Summary
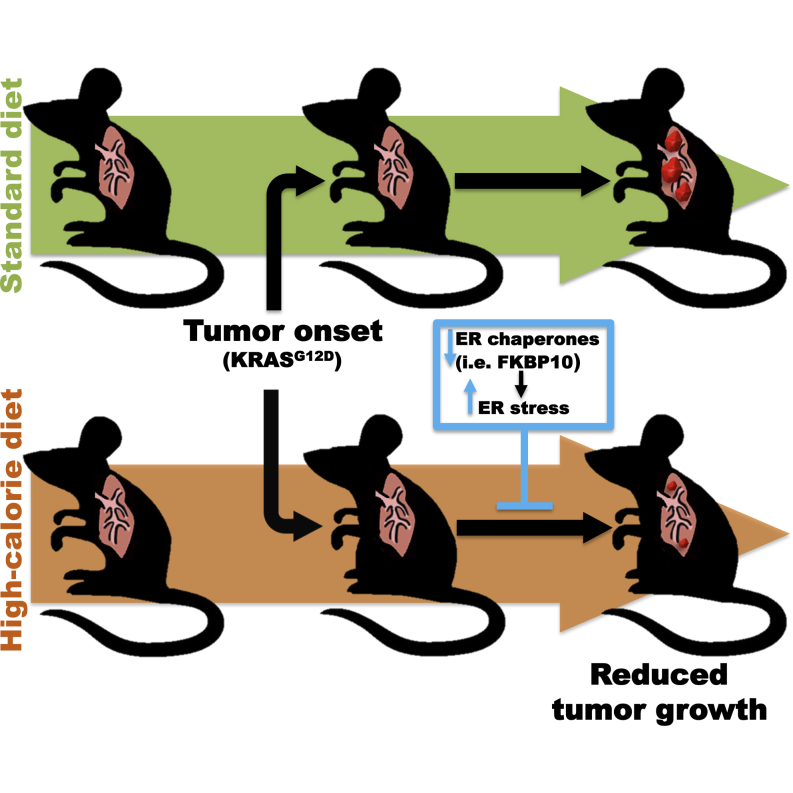
Dietary effects on tumor biology can be exploited to unravel cancer vulnerabilities. Here, we present surprising evidence for anti-proliferative action of high-calorie-diet (HCD) feeding on KRAS-driven lung tumors. Tumors of mice that commenced HCD feeding before tumor onset displayed defective unfolded protein response (UPR) and unresolved endoplasmic reticulum (ER) stress. Unresolved ER stress and reduced proliferation are reversed by chemical chaperone treatment. Whole-genome transcriptional analyses revealed FKBP10 as one of the most downregulated chaperones in tumors of the HCD-pre-tumor-onset group. FKBP10 downregulation dampens tumor growth in vitro and in vivo. Providing translational value to these results, we report that FKBP10 is expressed in human KRAS-positive and -negative lung cancers, but not in healthy parenchyma. Collectively, our data shed light on an unexpected anti-tumor action of HCD imposed before tumor onset and identify FKBP10 as a putative therapeutic target to selectively hinder lung cancer.
Graphical Abstract
Highlights
-
•
HCD imposed before tumor onset inhibits KRAS-driven lung tumorigenesis
-
•
HCD imposed before tumor onset causes unresolved ER stress in lung tumors
-
•
TUDCA or PBA treatment reverses the anti-tumor action of HCD
-
•
Downregulation of the chaperone FKBP10 inhibits tumor growth
Dietary effects on tumor biology can be exploited to unravel cancer vulnerabilities. Here, Ramadori et al. exploited the surprising anti-proliferative action of high-calorie-diet feeding on KRAS-driven lung tumors to unmask the chaperone FKBP10 as a putative therapeutic target to selectively hinder lung cancer.
Introduction
Feeding on a high-calorie diet (HCD) causes obesity and diabetes (Vianna and Coppari, 2011), which are known cancer risk factors (Bianchini et al., 2002; Calle and Kaaks, 2004; Calle and Thun, 2004; Khandekar et al., 2011). Thus, gaining a better understanding of the relationship between increased calorie intake and cancer is urgent, as it will provide insights into the mechanisms of cancer pathogenesis.
Increased anabolic signaling caused by HCD may explain higher cancer risk in obesity (Taubes, 2012). For example, HCD increases blood levels of anabolic citokines (e.g., insulin, insulin-like growth factors, and leptin) that are known to promote tumor growth and survival by signaling via the PI3K-AKT-mTOR and RAS-MAPK pathways (Banks et al., 2000; Engelman, 2009; Schubbert et al., 2007; Vansaun, 2013; Zoncu et al., 2011). Another well-established event associated with HCD is the increased inflammatory (e.g., TNF-α) signaling, which is also known to favor tumorigenesis (Park et al., 2010a).
Lung cancer is the leading cause of cancer-related death worldwide, with non-small cell lung cancer (NSCLC) representing almost 85% of all cases. Activating mutations of the proto-oncogene KRAS (mutant KRAS) occur in ∼30% of NSCLC cases (Pylayeva-Gupta et al., 2011). Mutant KRAS-driven tumors are associated with an aggressive phenotype, resistance to therapy, and poor outcome (Pao et al., 2005). The results from epidemiological studies assessing the effect of obesity on lung cancer risk are still inconclusive (Calle and Kaaks, 2004; Dahlberg et al., 2013; Leung et al., 2011; Yang et al., 2013).Yet, calorie restriction exerts a robust anti-tumor effect on KRAS-driven lung cancer in rodents (Kalaany and Sabatini, 2009), indicating that this type of tumor is sensitive to dietary changes. However, preclinical studies assessing KRAS-driven lung tumorigenesis in the context of increased calorie intake are missing. We reasoned that if HCD feeding interferes with KRAS-driven lung tumorigenesis, then the study of this contextual effect could be exploited to unmask key cancer vulnerabilities. Hence, we assessed the impact of HCD feeding on KRAS-driven lung tumors in mice.
Results
A Dual Effect of Chronic HCD Feeding on KRAS-Driven Lung Tumorigenesis
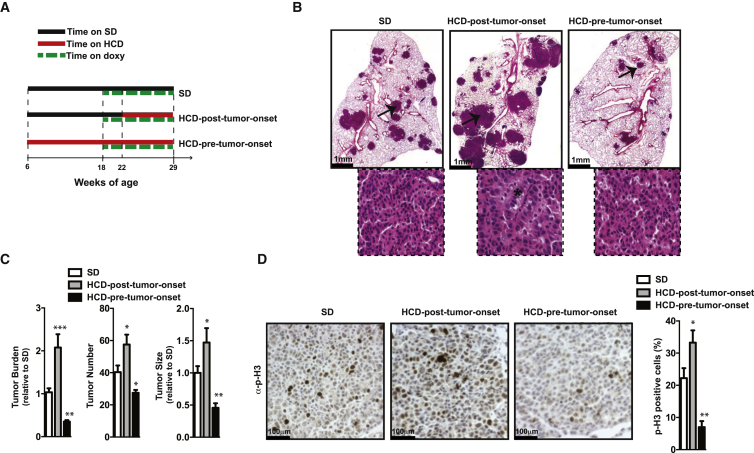
To comprehensively analyze the effect of HCD feeding on lung tumors in vivo, we fed genetically modified mice that develop KRAS-driven lung tumors (i.e., KrasG12D mice) with HCD starting before or after tumor onset. KrasG12D mice were obtained by breeding a transgene encoding KrasG12D under the control of the tetracycline operator (Tet-op-Kras) to a transgene expressing the reverse tetracycline transactivator in the respiratory epithelium under the control of the Clara cell secretory protein promoter (CCSP-rtTA). The resulting bi-transgenic KrasG12D mice develop lung cancer with 100% penetrance following continuous doxycycline (doxy) administration (Fisher et al., 2001). The effect of the different dietary treatments on lung tumors was assessed by comparing neoplastic lesion profiles between three groups: (i) KrasG12D mice constantly fed a standard diet (SD KrasG12D group), (ii) KrasG12D mice switched and maintained on a HCD starting 4 weeks after tumor induction (HCD-post-tumor-onset KrasG12D group), and (iii) KrasG12D mice switched and maintained on a HCD starting 12 weeks before tumor induction (HCD-pre-tumor-onset KrasG12D group) (Figure 1A). In all groups tumorigenesis was induced at the age of 18 weeks by doxy treatment, and endpoint analyses were performed at the age of 29 weeks (Figure 1A).
Figure 1.
A Dual Effect of Chronic HCD Feeding on KRAS-Driven Lung Tumorigenesis
(A) Timetable of mouse treatments.
(B) Representative images of lung sections stained with hematoxylin and eosin (H&E) (inserts indicate area selected by arrows for higher magnification images; asterisk indicates the presence of nests of cells with large atypical nuclei).
(C) Histograms showing quantification of tumor burden, size, and number.
(D) Representative images of lung sections stained with anti-p-H3 (p-H3-positive cells in dark brown) and histograms indicating the percentage of p-H3-positive cells/tumor cells.
Data shown in (B)–(D) are from 29-week-old KrasG12D mice treated as in (A) (females, n = 6–7/ group). Error bars represent SEM. Statistical analyses were done using one-way ANOVA (Tukey’s post test). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S1.
HCD-pre-tumor-onset KrasG12D mice (18 and 29 weeks old) had increased body weight, hyperinsulinemia, and hyperleptinemia, while these parameters were all normal in HCD-post-tumor-onset KrasG12D mice compared to age-matched SD KrasG12D mice. However, at the age of 29 weeks both HCD-fed groups displayed increased hepatic lipid accumulation (Figures S1A–S1C, available online).
Strikingly, endpoint analysis of the lungs revealed opposite effects of the two different HCD feeding regimens on neoplastic lesions. The HCD-post-tumor-onset KrasG12D mice had a significant increase in tumor burden owing to increased tumor size and number, while HCD-pre-tumor-onset KrasG12D mice had these parameters significantly reduced compared to those of SD KrasG12D mice (Figures 1B and 1C). Notably, the tumor burden-, size-, and number-suppressing effect of pretreatment with HCD applied to both genders (Figures 1B, 1C, and S1D).
Histopathological examination indicated that neoplastic lesions from all groups were adenomas (Figure 1B).Yet, lesions from HCD-post-tumor-onset KrasG12D mice were distinguished by the presence of nests of cells containing large atypical nuclei (Figure 1B), indicating a more advanced tumor grade.
Next, we set out to determine the underlying mechanism of HCD effect on tumor growth. As the tumor formation and maintenance in KrasG12D mice is strictly dependent on oncogenic KrasG12D expression (Fisher et al., 2001), we measured KrasG12D mRNA level in tumor lesions. This parameter was similar between groups (Figure S1E), hence ruling out the idea that the different tumor growth observed between groups was the consequence of dietary effects on KrasG12D expression.
Changes in tumor growth could be the result of altered cellular death, altered cellular proliferation, or both. Notably, while the status of apoptosis markers (assessed by TUNEL assay and immunostaining for cleaved caspase-3) was unchanged (Figures S1F and S1G), the expression levels of proliferation markers (p-H3 and cyclin-D1) were significantly reduced in tumors of HCD-pre-tumor-onset compared to SD KrasG12D mice (Figures 1D and S1H). Conversely, cellular proliferation was increased in HCD-post-tumor-onset compared to SD KrasG12D mice (Figure 1D). HCD feeding could also impact tumor initiation. For instance, inflammation caused by dietary obesity is an effective tumor promoter in a model of hepatocellular carcinoma (Park et al., 2010a). In addition, recent studies have shown that chronic pancreatitis is required for KRAS-induced pancreatic ductal adenocarcinomas in adult mice (Guerra et al., 2007). Therefore, nongenetic events associated with HCD feeding could modulate tumor initiation when the latter is driven by a defined oncogenic mutation including KRAS (Guerra et al., 2007; Park et al., 2010a). Notably, lungs of SD or HCD-pre-tumor-onset KrasG12D mice treated with doxy for 4 weeks had no differences in tumor size and multiplicity (Figure S1I). Combined, these findings indicate that HCD feeding imposed before tumor onset affects tumor progression rather than initiation.
Collectively, our results show that HCD feeding started before or after tumor onset can inhibit or promote KRAS-driven lung tumorigenesis by mechanisms that involve suppression or enhancement of cellular proliferation, respectively.
Chronic HCD Feeding Causes Unresolved ER Stress in KRAS-Driven Lung Tumors
We uncovered a surprising and potent anti-tumor effect of HCD imposed before tumor onset. This finding prompted us to look for the underlying mechanism because it could unveil key vulnerabilities of KRAS-driven lung tumors. To this end, we first analyzed the activation status of anabolic signaling pathways known to promote tumorigenesis and be altered in the context of increased calorie intake (i.e., AKT, ERK, and STAT3) (Banks et al., 2000; Engelman, 2009; Schubbert et al., 2007; Taubes, 2012; Vansaun, 2013; Zoncu et al., 2011). For instance, chronic HCD feeding induces systemic insulin resistance characterized by reduced insulin/AKT signaling in metabolically relevant tissues (Ramadori et al., 2011). Thus, reduced AKT signaling in tumors from HCD-pre-tumor-onset KrasG12D mice could in principle explain the reduced neoplastic growth. However, while we found reduced insulin-induced phosphorylation of AKT, the basal level of phosphorylated AKT and of one of its downstream targets ribosomal protein S6 was similar between tumors of HCD-pre-tumor-onset and SD KrasG12D mice (Figures S2A and S2B). These data suggest that exacerbated insulin resistance in tumors of HCD-pre-tumor-onset KrasG12D mice is not sufficient to dampen basal AKT signaling. Also, the status of phosphorylation and thus activation of ERK and STAT3 signaling was unchanged in HCD-pre-tumor-onset compared to SD KrasG12D mice (Figure S2B).
An expected consequence of chronic HCD feeding is increased inflammatory signaling. Indeed, we found that the serum level of the proinflammatory cytokine interleukin-6 (IL-6) and the mRNA level of intracellular inflammatory readouts (e.g., Ik-Βα and Tnf-α) were elevated in tumors of HCD-pre-tumor-onset compared to SD KrasG12D mice (Figures S2C–S2E). Collectively, the aforementioned results suggest that changes in basal AKT, ERK, STAT3, or inflammatory signaling are likely not underlying the antiproliferative effects of nutritional overload on KRAS-driven lung tumors.
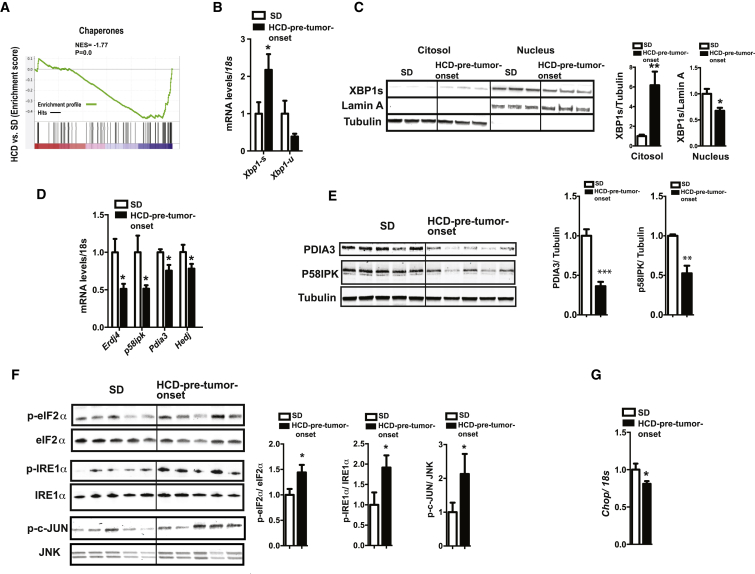
Next, we assessed whole-genome expression profile of microdissected lung tumors and found a significantly reduced expression of chaperones in lung tumors of HCD-pre-tumor-onset compared to SD KrasG12D mice (Figures 2A and S2F). Several of these chaperones are established or predicted ER residents. ER chaperones are essential for normal protein folding and key components of the ER-induced UPR (UPRER), a cellular mechanism limiting the degree of ER stress due to the accumulation of unfolded proteins in the ER (Walter and Ron, 2011). Thus, we hypothesized that tumor cells in HCD-pre-tumor-onset KrasG12D mice may be unable to mount an effective UPRER. To directly address this idea, we further characterized the status of the UPRER in tumors of these mice. X-box binding protein 1 (XBP1) is an essential component of the UPR, and in response to ER stress it is spliced to produce the nuclear-localized active transcription factor XBP1s (Walter and Ron, 2011). Similarly, chronic HCD feeding induced Xbp1 mRNA splicing in lung tumor tissue of HCD-pre-tumor-onset KrasG12D, as indicated by increased and decreased Xbp1s and Xbp1-unspliced (Xbp1-u) mRNAs, respectively (Figure 2B). However, despite the increased amount of cytosolic XBP1s, the amount of nuclear XBP1s was lower in lung tumors of HCD-pre-tumor-onset compared to SD KrasG12D mice (Figure 2C). This result is in line with the reduced mRNA level of the ER chaperones Erdj4, p58ipk, Pdia3, and Hedj (which are known transcriptional targets of XBP1s) in tumors of HCD-pre-tumor-onset compared to SD-fed KrasG12D mice (Figure 2D). Consistently, protein content of the same chaperones was also reduced (Figure 2E). Collectively, these results indicate a defective UPRER in tumors of HCD-pre-tumor-onset mice.
Figure 2.
Unresolved ER Stress in Lung Tumors of HCD-Pre-Tumor-Onset KrasG12D Mice
(A) Gene set enrichment analysis (GSEA) plot showing chaperone enrichment score depicted by NES and nominal p value (the gene set used for GSEA is GO: 0006457).
(B) Xbp1s and Xbp1u mRNA levels.
(C) Immunoblot images and quantifications of cytosolic and nuclear XBP1 protein content (Lamin A and Tubulin were used as nuclear and cytosolic markers, respectively).
(D) mRNA levels of ER chaperones.
(E) Immunoblot images and quantification of ER chaperone protein content in total cell lysate.
(F) Immunoblot images and quantification of the phosphorylation status of eIF2α, IRE1α, and c-JUN.
(G) Chop mRNA levels.
All panels show data from microdissected lung tumors of 29-week-old KrasG12D males treated as described in Figure 1A (n = 5–6/group). Error bars represent SEM. Statistical analyses were done using two-tailed unpaired Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S2.
To independently assay ER stress level, we measured the status of several ER stress transducers. Our data show that the phosphorylation level of eukaryotic translational initiation factor 2α (eIF2α), of ER transmembrane kinase/endoribonuclease (RNase) IRE1α, and activation of c-Jun N-terminal kinase (JNK), all of which are found to be increased upon ER stress (Ozcan et al., 2004; Ozcan et al., 2006), were significantly higher in tumors of HCD-pre-tumor-onset compared to SD-fed KrasG12D mice (Figure 2F). Notably, increased p-eIF2α is expected to lead to cell-cycle attenuation or even arrest in proliferating cells (Ranganathan et al., 2008; Ron, 2002), a result consistent with reduced tumor proliferation in HCD-pre-tumor-onset KrasG12D mice (Figures 1D and S1H).
Interestingly, mRNA level of another component of the UPRER, and a major component of the ER stress-mediated apoptosis pathway, C/EBP homologous protein (CHOP) was downregulated in tumors of HCD-pre-tumor-onset compared to SD KrasG12D mice (Figure 2G). These data are consistent with reduced expression of death receptor-5 (Dr5) (Figure S2G), a transcriptional target of CHOP and an important mediator of ER-stress-mediated apoptosis (Lu et al., 2014). CHOP regulates ER-stress-induced apoptosis also by repressing Bcl2 gene expression (Szegezdi et al., 2006) whose expression was similar in tumors of the different dietary groups (Figure S2G), another result that does not support CHOP-induced activation of apoptotic signaling. We suggest that reduced Chop expression may explain, at least in part, why unresolved ER stress does not lead to apoptosis in tumors of HCD-pre-tumor-onset KrasG12D mice.
Healthy lung tissue (tumor free) from HCD-pre-tumor-onset KrasG12D mice had increased ER stress compared to SD controls, as determined by Xbp1s, p-eIF2α, and p-IRE1α level (Figures S2H and S2I). However, the increased nuclear accumulation of XBP1s and the increased expression of ER chaperones and Chop (Figures S2J–S2L) indicated normal UPRER activation in this tissue. Notably, the levels of the aforementioned ER stress and UPRER activation markers and XBP1s protein contents were not different between tumors from HCD-post-tumor-onset and SD KrasG12D mice (Figures S2M–S2Q). Altogether, our data indicate a tumor-specific defect in ER chaperone expression in lungs of KrasG12D mice that strongly depends on the time HCD feeding is initiated relative to tumor onset.
Chaperones are also essential for the mitochondrial-induced UPR (UPRmt), a response aimed at maintaining proper protein folding in the mitochondrial matrix (Pellegrino et al., 2013). UPRmt is activated when mitochondrial protein balance is disrupted and is associated with mitochondrial and nuclear gene expression imbalance (Mouchiroud et al., 2013; Pellegrino et al., 2013). Our data show reduced expression of mitochondrial matrix chaperones in tumors of HCD-pre-tumor-onset KrasG12D mice compared to their lean controls (Figure S2R). However, no changes in the ratio between nuclear DNA-encoded Atp5a1 and mitochondrial DNA-encoded Mtco1 (Figure S2S) were observed. Moreover, level of protein carbonylation and expression of detoxification enzymes catalase (Cat) and superoxide dismutase 2 (Sod2) did not differ between tumors from different dietary groups (Figures S2T and S2U), indicating a similar level of reactive oxygen species. These results suggest that reduced expression of mitochondrial chaperones in tumors of HCD-pre-tumor-onset compared to SD KrasG12D mice did not cause significant changes in UPRmt and mitochondrial function; hence, it is not likely to be the reason for the anti-tumor effect of HCD feeding.
Collectively, our data indicate that HCD feeding started before tumor onset causes impaired UPRER response and consequentially unresolved ER stress in KRAS-driven lung tumors.
Treatment with Chemical Chaperones Reverses the Anti-Tumor Effect of HCD Feeding on KRAS-Driven Lung Tumors
Our results prompted us to directly test whether the anti-tumor action of HCD on KRAS-driven lung tumors is the result of unresolved ER stress. If unresolved ER stress underlies the antiproliferative effect of HCD feeding, its rescue in HCD-pre-tumor-onset KrasG12D mice should boost tumor growth to a level similar to that of SD KrasG12D mice. Administration of chemical chaperones, such as taurorsodeoxycholic acid (TUDCA) or 4-phenyl butyric acid (4-PBA), resolves ER stress induced by increased calorie intake (Ozcan et al., 2006). Thus, we tested whether treatment with TUDCA or 4-PBA reverses the anti-tumor action of HCD on Kras-driven lung tumors.
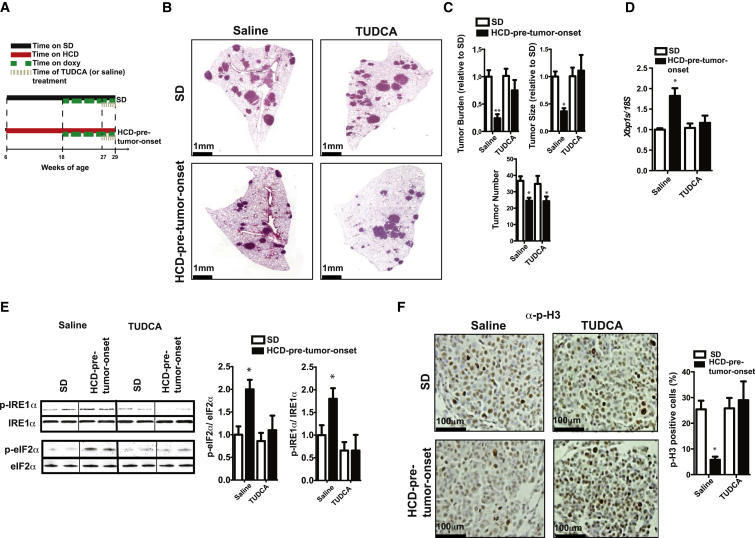
During the last 2 weeks of doxy treatment, HCD-pre-tumor-onset KrasG12D mice were additionally treated daily with TUDCA (or saline) (Figure 3A). While tumor burden was again significantly reduced in saline-injected HCD-pre-tumor-onset compared to SD KrasG12D mice, it was comparable between these groups following TUDCA administration (Figures 3B and 3C). The increased tumor burden in TUDCA-injected HCD-pre-tumor-onset KrasG12D mice was the result of increased tumor size, but not tumor number (Figure 3C). Tumor burden, number, and size were not affected by TUDCA administration in SD KrasG12D mice (Figures 3B and 3C). Pharmacokinetic assessments revealed a similar level of TUDCA accumulation in lung tumors between the different dietary groups (Figures S3A and S3B). Moreover, the treatment of HCD-pre-tumor-onset KrasG12D mice with TUDCA resulted in attenuation of ER stress to a level similar to that of SD KrasG12D mice, as evidenced by expression of ER stress markers (Figures 3D, 3E, and S3C). These effects were not secondary to changes in body weight, insulinemia, and leptinemia, as these parameters were not different between TUDCA-treated and saline-treated HCD-pre-tumor-onset KrasG12D mice (data not shown). In line with the fact that chronic HCD feeding caused reduced cellular proliferation (Figures 1D and S1H), TUDCA reversed the antiproliferative effect of chronic HCD pretreatment on KRAS-driven lung tumors, as indicated by increased expression of p-H3 (Figure 3F). Notably, the tumor-proliferating effect of TUDCA was not associated with changes in mitochondrial:nuclear gene expression ratio (Figure S3D) or in protein carbonylation level (Figure S3E).
Figure 3.
TUDCA Treatment Reverses the Anti-Tumor Effect of HCD Feeding on KRAS-Driven Lung Tumors
(A) Timetable of mouse treatments.
(B) Representative images of lung sections stained with H&E.
(C) Histograms indicating quantification of tumor burden, size, and number.
(D) mRNA levels of Xbp1s.
(E) Representative immunoblot against phospho- and total IRE1α and eIF2α.
(F) Representative images of lung sections stained with anti-p-H3 (p-H3-positive cells in brown) and histograms indicating the percentage of p-H3-positive cells/tumor cells of microdissected tumors.
Data shown in (B)–(F) are from 29-week-old KrasG12D mice treated as indicated in (A) (females, n = 5/group). Error bars represent SEM. Statistical analyses were done using one-way ANOVA (Tukey’s post test). ∗p < 0.05, ∗∗p < 0.01. See also Figure S3.
Similar treatment with another structurally unrelated chemical chaperone 4-PBA (Figure S3F) essentially reproduced the effect of TUDCA by relieving ER stress and partially rescuing proliferation and growth of tumors of HCD-pre-tumor-onset KrasG12D mice (Figures S3G–S3I and data not shown).
Altogether, our data indicate that chronic HCD feeding impairs UPRER and consequently causes unresolved ER stress that hinders the growth of KrasG12D-driven lung tumors.
Unraveling FKBP10 as a Target for KRAS-Driven Lung Cancer
Our data suggest that the inhibition of ER chaperones whose expression is negatively affected by HCD pretreatment may represent an effective antiproliferative therapeutic avenue against KRAS-driven lung cancer. A major drawback of current treatments against KRAS-driven lung cancer is the toxic side effects of the drugs due to the lack of selectivity between cancer and normal cells. Therefore, we reasoned that an ideal target against KRAS-driven lung cancer could be an ER chaperone expressed in tumor lesions but not healthy parenchyma.
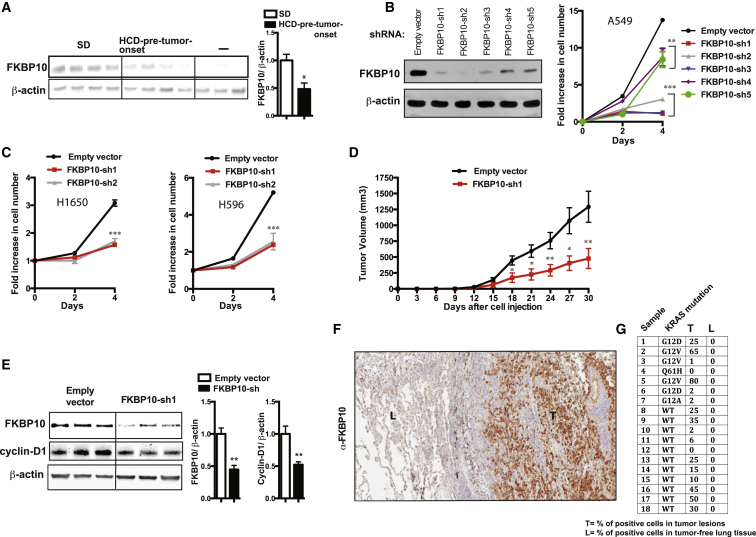
In order to determine a candidate target, we analyzed our microarray data searching for chaperones that are (i) a resident of the ER, (ii) significantly downregulated in tumors of HCD-pre-tumor-onset KrasG12D mice compared to SD controls, and (iii) specifically expressed in tumor lesions. We found several ER chaperones downregulated in tumors of HCD-pre-tumor-onset KrasG12D mice (Figure S2F). One of them, namely FKBP10, has been reported not to be expressed in lung during adulthood (Lietman et al., 2014; Patterson et al., 2000). Our data indicate that FKBP10 is (i) expressed in tumors of KrasG12D mice, (ii) reduced in tumors of HCD-pre-tumor-onset mice compared to their SD controls, and (iii) barely detectable in normal lung parenchyma (Figure 4A). Therefore, FKBP10 could be an ideal cancer-selective target against Kras-driven lung tumorigenesis. To our knowledge, the role of FKBP10 in tumorigenesis is unknown.
Figure 4.
Unraveling FKBP10 as a Therapeutic Target for KRAS-Driven Lung Cancer
(A) Expression of FKBP10 in lung tumors of 29-week-old KrasG12D mice treated as in Figure 1A (males, n = 5–6/group) (as negative control [−], we loaded a similar amount of lysate from wild-type lung tissue).
(B) Knockdown in A549 cells (KRAS mutation) of FKBP10 using five different shRNAs and relative proliferation curves.
(C) Antiproliferative effect of FKBP10 knockdown in H1650 (EGFR mutation) and A596 (PI3K mutation) cell lines.
(D) Xenograft growth of A549 cells in SCID mice injected subcutaneously with 1 × 106 cells as indicated (n = 5 for group).
(E) Immunoblot from tumors of mice treated as indicated in (D).
(F) Representative images of a human lung section stained against FKBP10 (“L” indicates tumor-free lung parenchyma; “T” indicates lung tumor lesion).
(G) Table indicating the expression of FKBP10 in human specimens including tumor lesions and adjacent tumor-free tissue. Error bars represent SEM. Statistical analyses were done using two-tailed unpaired Student’s t test or using one-way ANOVA (Tukey’s post test). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S4.
Next, we assessed the significance of FKBP10 in human lung cancer cell lines and found that it is required for proliferation of lines bearing KRAS mutation or mutations different from KRAS (i.e., EGFR or PI3K oncogenic mutations) (Figures 4B and 4C). Our in vivo data also show that knockdown of FKBP10 is sufficient to hinder tumor growth (Figure 4D). This anti-tumor effect is caused by reduced cellular proliferation, as determined by reduced cyclin-D1 and p-H3 (Figures 4E and S4A), and not an increased rate of apoptosis (Figure S4B). Notably, our data indicate that the antiproliferative effect of FKBP10 knockdown is not restricted to lung cancer cell lines but extends to cancer cells derived from other tissues (i.e., breast and pancreas) (Figures S4C and S4D).
To add translational value to our preclinical studies, we assessed expression of FKBP10 in surgically collected human lung samples containing tumor lesions and healthy parenchyma. Importantly, our results indicate that FKBP10 is expressed in KRAS-positive and -negative lung carcinoma, but not in healthy lung parenchyma (Figures 4F and 4G). Collectively, our data suggest that inhibition of FKBP10 is a promising avenue to selectively hinder lung cancer growth while sparing healthy lung tissue function.
Discussion
Our data challenge the common wisdom that HCD feeding exerts only deleterious effects. In fact, we demonstrate that if HCD starts before tumor onset it dampens growth of KRAS-driven lung tumors (Figures 1A–1C). However, an opposite outcome is caused by HCD starting after tumor onset (Figures 1A–1C). Hence, the anti-tumor effect is not due to the diet per se but depends on when HCD feeding is initiated relative to tumor onset.
Our results indicate that impaired UPRER underlies, at least in part, the anti-tumor action of HCD imposed before tumor onset. We suggest that a contributor to impaired UPRER is the poor cytosol-to-nucleus translocation of XBP1s, a defect consistent with insulin resistance in tumors of HCD-pre-tumor-onset compared to SD KrasG12D mice (Figure S2A) (Lee et al., 2011; Park et al., 2010b, 2014). Additional studies are needed to test this possibility.
Why does HCD feeding imposed before tumor onset hinder tumor progression but not tumor initiation (Figures 1B, 1C, and S1I)? We propose that this could be explained, at least in part, by an effect of the HCD on tumor vascularization, as CD31-positive cells were found to be diminished in tumors of HCD-pre-tumor-onset mice compared to the SD KrasG12D mice (G.R., G.K., and R.C. unpublished data). In fact, this difference may not lead to relevant effects at the initial stage of the tumorigenesis (when tumor lesions are sufficiently vascularized by the vessels in the parenchyma) but may become a limiting factor for further tumor progression, a hypothesis that warrants future experimental testing.
The clinical relevance of our results is at least 2-fold. First, our data suggest that inhibition of chaperones downregulated by HCD pretreatment (e.g., FKBP10) may reduce KRAS-driven lung tumor growth. Second, our data suggest that TUDCA or PBA treatment improves ER stress, therefore leading to increased growth of already-formed KRAS-driven lung tumors in the context of obesity. Because TUDCA and PBA are currently undergoing evaluation (http://clinicaltrials.gov identifiers: NCT00771901, NCT01877551) on insulin-resistant subjects (a population typically also affected by increased body adiposity), our results caution for evaluation of cancer risk in these patients.
It is important to note that we have studied the effects of relatively short-term HCD on KRAS-driven lung tumors. Thus, long-term outcomes and the contribution of obesity and/or other metabolic defects caused by HCD to tumor behavior remain to be evaluated. Also, while our pharmacological data shown in Figures 3A–3F and S3A–S3I suggest that unresolved ER stress underlies reduced tumor growth in HCD-pre-tumor-onset KrasG12D mice, further genetic studies will be needed to fully dissect the underpinning mechanisms. Lastly, our data warrant thorough epidemiological assessment of lung cancer risk in obese people.
In summary, we unveiled an anti-tumor effect of HCD feeding on KRAS-driven lung tumorigenesis. Unresolved ER stress owing to impaired UPRER underlies, at least in part, the anti-tumor action of HCD. Hence, we propose that this vulnerability of KRAS-driven lung tumors could be targeted for therapeutic purposes. Importantly, we also identified FKBP10 as a putative anti-cancer target.
Experimental Procedures
Mouse Generation and Studies
CCSP-rtTA/Tet-op-K-ras mice (FVB/SV129 mixed background) were generated as previously described (Konstantinidou et al., 2013) and housed in groups of 4–5 with food (either SD or HCD; D12331 from Research Diets) and water available ad libitum in light- and temperature-controlled environments. Drinking water was supplemented with doxycycline at the concentration of 200 μg/ml. TUDCA was administered by intraperitoneal injection (250 mg/kg at 8 a.m. and 250 mg/kg at 8 p.m.). 4-PBA was administered at the same time points (500 mg/kg) by oral gavage. Care of mice at UTSW Medical Center at Dallas and at University of Geneva was within the procedures approved by Institutional Animal Care and Use Committee and by the animal care and experimentation authorities of the Canton of Geneva, respectively. Xenograft experiments were performed by subcutaneous inoculation of cells into 8-week-old male SCID mice.
Immunoblotting, Immunohistochemistry, and Quantitative Real-Time PCR
These procedures were performed as previously described (Konstantinidou et al., 2013). Clinical data were collected at Università Politecnica delle Marche from samples from patients who signed a waiver of authorization.
Expression Profiling
Gene expression profiles were obtained from microdissected lung tumors using MouseWG-6 v2.0 Expression BeadChips (Illumina).
shRNAs, Virus Production, and Transduction of Cell Lines
pLKO vectors (Open Biosystems) were used to produce recombinant lentiviruses using TransIT-293T Mirus reagent following manufacturer’s instructions. Human cell lines A549, H596, H1650, ASPC, and MDA-MB231 were transduced as previously described (Konstantinidou et al., 2013).
Carbonylated Protein Determination
Levels of carbonylated proteins were determined by ELISA (Enzo Life Sciences) following manufacturer’s instructions.
Pharmacokinetics Assessments
Analytical assay of plasma, lung, and tumor samples was performed using a QTRAP 4000 LC-MS/MS system.
Statistical Analysis
Two-tail unpaired Student’s t test or one-way ANOVA (Tukey’s post test) was used when comparing two groups or more than two groups, respectively. Error bars represent SEM.
Author Contributions
G.R., G.K., P.P.S., and R.C. conceived the study and wrote the paper. G.R., G.K., N.V., T.B., L.M., M.G., N.S.W., M.L., and A.S. performed the experiments and analyzed and interpreted the data.
Acknowledgments
We thank Ariane Widmer, Anne Charollais, Carolyn Heckenmeyer, and Laurent Vinet for technical support; Drs. Claes Wollheim and Pedro Herrera (UNIGE) for critical reading of the manuscript; and Dr. James Richardson (UTSW) for pathological examination of the lungs. This work was supported by CPRIT RP101496 (G.K.), ACS Award 13-068-01-TBG and Lung Cancer SPORE P50CA70907 (P.P.S.), by European Commission (Marie Curie CIG 320898 and ERC-Consolidator 614847), and by Swiss National Science Foundation (310030_146533/1) (R.C.). This work has also received the unrestricted support of the Louis-Jeantet Foundation (R.C.).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Contributor Information
Pier Paolo Scaglioni, Email: pier.scaglioni@utsouthwestern.edu.
Roberto Coppari, Email: roberto.coppari@unige.ch.
Accession Numbers
Data have been deposited in the NCBI Gene Expression Omnibus and are accessible through the accession number GSE56260.
Supplemental Information
References
- Banks A.S., Davis S.M., Bates S.H., Myers M.G., Jr. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- Bianchini F., Kaaks R., Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- Calle E.E., Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Calle E.E., Thun M.J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- Dahlberg S.E., Schiller J.H., Bonomi P.B., Sandler A.B., Brahmer J.R., Ramalingam S.S., Johnson D.H. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J. Thorac. Oncol. 2013;8:1121–1127. doi: 10.1097/JTO.0b013e31829cf942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman J.A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Fisher G.H., Wellen S.L., Klimstra D., Lenczowski J.M., Tichelaar J.W., Lizak M.J., Whitsett J.A., Koretsky A., Varmus H.E. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C., Schuhmacher A.J., Cañamero M., Grippo P.J., Verdaguer L., Pérez-Gallego L., Dubus P., Sandgren E.P., Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Kalaany N.Y., Sabatini D.M. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar M.J., Cohen P., Spiegelman B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- Konstantinidou G., Ramadori G., Torti F., Kangasniemi K., Ramirez R.E., Cai Y., Behrens C., Dellinger M.T., Brekken R.A., Wistuba I.I. RHOA-FAK is a required signaling axis for the maintenance of KRAS-driven lung adenocarcinomas. Cancer Discov. 2013;3:444–457. doi: 10.1158/2159-8290.CD-12-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Sun C., Zhou Y., Lee J., Gokalp D., Herrema H., Park S.W., Davis R.J., Ozcan U. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat. Med. 2011;17:1251–1260. doi: 10.1038/nm.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.C., Lam T.H., Yew W.W., Chan W.M., Law W.S., Tam C.M. Lower lung cancer mortality in obesity. Int. J. Epidemiol. 2011;40:174–182. doi: 10.1093/ije/dyq134. [DOI] [PubMed] [Google Scholar]
- Lietman C.D., Rajagopal A., Homan E.P., Munivez E., Jiang M.M., Bertin T.K., Chen Y., Hicks J., Weis M., Eyre D. Connective tissue alterations in Fkbp10-/- mice. Hum. Mol. Genet. 2014;23:4822–4831. doi: 10.1093/hmg/ddu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Lawrence D.A., Marsters S., Acosta-Alvear D., Kimmig P., Mendez A.S., Paton A.W., Paton J.C., Walter P., Ashkenazi A. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Cantó C., Mottis A., Jo Y.S., Viswanathan M., Schoonjans K. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Görgün C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O., Görgün C.Z., Hotamisligil G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W., Wang T.Y., Riely G.J., Miller V.A., Pan Q., Ladanyi M., Zakowski M.F., Heelan R.T., Kris M.G., Varmus H.E. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.J., Lee J.H., Yu G.Y., He G., Ali S.R., Holzer R.G., Osterreicher C.H., Takahashi H., Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.W., Zhou Y., Lee J., Lu A., Sun C., Chung J., Ueki K., Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.W., Herrema H., Salazar M., Cakir I., Cabi S., Basibuyuk Sahin F., Chiu Y.H., Cantley L.C., Ozcan U. BRD7 regulates XBP1s’ activity and glucose homeostasis through its interaction with the regulatory subunits of PI3K. Cell Metab. 2014;20:73–84. doi: 10.1016/j.cmet.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C.E., Schaub T., Coleman E.J., Davis E.C. Developmental regulation of FKBP65. An ER-localized extracellular matrix binding-protein. Mol. Biol. Cell. 2000;11:3925–3935. doi: 10.1091/mbc.11.11.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M.W., Nargund A.M., Haynes C.M. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta. 2013;1833:410–416. doi: 10.1016/j.bbamcr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y., Grabocka E., Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat. Rev. Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Fujikawa T., Anderson J., Berglund E.D., Frazao R., Michán S., Vianna C.R., Sinclair D.A., Elias C.F., Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan A.C., Ojha S., Kourtidis A., Conklin D.S., Aguirre-Ghiso J.A. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res. 2008;68:3260–3268. doi: 10.1158/0008-5472.CAN-07-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Translational control in the endoplasmic reticulum stress response. J. Clin. Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S., Shannon K., Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Szegezdi E., Logue S.E., Gorman A.M., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubes G. Cancer research. Unraveling the obesity-cancer connection. Science. 2012;335:28–30–32. doi: 10.1126/science.335.6064.28. [DOI] [PubMed] [Google Scholar]
- Vansaun M.N. Molecular pathways: adiponectin and leptin signaling in cancer. Clin. Cancer Res. 2013;19:1926–1932. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna C.R., Coppari R. A treasure trove of hypothalamic neurocircuitries governing body weight homeostasis. Endocrinology. 2011;152:11–18. doi: 10.1210/en.2010-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Yang Y., Dong J., Sun K., Zhao L., Zhao F., Wang L., Jiao Y. Obesity and incidence of lung cancer: a meta-analysis. Int. J. Cancer. 2013;132:1162–1169. doi: 10.1002/ijc.27719. [DOI] [PubMed] [Google Scholar]
- Zoncu R., Efeyan A., Sabatini D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.