Abstract
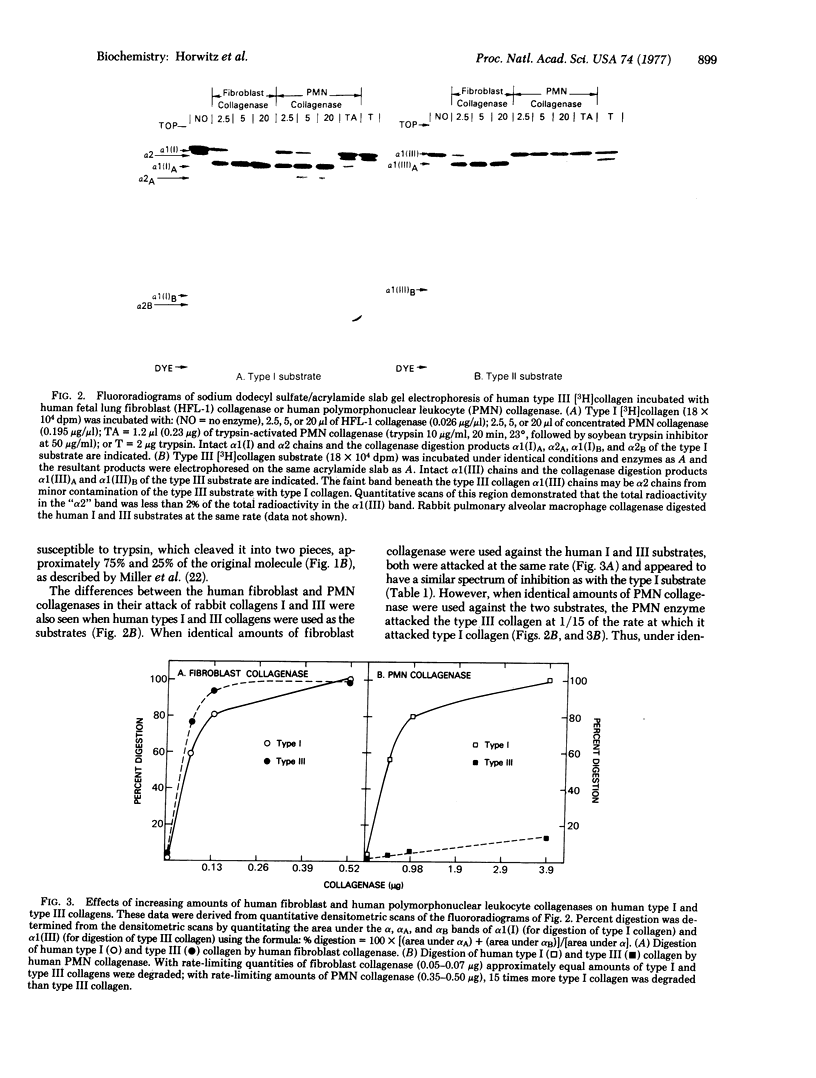
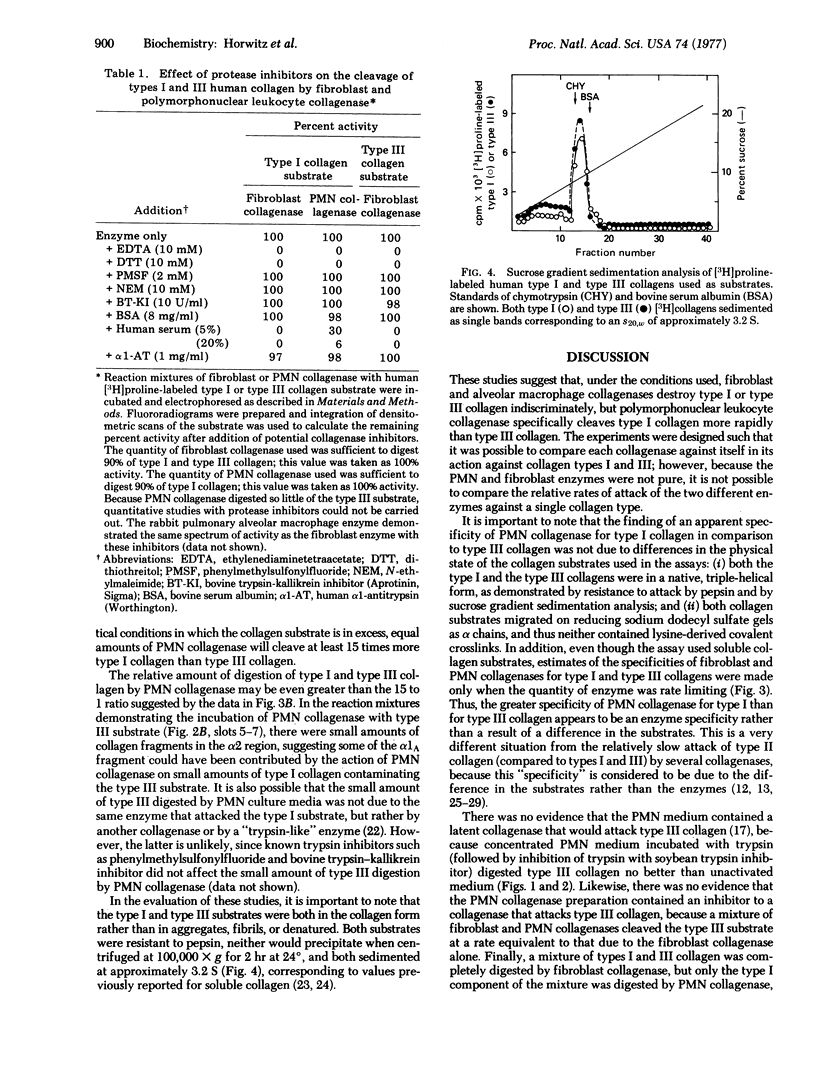
Collagenases produced by human polymorphonuclear leukocytes, human lung fibroblasts, and rabbit pulmonary alveolar macrophages were compared in their ability to digest soluble native type I and type III collagens. While the fibroblast and macrophage collagenases attacked the two substrates at approximately equal rates, the leukocyte collagenase attacked type I collagen preferentially (15:1) in comparison to type III collagen. This was true with human or rabbit collagen substrates. Thus, proteolysis of collagen, particularly in acute inflammation, may have a significant role in controlling the types of collagen present in connective tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Bazin S., Sims T. J., Le Lous M., Nicoletis C., Delaunay A. Characterization of the collagen of human hypertrophic and normal scars. Biochim Biophys Acta. 1975 Oct 20;405(2):412–421. doi: 10.1016/0005-2795(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Sims T. J., Le Lous, bazin S. Collagen polymorphism in experimental granulation tissue. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1160–1165. doi: 10.1016/0006-291x(75)90480-5. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Stricklin G. P., Jeffrey J. J., Eisen A. Z. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Epstein E. H., Jr, Munderloh N. H. Isolation and characterization of CNBr peptides of human (alpha 1 (III) )3 collagen and tissue distribution of (alpha 1 (I) )2 alpha 2 and (alpha 1 (III) )3 collagens. J Biol Chem. 1975 Dec 25;250(24):9304–9312. [PubMed] [Google Scholar]
- Hance A. J., Bradley K., Crystal R. G. Lung collagen heterogeneity. Synthesis of type I and type III collagen by rabbit and human lung cells in culture. J Clin Invest. 1976 Jan;57(1):102–111. doi: 10.1172/JCI108250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Faulkner C. S., 2nd, Brown F. E. Collagenolytic systems in rheumatoid arthritis. Clin Orthop Relat Res. 1975 Jul-Aug;(110):303–316. doi: 10.1097/00003086-197507000-00041. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Cartilage collagen: substrate in soluble and fibrillar form for rheumatoid collagenase. Trans Assoc Am Physicians. 1973;86:82–94. [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Horwitz A. L., Crystal R. G. Collagenase from rabbit pulmonary alveolar macrophages. Biochem Biophys Res Commun. 1976 Mar 22;69(2):296–303. doi: 10.1016/0006-291x(76)90521-0. [DOI] [PubMed] [Google Scholar]
- KUEHN K., ENGEL J., ZIMMERMANN B., GRASSMANN W. RENATURATION OF SOLUBLE COLLAGEN. 3. REORGANIZATION OF NATIVE COLLAGEN MOLECULES FROM COMPLETELY SEPARATED UNITS. Arch Biochem Biophys. 1964 May;105:387–403. doi: 10.1016/0003-9861(64)90023-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Brown R. S., Bladen H. A., Fullmer H. M. Degradation of collagen by a human granulocyte collagenolytic system. J Clin Invest. 1968 Dec;47(12):2622–2629. doi: 10.1172/JCI105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery P. A., Richards J. F., Harris E. D., Jr Purification and characterization of a collagenase extracted from rabbit tumours. Biochem J. 1975 Oct;152(1):131–142. doi: 10.1042/bj1520131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh K. A., Balian G. Collagen characterisation and cell transformation in human atherosclerosis. Nature. 1975 Nov 6;258(5530):73–75. doi: 10.1038/258073a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Finch J. E., Jr, Chung E., Butler W. T., Robertson P. B. Specific cleavage of the native type III collagen molecule with trypsin. Similarity of the cleavage products to collagenase-produced fragments and primary structure at the cleavage site. Arch Biochem Biophys. 1976 Apr;173(2):631–637. doi: 10.1016/0003-9861(76)90300-3. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Harris E. D., Jr, Chung E., Finch J. E., Jr, McCroskery P. A., Butler W. T. Cleavage of Type II and III collagens with mammalian collagenase: site of cleavage and primary structure at the NH2-terminal portion of the smaller fragment released from both collagens. Biochemistry. 1976 Feb 24;15(4):787–792. doi: 10.1021/bi00649a009. [DOI] [PubMed] [Google Scholar]
- Oronsky A. L., Perper R. J., Schroder H. C. Phagocytic release and activation of human leukocyte procollagenase. Nature. 1973 Dec 14;246(5433):417–419. doi: 10.1038/246417a0. [DOI] [PubMed] [Google Scholar]
- RICE R. V., CASASSA E. F., KERWIN R. E., MASER M. D. ON THE LENGTH AND MOLECULAR WEIGHT OF TROPOCOLLAGEN FROM CALF SKIN. Arch Biochem Biophys. 1964 May;105:409–423. doi: 10.1016/0003-9861(64)90025-6. [DOI] [PubMed] [Google Scholar]
- Robertson P. B., Miller E. J. Cartilage collagen: inability to serve as a substrate for collagenases active against skin and bone collagen. Biochim Biophys Acta. 1972 Nov 10;289(1):247–250. doi: 10.1016/0005-2744(72)90129-5. [DOI] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J Clin Invest. 1976 Jun;57(6):1498–1507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L. Changes in guinea-pig dermal collagen during development. Eur J Biochem. 1975 Jul 1;55(2):391–395. doi: 10.1111/j.1432-1033.1975.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L., Jackson D. S. Comparison of the cyanogen bromide peptides of insoluble guinea-pig skin and scar collagen. Biochim Biophys Acta. 1975 Jan 30;379(1):207–216. doi: 10.1016/0005-2795(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Vinson W. C., Seyer J. M. Synthesis of type 3 collagen by embryonic chick skin. Biochem Biophys Res Commun. 1974 May 7;58(1):58–65. doi: 10.1016/0006-291x(74)90890-0. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Lindberg K. A., Bailey A. J., Evanson J. M. Action of human skin collagenase on cartilage collagen. FEBS Lett. 1973 Aug 15;34(2):267–269. doi: 10.1016/0014-5793(73)80809-9. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Lindberg K. A., Glanville R. W., Evanson J. M. Action of rheumatoid synovial collagenase on cartilage collagen. Different susceptibilities of cartilage and tendon collagen to collagenase attack. Eur J Biochem. 1975 Jan 2;50(2):437–444. doi: 10.1111/j.1432-1033.1975.tb09821.x. [DOI] [PubMed] [Google Scholar]