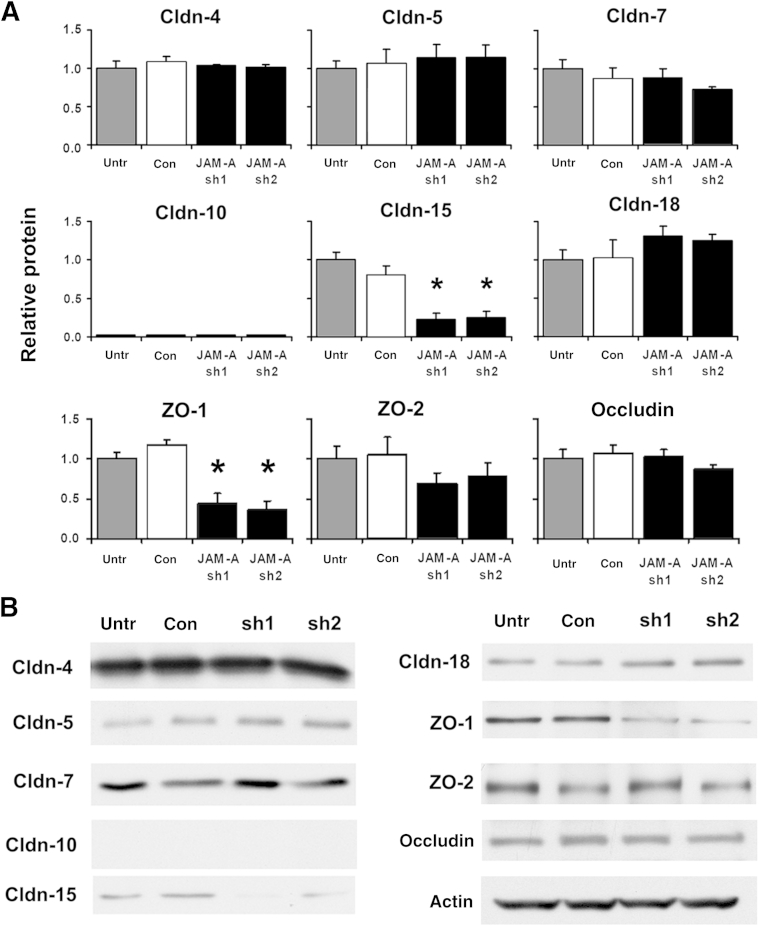
We then examined the effects of decreased JAM-A expression on alveolar epithelial tight junction proteins (Figure 3). Consistent with measurements of total lung ZO-1 protein levels in JAM-A−/− mice, shRNA-mediated depletion of JAM-A had a significant effect on total alveolar epithelial ZO-1 levels. Mean ± SD ZO-1 protein levels were reduced to 44% ± 13% and 36% ± 11% (n = 3) of control values by JAM-A sh1 and sh2, respectively. Similarly, decreases in ZO-1 levels paralleled decreases in alveolar epithelial transepithelial resistance induced by these JAM-A–targeting shRNAs. Total cellular levels of ZO-2 were not affected by reduced JAM-A expression in alveolar epithelial cells. This was an unexpected finding given the recent report that ZO-2 has a direct association with JAM-A in intestinal epithelium.15
Figure 3.
JAM-A depletion of alveolar epithelial cells decreases zonula occludens 1 (ZO-1) expression. A: Model type I cells on Transwell permeable supports that were either untreated (Untr) or transduced with lentiviral vectors encoding for control scrambled shRNA (Con) or were one of two different JAM-A–specific shRNAs (sh1, sh2) were harvested and processed, and the effect on claudin-4 (Cldn-4), Cldn-5, Cldn-7, Cldn-10 Cldn-15, Cldn-18, ZO-1, ZO-2, and occludin levels was measured by immunoblot analysis and was quantified. The major tight junction protein affected by JAM-A was ZO-1, which was decreased. Cldn-15 also showed a significant decrease in JAM-A–depleted cells. B: Representative immunoblots. Data are expressed as means ± SD. n = 3. ∗P < 0.05.
We next examined the effect of decreased JAM-A expression on other alveolar epithelial tight junction proteins. JAM-A protein depletion had no measurable effect on alveolar epithelial expression of claudin-4, claudin-5, claudin-7, or claudin-18. In contrast to total mouse lung, claudin-10 was not detected in either control or JAM-A–depleted rat alveolar epithelial cells, as determined by immunoblot analysis using an antibody that recognizes both isoforms (claudin-10a/b). Claudin-15 expression by rat alveolar epithelial cells was decreased when JAM-A was depleted, which contrasts with intestinal epithelial cells, where loss of JAM-A has been reported to result in increased claudin-15 expression.20 This also differed from the effects of JAM-A deficiency on total lung claudin-15 levels, which was unchanged in JAM-A−/− mice, and may be due to a cell-, tissue-, or species-specific difference in claudin-15 regulation. Critically, in neither case did claudin-15 expression increase compared with claudin-15 expression in JAM-A–deficient intestinal epithelium.20 In addition to the examination of claudin protein expression, we also assessed the level of occludin protein and determined that there was no change in JAM-A–depleted alveolar epithelial cells (Figure 3).
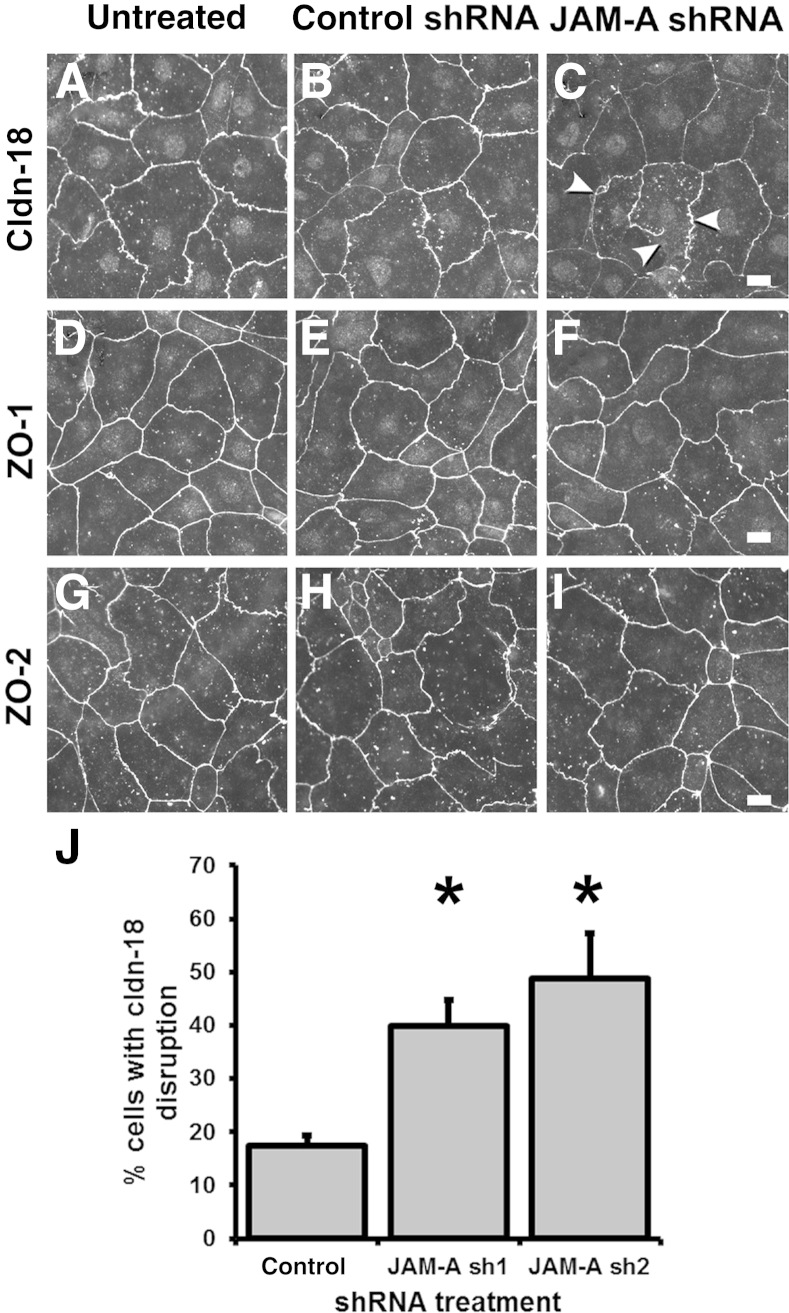
Claduin-18 has a critical role in regulating lung barrier function.23, 24 Thus, we examined claudin-18 localization in alveolar epithelial cells. Immunofluorescence analysis confirmed that depletion of JAM-A disrupted localization of claudin-18 to tight junctions at sites of cell-cell contact (Figure 4). Specifically, cells transduced with JAM-A shRNA and immunolabeled for claudin-18 showed apparent tight junction strand breaks and other morphologic abnormalities (Figure 4). Quantitative analysis of cells transduced with either control or JAM-A shRNA revealed that there was a significant increase in epithelial cells with disrupted claudin-18 localization. In contrast to the overt changes observed with claudin-18, junctional localization of ZO-1 and ZO-2 was largely unaffected in JAM-A–depleted alveolar epithelial cells in culture (Figure 4), despite the net decrease in total ZO-1 protein levels (Figure 3). However, we cannot rule out rearrangements in ZO-1 and/or ZO-2 that are below the level of resolution obtainable by standard confocal immunofluorescence microscopy.
Figure 4.
Claudin-18 (Cldn-18) localization is disrupted by JAM-A depletion. Model type I cells on Transwell permeable supports that were either untreated (A, D, and G) or transduced with lentiviral vectors encoding for control scrambled shRNA (B, E, and H) or JAM-A–specific shRNA-1 (C, F, and I) were fixed, permeabilized, and immunolabeled for Cldn-18 (A–C), zonula occludens 1 (ZO-1) (D–F), or ZO-2 (G–I) and imaged. Arrowheads in C denote areas where tight junction localization of Cldn-18 was impaired or absent owing to JAM-A depletion. J: Images from cells treated with either control scrambled shRNA or one of the two different JAM-A–targeted shRNAs were quantified to determine the number of cells with disrupted Cldn-18 localization. JAM-A depletion significantly increased the number of cells with impaired Cldn-18. Data are expressed as means ± SD. n = 5 to 7 fields from two independent experiments. ∗P < 0.05 versus control. Scale bars: 10 μm.
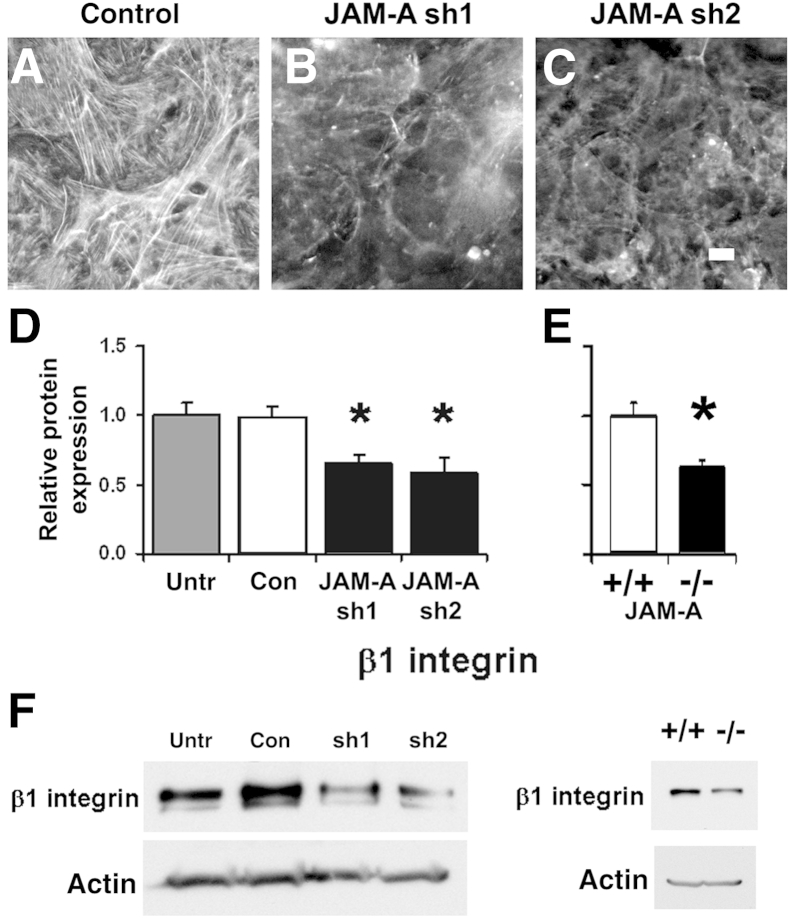
Given the known central role for claudin-18 in the formation of alveolar tight junctions, these data from cultured cells suggest that JAM-A regulates claudin-18 processing and, thereby, maintains alveolar barrier function. JAM-A has previously been linked to regulation of the actin cytoskeleton via control of β1 integrin expression.15, 40, 41 Given this, and the demonstration that β1 integrin is necessary for optimal epithelial barrier function,42 we investigated the effect of JAM-A deficiency on actin and β1 integrin expression in alveolar epithelial cells. JAM-A–depleted cells had decreased levels of filamentous actin compared with control cells, whereas the total actin protein levels remained unchanged (Figure 5). JAM-A–depleted alveolar epithelial cells also showed decreased β1 integrin expression, and cells transduced with JAM-A sh1 or JAM-A sh2 had β1 integrin protein levels that were a mean ± SD of 66% ± 6% and 59% ± 10% of control cells, respectively. A comparable decrease in β1 integrin protein expression was observed in JAM-A−/− lungs, which had a mean ± SD of 63% ± 5% (n = 4) of the β1 integrin content found in wild-type lungs, consistent with a link between JAM-A and β1 integrin protein expression.
Figure 5.
JAM-A depletion disrupts actin and β1 integrin expression. A–C: Model type I cells on Transwell permeable supports that were transduced with lentiviral vectors encoding for control scrambled shRNA or one of two different JAM-A–specific shRNAs (sh1, sh2) were fixed, permeabilized, and stained using rhodamine phalloidin. B and C: JAM-A depletion disrupted the formation of filamentous actin. D: JAM-A–depleted cells also showed a significant decrease in β1 integrin levels as measured by immunoblot and quantified. E: β1 integrin expression was significantly decreased in JAM-A−/− lungs. F: Representative immunoblots. Data are expressed as means ± SD. n = 3 (D); n = 4 (E). ∗P < 0.05 versus control. Scale bar = 10 μm. Con, control; Untr, untreated.
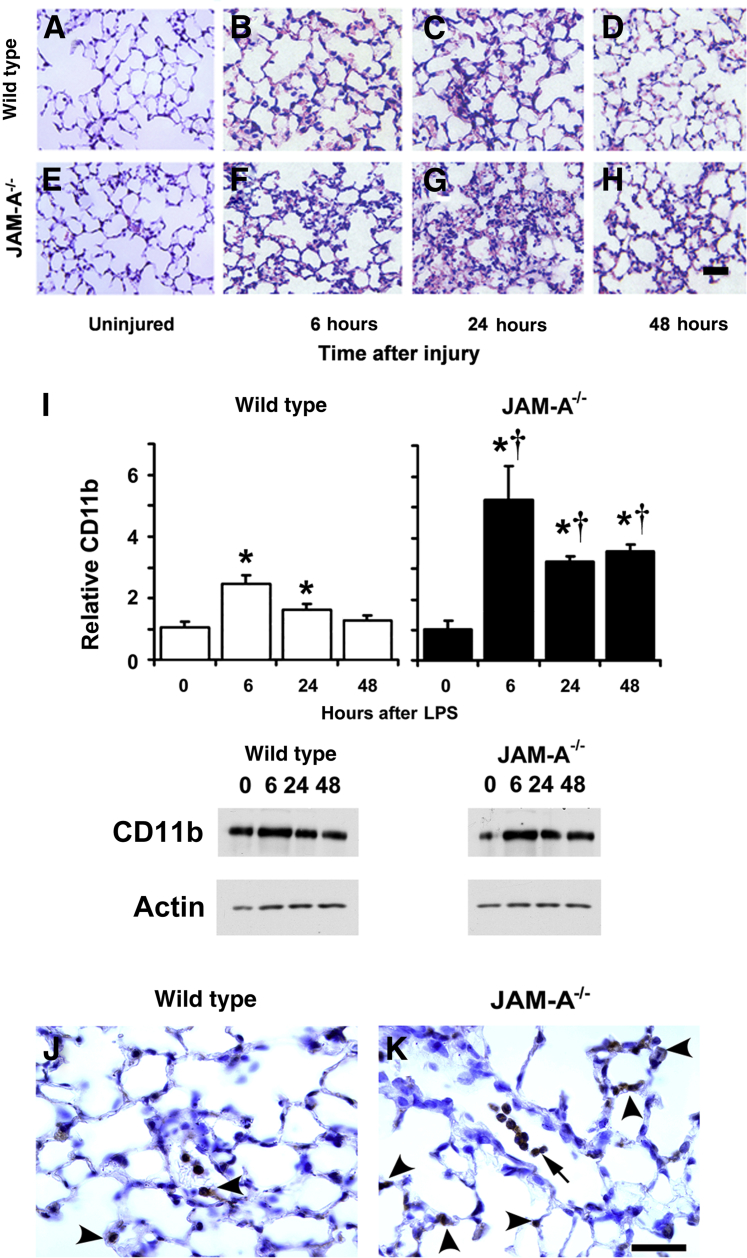
Given that alveolar barrier function was impaired in JAM-A–depleted alveolar epithelial cells, we examined whether JAM-A−/− mice were more susceptible to pulmonary injury in response to a mild form of endotoxemia induced by i.p. injection of LPS.43 Histologic hematoxylin and eosin analyses of lung sections showed that JAM-A−/− mice challenged with i.p. LPS had more cell infiltrate into lung air spaces compared with wild-type controls (Figure 6, A–H). We further confirmed this observation by immunoblot analysis of total lung tissue using a broad spectrum marker for leukocytes (CD11b) that recognizes monocytes, neutrophils, and macrophages.44 Wild-type and JAM-A−/− mice showed a transient, significant increase in CD11b-staining cells that peaked 6 hours after i.p. administration of LPS (Figure 6I). By this measure, JAM-A−/− mice (n = 6) 6 hours after i.p. LPS injection had a significant relative increase in cellular infiltrates that were a mean ± SD of 2.1 ± 0.5 fold higher than that of wild-type mice, consistent with hematoxylin and eosin images. These results suggest that JAM-A−/− mice had increased sensitivity to mild systemic endotoxemia compared with wild-type animals, although it is likely that enhanced motility of JAM-A–deficient CD11b-positive cells may also contribute to this response.45 We also examined lungs 6 hours after injury using an antibody (NIMP-R14) that recognizes the neutrophil marker Ly6G. Six hours after injury, there were Ly6G-positive cells in the air spaces and in interstitial tissues of wild-type and JAM-A−/− lungs, qualitatively demonstrating that both mouse strains showed neutrophil recruitment to lungs in response to systemic endotoxemia (Figure 6, J and K).
Figure 6.
JAM-A−/− mice have increased leukocyte recruitment in response to mild systemic endotoxemia. A–H: Wild-type (A–D) or JAM-A−/− (E–H) lungs from uninjured controls (A and E) or mice 6 hours (B and F), 24 hours (C and G), or 48 hours (D and H) after i.p. injection with 1 mg/kg endotoxin [lipopolysaccharide (LPS)] were harvested, paraffin embedded, processed, stained with hematoxylin and eosin, and imaged as described in Materials and Methods. JAM-A−/− mice showed more cell infiltrate than comparably treated wild-type mice, particularly 24 hours after treatment (G versus C). I: Lungs from littermate control mice (wild-type) or JAM-A−/− mice were harvested either immediately (0 hours) or 6, 24, or 48 hours after i.p. injection with 1 mg/kg endotoxin (LPS). The lungs were homogenized in a protease inhibitor cocktail, and then protein expression was analyzed by immunoblot and quantified. Wild-type and JAM-A−/− mice had a significant increase in CD11b after injury in response to i.p. LPS compared with uninjured mice. The relative increase in CD11b for JAM-A−/− mice after injury was significantly greater than the relative increase in CD11b for wild-type mice. J and K: Lungs from wild-type (J) and JAM-A−/− (K) mice 6 hours after i.p. LPS were harvested, processed for immunohistochemical analysis, and immunolabeled for the neutrophil marker NIMP-R14. Both wild-type and JAM-A−/− mice had NIMP-R14–positive neutrophils associated with alveolar septa (arrowheads). Neutrophils were more apparent in air spaces of injured JAM-A−/− mice (arrow). Data are expressed as means ± SD. n = 4 to 6 (I). ∗P < 0.05 versus uninjured mice; †P < 0.05 versus comparably treated wild-type mice. Scale bars: 100 μm.
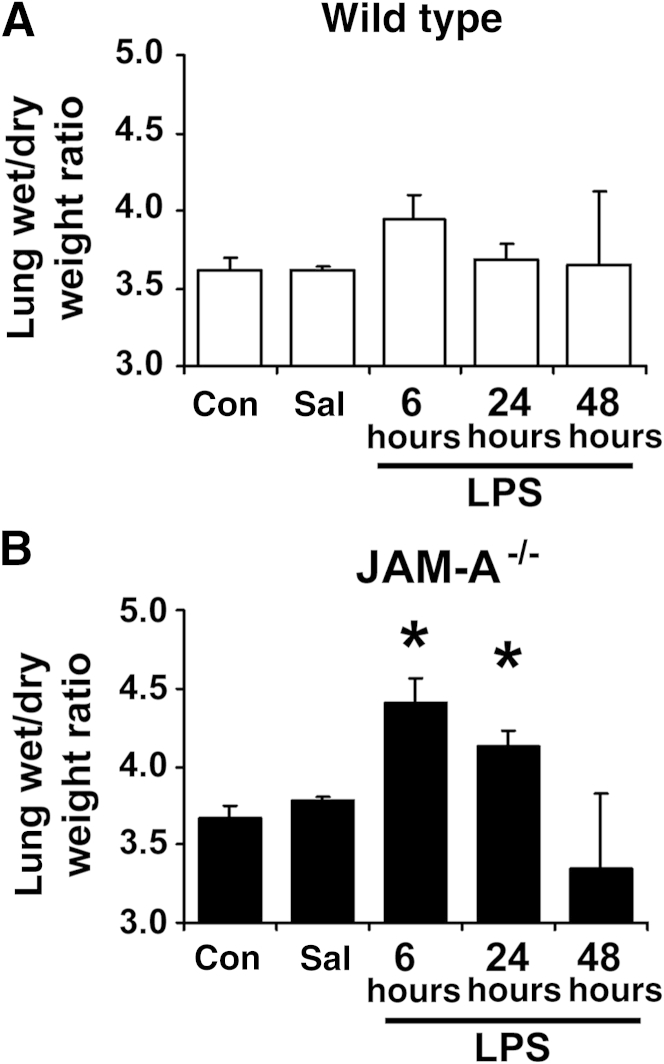
Pulmonary edema is also linked to lung injury. Wild-type littermate controls had a slight, brief increase in pulmonary edema 6 hours after injection (P = 0.07, n = 6) based on wet/dry lung weight (Figure 7). In contrast, JAM-A−/− mice had enhanced edema that was apparent in significantly increased wet/dry lung weights at 6 and 24 hours. Consistent with mild lung injury, pulmonary edema in wild-type and JAM-A−/− mice was transient and resolved 48 hours after i.p. administration of LPS.
Figure 7.
JAM-A−/− mice have increased lung edema to mild systemic endotoxemia. Lungs from littermate control mice (wild-type, A) or JAM-A−/− mice (B) were harvested immediately (Con), 6 hours after i.p.-injected saline (Sal), or 6, 24, or 48 hours after i.p. injection with 1 mg/kg endotoxin [lipopolysaccharide (LPS)]. The lungs were weighed, dried overnight in an oven at 65°C, and weighed again, and the ratio of lung wet/dry weight was calculated. JAM-A−/− mice (B) showed a significant increase in wet/dry weight, indicating pulmonary edema. Data are expressed as means ± SD. n = 7. ∗P < 0.05 versus uninjured mice.
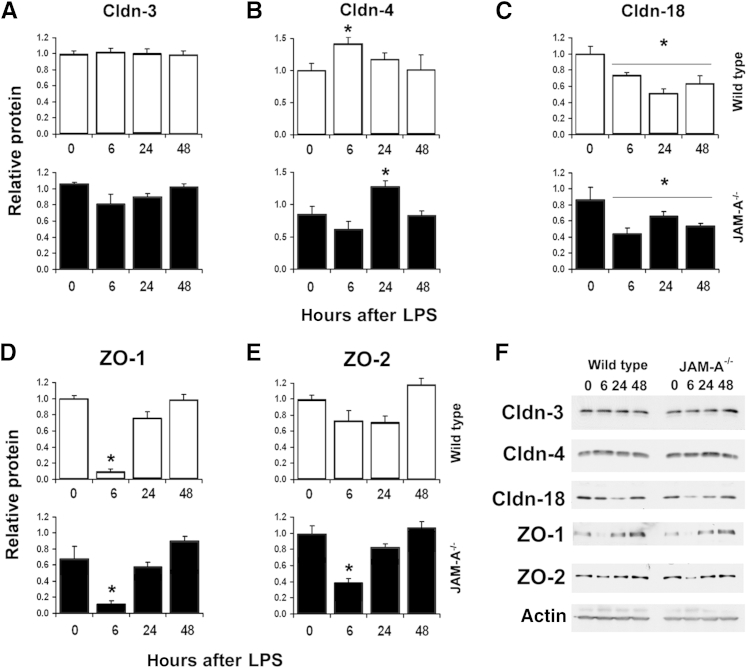
The increased pulmonary edema induced by lung injury between wild-type and JAM-A−/− mice is consistent with dysregulation of tight junctions in JAM-A−/− mice. Thus, we examined the effect of systemic endotoxemia on lung tight junction protein expression in JAM-A−/− mice, initially focusing on the major claudins expressed by alveolar epithelial cells: claudin-3, claudin-4, and claudin-18 (Figure 8, A–C and F). Lung claudin-3 expression, which is largely restricted to type II alveolar epithelial cells, was unaffected in lungs of either wild-type or JAM-A−/− mice in response to injury (Figure 8A). However, levels of claudin-4 protein in lungs showed a significant change in response to i.p.-administered LPS for wild-type and JAM-A−/− mice (Figure 8B). Lungs from wild-type mice showed a transient increase in claudin-4 protein expression 6 hours after i.p. LPS, with levels returning to baseline 24 hours after LPS challenge. Lungs from JAM-A−/− mice also showed a transient increase in claudin-4 protein expression; however, the peak occurred 24 hours after i.p. LPS, as opposed to 6 hours for wild-type mice (Figure 8B). In wild-type and JAM-A−/− mice, claudin-4 expression returned to baseline 48 hours after injury. Given the critical role of claudin-4 in promoting lung fluid clearance,25, 28, 46 this observed delay in up-regulation of claudin-4 by JAM-A−/− mice has clinical significance. Claudin-18 protein levels were also affected by i.p. LPS. Lungs from wild-type and JAM-A−/− mice exhibited a comparable, persistent (approximately 30% to 40%) decrease in claudin-18 protein expression (Figure 8C).
Figure 8.
JAM-A−/− mice have an impaired tight junction remodeling response to mild systemic endotoxemia. Lungs from littermate control mice (wild-type) or JAM-A−/− mice were harvested immediately (0 hours), 6 hours after i.p.-injected saline (Sal), or 6, 24, or 48 hours after i.p. injection with 1 mg/kg endotoxin [lipopolysaccharide (LPS)]. The lungs were homogenized in a protease inhibitor cocktail, and then protein expression was analyzed by immunoblot for claudin-3 (Cldn-3) (A), Cldn-4 (B), Cldn-18 (C), zonula occludens 1 (ZO-1) (D), and ZO-2 (E) and quantified. Time 0 quantitation was reproduced from Figure 1. Both wild-type and JAM-A−/− showed a transient increase in Cldn-4 expression; however, this response was delayed in JAM-A−/− mice. Cldn-18 expression is uniformly decreased during the entire course of recovery. Both wild-type and JAM-A−/− showed a transient decrease in ZO-1 levels. In contrast, only JAM-A−/− mice showed a significant decrease in ZO-2 expression 6 hours after i.p. LPS injection. F: Representative immunoblots. Data are expressed as means ± SD. n = 4 to 6. ∗P < 0.05 versus uninjured mice.
Levels of the tight junction–associated PDZ binding scaffold proteins ZO-1 and ZO-2 were also assessed during the course of lung injury and resolution. Six hours after the administration of LPS there was a dramatic decrease in total lung ZO-1 protein levels for wild-type and JAM-A−/− mice (Figure 8, D and F). This decrease was transient, and both strains of mice showed normal levels of lung ZO-1 24 hours after LPS challenge. Whereas lungs from wild-type mice showed little change in ZO-2 protein levels, lungs from JAM-A−/− mice showed a transient decrease in ZO-2 levels in response to injury (Figure 8, E and F). The changes observed in lung ZO-2 protein levels mirrored those of ZO-1 levels, with decreases in ZO-2 protein levels 6 hours after injury and complete recovery of protein levels 24 hours after the administration of LPS. Thus, wild-type and JAM-A−/− mice showed differential regulation of ZO-2 in response to injury. In particular, the combined effects of simultaneous decreases in ZO-1 and ZO-2 levels in JAM-A−/− mice correlated with increased edema, consistent with roles for these scaffold proteins in regulating epithelial barrier function.47, 48
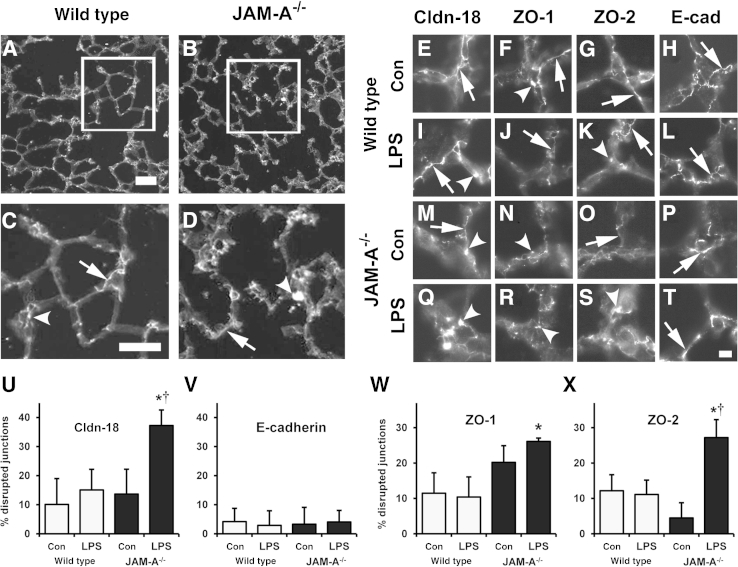
Given the differential changes in lung tight junction protein content between wild-type and JAM-A−/− mice and the effects of JAM-A depletion on claudin-18 localization, we examined the localization of these tight junction proteins in lung sections (Figures 9 and 10). Wild-type mice showed a predominantly linear staining pattern of localization for claudin-18, ZO-1, and ZO-2 (Figure 9, A, C, and E–G). However, there were also areas where junctional localization of these proteins was disrupted. Uninjured JAM-A−/− mice showed a pattern of claudin-18, ZO-1, and ZO-2 localization comparable with that of wild-type mice (Figure 9, B, D, and M–O). However, 6 hours after LPS challenge, JAM-A−/− mice showed a significant level of tight junction disruption, where claudin-18, ZO-1, and ZO-2 seemed to form aggregates as opposed to linear arrays (Figure 9, Q–S). This was in contrast to wild-type lungs, where localization of these tight junction proteins was largely unaffected (Figure 9, I–K). Changes to tight junction proteins in injured JAM-A−/− mice were specific because localization of the adherens junction protein E-cadherin was unaffected by injury in either wild-type or JAM-A−/− mice (Figure 9, L and T).
Figure 9.
Claudin-18 (Cldn-18), zonula occludens 1 (ZO-1), and ZO-2 localization are disrupted in injured JAM-A−/− mice. Sections from wild-type (A and C) and JAM-A−/− (B and D) lungs were processed and immunolabeled for Cldn-18. C and D: Magnified insets. Cldn-18 shows normal junctional localization (arrow) and a pattern indicating disrupted localization (arrowhead). E–T: Lung sections from wild-type (E–L) or JAM-A−/− (M–T) mice that were either uninjured (Con) (E–H and M–P) or 6 hours after lipopolysaccharide (LPS) challenge (I–L and Q–T) were processed and immunolabeled for Cldn-18 (E, I, M, and Q), ZO-1 (F, J, N, and R), ZO-2 (G, K, O, and S), or E-cadherin (E-cad) (H, L, P, and T). Lungs from injured JAM-A−/− mice showed more disrupted Cldn-18 (Q), ZO-1 (R), and ZO-2 (S) (arrowheads) than lung sections from wild-type or uninjured JAM-A−/− mice which instead had more regions with normal junction morphology (arrows). U–X: Images were quantified to determine the percentage of disrupted junctions for Cldn-18 (U), E-cad (V), ZO-1 (W), and ZO-2 (X). For injured JAM-A−/− mice, Cldn-18, ZO-1, and ZO-2 were significantly more disrupted than in uninjured wild-type mice. Cldn-18 and ZO-2 in injured JAM-A−/− mice were significantly more disrupted than in uninjured JAM-A−/− mice. Data are expressed as means ± SD. ∗P < 0.05 versus uninjured wild-type mice. †P < 0.05 versus uninjured JAM-A−/− mice. Scale bars: 100 μm (A and C); 20 μm (T).
Figure 10.
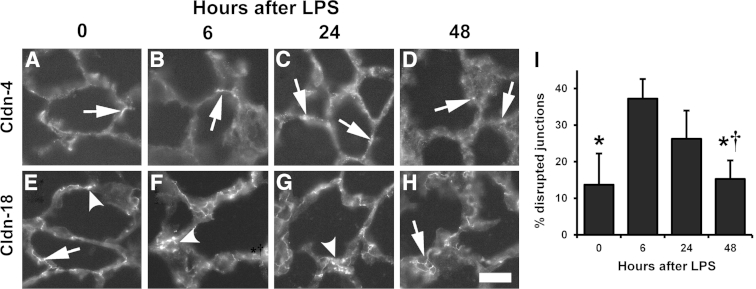
Recovery of lung claudin (Cldn) localization by JAM-A−/− mice 48 hours after lipopolysaccharide (LPS) challenge. Sections from JAM-A−/− lungs were processed and immunolabeled for Cldn-4 (A–D) or Cldn-18 (E–H) 0 (A and E), 6 (B and F), 24 (C and G), or 48 hours (D and H) after LPS challenge. Cldn-4 shows a limited localization pattern (arrows), as opposed to Cldn-18, which is more prominently expressed. Cldn-18 6 and 24 hours after LPS challenge (F and G) is more frequently disrupted (arrowheads) than in uninjured JAM-A−/− mice (E) or in mice 48 hours after injury (H). I: Images were quantified to determine the percent disrupted junctions for claudin-18 during the injury time course. Time 0 and 6 hours quantitation is reproduced from Figure 9. At 48 hours, mice show significantly less Cldn-18 disruption than lungs either 6 or 24 hours after LPS challenge, suggesting full recovery from systemic endotoxemia. Data are expressed as means ± SD. ∗P < 0.05 versus 6 hours; †P < 0.05 versus 24 hours. Scale bar = 50 μm.
We then further examined changes to claudin-4 and claudin-18 localization during the time course of injury in JAM-A−/− mice. Claudin-4 showed a patchy distribution in lung sections consistent with previous results examining claudin-4 localization in normal rat lung (Figure 10, A–D).33 The distribution of claudin-4 did not change much, although qualitatively there did seem to be more claudin-4–positive regions of JAM-A−/− lungs 24 hours after LPS challenge compared with the other time points examined (Figure 10C). Twenty-four hours after the onset of endotoxemia, claudin-18 localization was comparable with the 6-hour time point and remained disrupted in JAM-A−/− mice (Figure 10, F and G). Critically, claudin-18 morphologic features renormalized by 48 hours (Figure 10, E and H), which correlated with the return to normal lung barrier function after injury (Figure 7).
Taken together, these results support a role for JAM-A in regulating tight junction protein expression, processing, and function. In particular, these data suggest that JAM-A plays a key role in preventing acute lung injury by enabling alveolar tight junctions to resist aberrant remodeling in response to systemic endotoxemia.
Discussion
Tight junctions are the structural components that regulate paracellular permeability. To form a suitable and effective tight junction barrier, the functional coordination of several classes of transmembrane, scaffold, and cytoskeletal proteins is required. Herein, we showed that JAM-A expression plays a key role in the regulation of tight junction dynamics in alveolar epithelial cells in situ and in cell culture. Specifically, depletion of JAM-A led to diminished claudin-18 localization to tight junctions, decreased ZO-1 and β1 integrin expression, and compromised actin cytoskeleton assembly. JAM-A deficiency also resulted in misregulation of claudin-4 and ZO-2 in response to lung injury. Thus, JAM-A plays a key role in promoting alveolar epithelial tight junction homeostasis and lung barrier function by coordinating interactions between claudins, the tight junction scaffold, and the cytoskeleton.
JAM-A−/− mice did not exhibit spontaneous lung injury in the absence of an added insult, suggesting compensatory changes to adaptive immunity, such as the increase in CD4+ T cells observed in the intestines of JAM-A−/− mice.49 However, JAM-A−/− mice showed significantly more lung edema when challenged with systemic endotoxemia as opposed to wild-type control mice. Increased sensitivity of JAM-A−/− mice to injury was due to differences in the regulation of several tight junction proteins after injury that caused significant rearrangement of several tight junction proteins, including claudin-18, ZO-1, and ZO-2 (Figure 9, U–X). The enhanced level of tight junction disruption in JAM-A−/− mice compared with wild-type mice in response to LPS challenge provides a direct mechanism for the increased sensitivity of JAM-A−/− mice to endotoxemia, particularly when considering the effects of JAM-A depletion on claudin-18 localization and barrier function in isolated alveolar epithelial cells (Figures 2C and 4C).
There also was a delay in the up-regulation of lung claudin-4 in JAM-A−/− mice after injury. Claudin-4 has been shown to play a key role in preventing pulmonary edema by promoting lung fluid clearance.25, 28, 46 Thus, the delay in claudin-4 exhibited by JAM-A−/− mice was likely to contribute to enhanced lung edema observed in JAM-A knockout mice. Previously published studies show that increased claudin-4 expression promotes lung barrier function and fluid clearance.25, 26, 28 Consistent with a protective effect of claudin-4, this protein was increased at 6 hours, which prevented pulmonary edema in wild-type mice. Critically, this increase in claudin-4 expression was in the context of well-formed alveolar tight junctions. In contrast, JAM-A−/− not only had a delay in increased claudin-4 levels but also a significant disruption in tight junction assembly. Thus, any beneficial effect of increased claudin-4 expression by JAM-A−/− mice was blunted until claudin-18 was able to return to a normalized state of assembly (Figure 10H). This is critical in thinking about clinical strategies to improve lung barrier function in response to systemic endotoxemia because it implies that up-regulation of claudin-4 alone is not necessarily sufficient to rescue proper lung fluid balance in the absence of well-formed tight junctions. Consistent with this model, claudin-18–deficient mice up-regulate lung claudin-4 protein yet still have alveolar barrier dysfunction.24
Two prevailing, complementary models can account for the ability of JAM-A to enhance barrier function: by recruiting scaffold proteins to promote tight junction assembly14, 15 and by inducing signaling pathways that alter tight junction protein composition. It was recently demonstrated that the cytoplasmic domain of JAM-A binds directly to ZO-2, which, in turn, helps recruit ZO-1, afadin, PDZ-GTP exchange factor 1 (PDZ-GEF1), and rap2c into a multiprotein complex that inhibits cytoskeletal turnover, thereby stabilizing tight junctions.15 JAM-A has been shown to bind directly to ZO-2 with far greater affinity than to ZO-1,15 although a direct interaction of JAM-A with ZO-1 in some contexts cannot be completely ruled out.14, 50 We found that wild-type and JAM-A−/− mice showed dramatic decreases in total lung ZO-1 levels 6 hours after the onset of endotoxemia. However, lungs from JAM-A−/− mice also showed decreased levels of ZO-2. Because the presence of JAM-A helped stabilize ZO-2 expression in response to injury, the present data are consistent with a model in which JAM-A recruits ZO-2 to tight junctions. Given the complementary roles of ZO-1 and ZO-2 in recruiting claudins to tight junctions,47, 48, 51 the present data suggest a mechanism by which ZO-2 compensates for loss of ZO-1 in injured wild-type mice and implicates a protective role for JAM-A in this process.
A comparable JAM-A–dependent pathway that is regulated by the formation of a multiprotein scaffold protein complex has also been shown to modulate intestinal epithelial cell migration during wound repair. However, instead of co-clustering PDZ-GEF1 and rap2c to regulate tight junction permeability, cis-dimerization of JAM-A regulates cell migration through formation of an afadin/PDZ-GEF2 complex that activates rap1.41 Activated rap1 inhibits degradation of β1 integrin and promotes cell-matrix interactions, providing a link between JAM-A and regulation of cell migration.41 Although JAM-A clustering is a conserved mechanism of action for diverse cell processes, downstream effects depend on the nature of the signaling proteins recruited to the complex in response to JAM-A dimerization. In contrast to intestinal epithelium, endothelial barrier function is promoted by rap1 activation, indicating that there are tissue-specific components of JAM-A signaling that determine how downstream signaling is processed.52, 53
The cytoskeleton is a critical determinant of epithelial barrier function and serves several regulatory functions.54 Moreover, filamentous actin has previously been found to enhance alveolar barrier function, even in the absence of other changes in tight junction protein expression.55 Thus, by regulating actin, JAM-A has the capacity to alter tight junction structure and permeability. Herein, we found that in JAM-A–depleted cells there was decreased β1 integrin and a concomitant decrease in filamentous actin, demonstrating a link among JAM-A, actin, and β1 integrin expression. This is consistent with previous studies linking JAM-A to the regulation of β1 integrin expression.15, 40, 41 It is known that JAM-A induces afadin/rap signaling pathways to increase β1 integrin expression.40, 41 JAM-A signaling via a parallel afadin/rap pathway has also been shown to modulate RhoA and myosin light chain kinase to strengthen colonic epithelial barrier function by decreasing tight junction turnover.15 The present results extend the results of that study by showing that epithelial β1 integrin is regulated by JAM-A in alveolar epithelial cells. Similar to β1 integrin, additional integrins have been found to regulate barrier function. For example, endothelial tight junctions require signaling by αvβ3 and αvβ5 integrins.56, 57 Moreover, studies of MDCK cells demonstrate that there is a sizable pool of β1 integrin directly associated with tight junctions, which strengthens barrier function by promoting interactions between the cytoskeleton and tight junction proteins,58 and β1 integrin is necessary for optimal kidney epithelial barrier function.42 Recently, it has been shown that JAM-A, CD9, and αvβ5 integrin form a ternary complex that promotes vascular remodeling and endothelial cell migration.59 Whether a comparable complex involving β1 integrin is involved in tight junction assembly or epithelial barrier function is unknown.
A recent study examining the sensitivity of JAM-A−/− mice to direct intratracheal instillation of endotoxin showed that wild-type and JAM-A−/− mice developed comparable levels of lung edema 6 hours after injury.29 Although these data are in apparent contrast to the present finding that JAM-A−/− mice had increased edema in response to systemic endotoxemia, the method of lung injury used differed from that of the present study. In particular, intratracheal instillation of endotoxin directly injures lung epithelium to impair fluid clearance.60 In contrast, i.p. injection of LPS results in an indirect insult resulting in lung injury that is significantly milder43 and, thus, revealed increased sensitivity of JAM-A−/− mice to pulmonary edema. Moreover, we found that shRNA-mediated depletion of JAM-A recapitulated the deleterious effect of JAM-A depletion on tight junctions in vivo, consistent with a role for JAM-A in regulating lung fluid balance by maintaining alveolar barrier function.
Using a model for systemic endotoxemia,43 we found that JAM-A−/− mice have increased pulmonary edema in response to lung injury. The ability of JAM-A to promote alveolar epithelial tight junction assembly and remodeling in response to injury are likely to be the mechanism by which JAM-A protects the lung from edema that occurs during sepsis induced by distal organs such as the gut.39, 61, 62 Herein we provide the first direct evidence that JAM-A has a protective role in preventing lung injury and promoting fluid clearance. The important role of JAM-A in promoting alveolar barrier function suggests that lung inflammation may also be exacerbated under conditions in which JAM-A is pathologically impaired. For example, the airway epithelium of patients with cystic fibrosis contains 50% less JAM-A than do non–cystic fibrosis airway cells and are more susceptible to barrier dysfunction and paracellular leak in response to pro-inflammatory cytokines.63 However, the mechanism by which JAM-A expression is diminished in cystic fibrosis is unknown. Identification of pathways that increase the expression and function of JAM-A in cystic fibrosis, sepsis, and other conditions is likely to identify new approaches to promote barrier function in response to inflammation and injury.
Acknowledgments
We thank Mauricio Rojas for guidance related to establishing the systemic endotoxemia model and Barbara Schlingmann for critical reading of the manuscript.
Footnotes
Supported by Emory Alcohol and Lung Biology Center/NIH grant P50-AA013757 (M.K.); NIH grants R01-HL083120 and R01-HL116958 (to M.K.); AA-013528 (L.A.M.); R01-DK061379, R01-DK072564, and R01-DK079392 (to C.A.P.); and R0-DK089763 and R01-DK055679 (to A.N.); Emory University Research Committee (M.K.); and Emory-Children's Center of Excellence for Cystic Fibrosis Research (M.K.). Lentiviral vectors were packaged and amplified by the Emory Neuroscience National Institute of Neurological Disorders and Stroke Viral Vector Core funded by NIH grant P30-NS055077.
Disclosures: None declared.
References
- 1.Matthay M.A., Zemans R.L. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Ware L.B., Matthay M.A. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 5.Wilson T.A., Anafi R.C., Hubmayr R.D. Mechanics of edematous lungs. J Appl Physiol (1985) 2001;90:2088–2093. doi: 10.1152/jappl.2001.90.6.2088. [DOI] [PubMed] [Google Scholar]
- 6.Pittet J.F., Griffiths M.J., Geiser T., Kaminski N., Dalton S.L., Huang X., Brown L.A., Gotwals P.J., Koteliansky V.E., Matthay M.A., Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechara R.I., Brown L.A., Roman J., Joshi P.C., Guidot D.M. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med. 2004;170:188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- 8.Vogelmann R., Amieva M.R., Falkow S., Nelson W.J. Breaking into the epithelial apical-junctional complex: news from pathogen hackers. Curr Opin Cell Biol. 2004;16:86–93. doi: 10.1016/j.ceb.2003.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber C., Fraemohs L., Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 10.Mandell K.J., Parkos C.A. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Severson E.A., Parkos C.A. Structural determinants of Junctional Adhesion Molecule A (JAM-A) function and mechanisms of intracellular signaling. Curr Opin Cell Biol. 2009;21:701–707. doi: 10.1016/j.ceb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Severson E.A., Jiang L., Ivanov A.I., Mandell K.J., Nusrat A., Parkos C.A. Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell. 2008;19:1862–1872. doi: 10.1091/mbc.E07-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh M., Sasaki H., Furuse M., Ozaki H., Kita T., Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazzoni G., Martinez-Estrada O.M., Orsenigo F., Cordenonsi M., Citi S., Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275:20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro A.C., Sumagin R., Rankin C.R., Leoni G., Mina M.J., Reiter D.M., Stehle T., Dermody T.S., Schaefer S.A., Hall R.A., Nusrat A., Parkos C.A. JAM-A associates with ZO-2, Afadin and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;24:2849–2860. doi: 10.1091/mbc.E13-06-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelow S., Ahlstrom R., Yu A.S. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–F876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol. 2012;75:551–567. doi: 10.1146/annurev-physiol-030212-183809. [DOI] [PubMed] [Google Scholar]
- 18.Furuse M., Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Krause G., Winkler L., Mueller S.L., Haseloff R.F., Piontek J., Blasig I.E. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Laukoetter M.G., Nava P., Lee W.Y., Severson E.A., Capaldo C.T., Babbin B.A., Williams I.R., Koval M., Peatman E., Campbell J.A., Dermody T.S., Nusrat A., Parkos C.A. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soini Y. Claudins in lung diseases. Respir Res. 2011;12:70. doi: 10.1186/1465-9921-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta H., Chiba S., Ebina M., Furuse M., Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L193–L205. doi: 10.1152/ajplung.00349.2010. [DOI] [PubMed] [Google Scholar]
- 23.Lafemina M.J., Sutherland K.M., Bentley T., Gonzales L.W., Allen L., Chapin C.J., Rokkam D., Sweerus K.A., Dobbs L.G., Ballard P.L., Frank J.A. Claudin-18 deficiency results in alveolar barrier dysfunction and impaired alveologenesis in mice. Am J Respir Cell Mol Biol. 2014;51:550–558. doi: 10.1165/rcmb.2013-0456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G., Flodby P., Luo J., Kage H., Sipos A., Gao D., Ji Y., Beard L.L., Marconett C.N., Demaio L., Kim Y.H., Kim K.J., Laird-Offringa I.A., Minoo P., Liebler J.M., Zhou B., Crandall E.D., Borok Z. Knockout mice reveal key roles for claudin 18 in alveolar barrier properties and fluid homeostasis. Am J Respir Cell Mol Biol. 2014;51:210–222. doi: 10.1165/rcmb.2013-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell L.A., Overgaard C.E., Ward C., Margulies S.S., Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2011;301:L40–L49. doi: 10.1152/ajplung.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wray C., Mao Y., Pan J., Chandrasena A., Piasta F., Frank J.A. Claudin 4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–L227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kage H., Flodby P., Gao D., Kim Y.H., Marconett C.N., DeMaio L., Kim K.J., Crandall E.D., Borok Z. Claudin 4 knockout mice: normal physiologic phenotype with increased susceptibility to lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L524–L536. doi: 10.1152/ajplung.00077.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rokkam D., Lafemina M.J., Lee J.W., Matthay M.A., Frank J.A. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. Am J Pathol. 2011;179:1081–1087. doi: 10.1016/j.ajpath.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshmi S.P., Reddy A.T., Naik M.U., Naik U.P., Reddy R.C. Effects of JAM-A deficiency or blocking antibodies on neutrophil migration and lung injury in a murine model of ALI. Am J Physiol Lung Cell Mol Physiol. 2012;303:L758–L766. doi: 10.1152/ajplung.00107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobbs L.G., Gonzalez R., Williams M.C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 31.Abraham V., Chou M.L., DeBolt K.M., Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol. 1999;276:L825–L834. doi: 10.1152/ajplung.1999.276.5.L825. [DOI] [PubMed] [Google Scholar]
- 32.Koval M., Ward C., Findley M.K., Roser-Page S., Helms M.N., Roman J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am J Respir Cell Mol Biol. 2010;42:172–180. doi: 10.1165/rcmb.2008-0270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F., Daugherty B., Keise L.L., Wei Z., Foley J.P., Savani R.C., Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]
- 34.Daugherty B.L., Mateescu M., Patel A.S., Wade K., Kimura S., Gonzales L.W., Guttentag S., Ballard P.L., Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1266–L1273. doi: 10.1152/ajplung.00423.2003. [DOI] [PubMed] [Google Scholar]
- 35.Fasano C.A., Dimos J.T., Ivanova N.B., Lowry N., Lemischka I.R., Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Cera M.R., Del Prete A., Vecchi A., Corada M., Martin-Padura I., Motoike T., Tonetti P., Bazzoni G., Vermi W., Gentili F., Bernasconi S., Sato T.N., Mantovani A., Dejana E. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest. 2004;114:729–738. doi: 10.1172/JCI21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell P.O., Jensen J.S., Ritzenthaler J.D., Roman J., Pelaez A., Guidot D.M. Alcohol primes the airway for increased interleukin-13 signaling. Alcohol Clin Exp Res. 2009;33:505–513. doi: 10.1111/j.1530-0277.2008.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelaez A., Force S.D., Gal A.A., Neujahr D.C., Ramirez A.M., Naik P.M., Quintero D.A., Pileggi A.V., Easley K.A., Echeverry R., Lawrence E.C., Guidot D.M., Mitchell P.O. Receptor for advanced glycation end products in donor lungs is associated with primary graft dysfunction after lung transplantation. Am J Transplant. 2010;10:900–907. doi: 10.1111/j.1600-6143.2009.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoseph B.P., Breed E., Overgaard C.E., Ward C.J., Liang Z., Wagener M.E., Lexcen D.R., Lusczek E.R., Beilman G.J., Burd E.M., Farris A.B., Guidot D.M., Koval M., Ford M.L., Coopersmith C.M. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS One. 2013;8:e62792. doi: 10.1371/journal.pone.0062792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandell K.J., Babbin B.A., Nusrat A., Parkos C.A. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 41.Severson E.A., Lee W.Y., Capaldo C.T., Nusrat A., Parkos C.A. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–1925. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elias B.C., Mathew S., Srichai M.B., Palamuttam R., Bulus N., Mernaugh G., Singh A.B., Sanders C.R., Harris R.C., Pozzi A., Zent R. The integrin beta1 subunit regulates paracellular permeability of kidney proximal tubule cells. J Biol Chem. 2014;289:8532–8544. doi: 10.1074/jbc.M113.526509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas M., Woods C.R., Mora A.L., Xu J., Brigham K.L. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 44.Patarroyo M., Prieto J., Rincon J., Timonen T., Lundberg C., Lindbom L., Asjo B., Gahmberg C.G. Leukocyte-cell adhesion: a molecular process fundamental in leukocyte physiology. Immunol Rev. 1990;114:67–108. doi: 10.1111/j.1600-065x.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 45.Murakami M., Francavilla C., Torselli I., Corada M., Maddaluno L., Sica A., Matteoli G., Iliev I.D., Mantovani A., Rescigno M., Cavallaro U., Dejana E. Inactivation of junctional adhesion molecule-A enhances antitumoral immune response by promoting dendritic cell and T lymphocyte infiltration. Cancer Res. 2010;70:1759–1765. doi: 10.1158/0008-5472.CAN-09-1703. [DOI] [PubMed] [Google Scholar]
- 46.LaFemina M.J., Bentley T., Sutherland K., Rokkam D., Allen L., Dobbs L.G., Frank J. A role for lung-specific tight junction protein claudin-18 in alveolar epithelial barrier function. Am J Respir Crit Care Med. 2012;185:A6740. [Google Scholar]
- 47.Fanning A.S., Van Itallie C.M., Anderson J.M. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577–590. doi: 10.1091/mbc.E11-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita S., Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 49.Khounlotham M., Kim W., Peatman E., Nava P., Medina-Contreras O., Addis C., Koch S., Fournier B., Nusrat A., Denning T.L., Parkos C.A. Compromised intestinal epithelial barrier induces adaptive immune compensation that protects from colitis. Immunity. 2012;37:563–573. doi: 10.1016/j.immuni.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebnet K., Schulz C.U., Meyer Zu Brickwedde M.K., Pendl G.G., Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 51.Van Itallie C.M., Fanning A.S., Bridges A., Anderson J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan J., Li F., Ingram D.A., Quilliam L.A. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Mol Cell Biol. 2008;28:5803–5810. doi: 10.1128/MCB.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birukova A.A., Fu P., Xing J., Birukov K.G. Rap1 mediates protective effects of iloprost against ventilator induced lung injury. J Appl Physiol (1985) 2009;107:1900–1910. doi: 10.1152/japplphysiol.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanov A.I., Parkos C.A., Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lafemina M.J., Rokkam D., Chandrasena A., Pan J., Bajaj A., Johnson M., Frank J.A. Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L724–L734. doi: 10.1152/ajplung.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su G., Atakilit A., Li J.T., Wu N., Bhattacharya M., Zhu J., Shieh J.E., Li E., Chen R., Sun S., Su C.P., Sheppard D. Absence of integrin alphavbeta3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am J Respir Crit Care Med. 2012;185:58–66. doi: 10.1164/rccm.201108-1381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su G., Hodnett M., Wu N., Atakilit A., Kosinski C., Godzich M., Huang X.Z., Kim J.K., Frank J.A., Matthay M.A., Sheppard D., Pittet J.F. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol. 2007;36:377–386. doi: 10.1165/rcmb.2006-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tafazoli F., Holmstrom A., Forsberg A., Magnusson K.E. Apically exposed, tight junction-associated beta1-integrins allow binding and YopE-mediated perturbation of epithelial barriers by wild-type Yersinia bacteria. Infect Immun. 2000;68:5335–5343. doi: 10.1128/iai.68.9.5335-5343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peddibhotla S.S., Brinkmann B.F., Kummer D., Tuncay H., Nakayama M., Adams R.H., Gerke V., Ebnet K. Tetraspanin CD9 links junctional adhesion molecule-A to alphavbeta3 integrin to mediate basic fibroblast growth factor-specific angiogenic signaling. Mol Biol Cell. 2013;24:933–944. doi: 10.1091/mbc.E12-06-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J.W., Fang X., Gupta N., Serikov V., Matthay M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velasquez A., Bechara R.I., Lewis J.F., Malloy J., McCaig L., Brown L.A., Guidot D.M. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- 62.Cohen T.S., Gray Lawrence G., Margulies S.S. Cultured alveolar epithelial cells from septic rats mimic in vivo septic lung. PLoS One. 2010;5:e11322. doi: 10.1371/journal.pone.0011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coyne C.B., Vanhook M.K., Gambling T.M., Carson J.L., Boucher R.C., Johnson L.G. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]