Abstract
Aim
The Baltic Sea is one of the world's largest semi-enclosed brackish water bodies characterized by many special features, including endemic species that may be particularly threatened by climate change. We mapped potential distribution patterns under present and future conditions for a community with three trophic levels. We analysed climate-induced changes in the species' distribution patterns and examined possible consequences for the chosen food web.
Location
Baltic Sea and northern Europe.
Methods
We developed two open-source workflow-based analytical tools: one for ecological niche modelling and another for raster layer comparison to compute the extent and intensity of change in species' potential distributions. Individual ecological niche models were generated under present conditions and then projected into a future climate change scenario (2050) for a food web consisting of a guild of meso-grazers (Idotea spp.), their host algae (Fucus vesiculosus and Fucus radicans) and their fish predator (Gasterosteus aculeatus). We used occurrence data from the Global Biodiversity Information Facility (GBIF), literature and museum collections, together with five environmental layers at a resolution of 5 and 30 arc-minutes.
Results
Habitat suitability for Idotea balthica and Idotea chelipes in the Baltic Sea seems to be mostly determined by temperature and ice cover rather than by salinity. 2050 predictions for all modelled species show a northern/north-eastern shift in the Baltic Sea. The distribution ranges for Idotea granulosa and G. aculeatus are predicted to become patchier in the Baltic than in the rest of northern Europe, where the species will gain more suitable habitats.
Main conclusions
For the Baltic Sea, climate-induced changes resulted in a gain of suitable habitats for F. vesiculosus,I. chelipes and I. balthica, whereas lower habitat suitability was predicted for I. granulosa,F. radicans and G. aculeatus. The predicted north-eastern shift of I. balthica and I. chelipes into the distribution area of F. radicans in the Baltic Sea may result in increased grazing pressure. Such additional threats to isolated Baltic populations can lead to a higher extinction risk for the species, especially as climate changes are likely to be very rapid.
Keywords: Climate change, Baltic Sea, ecological niche modelling, e-Science, food web, Fucus radicans, Fucus vesiculosus, Gasterosteus aculeatus, Idotea, workflows
Introduction
Ecological niche modelling (ENM) has become a widely used approach for analysing species distributions and predicting changes in biodiversity patterns (Guinan et al., 2009; Kulhanek et al., 2011). Niche modelling techniques aim to recover the ranges of suitable habitat for a species by identifying environmental conditions associated with the species' occurrence (Peterson et al., 2011). Potential distribution (PD) models can be generated using relatively few variables to characterize the abiotic environment of the species. These variables are held in the form of geo-referenced raster layers. Biotic factors such as predation and/or competition also determine the niche of species, but in marine ecosystems abiotic factors (e.g. salinity and temperature) are considered the major features limiting the distribution of many species at the macroscale (Paavola et al., 2005; Gogina & Zettler, 2010). Because species' ranges conform closely to their thermal limits in aquatic systems, ecological niche modelling can yield a more accurate prediction of range shifts than on land (Sunday et al., 2012).
The Baltic Sea (BS) is one of the world's largest semi-enclosed brackish water bodies and is characterized by numerous special environmental features. Temperature and salinity, for instance, have very different ranges than in most other marine waters of the world, which results in unique water layer dynamics (Stipa & Vepsäläinen, 2002). The transition from the North Atlantic via the Skagerrak, Kattegat and Belt Sea to the entrance of the BS is characterized by a salinity gradient of 33–15 psu (practical salinity units). This gradient continues in the Baltic Proper with salinities around 10–8 psu in the Arkona Basin up to the Bothnian Bay and nearly freshwater in the Gulf of Finland (Fig.1).
Figure 1.
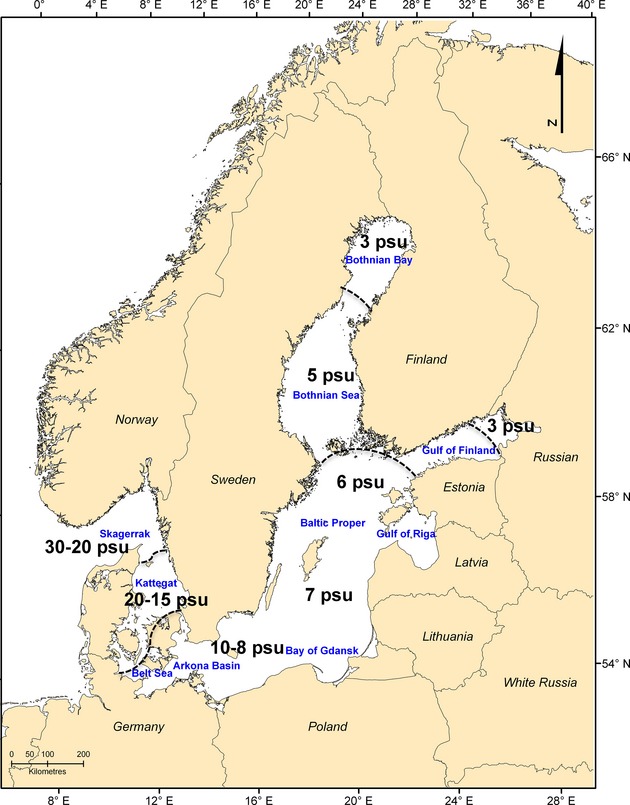
Study area of the meso-grazer guild. Mean sea-surface salinity values show the characteristic salinity gradient of the Baltic Sea. psu = practical salinity unit.
Following the latest deglaciation (c. 8–10 ka), immigrating species must have evolved a broad salinity tolerance to survive. Most of the marine species that have invaded the Baltic have gone through a bottleneck during colonization, with known losses in genetic variation, such as in the herring Clupea harengus and the harbour seal Phoca vitulina (Johannesson & André, 2006).
Biodiversity in the BS declines with the salinity gradient for species of both marine and freshwater origin (Bonsdorff, 2006). As the BS is a species-poor environment, communities tend to be less complex and often consist of only a few key species (Leidenberger et al., 2012). In this paper, we model climate-induced distribution changes in a community consisting of three trophic levels: two primary producers, three grazers and a predator.
Two important key taxa in the BS are the meso-grazers of the isopod genus Idotea and the macroalgae Fucus vesiculosus Linnaeus, 1753 and Fucus radicans Bergström et al., 2005 (Kautsky et al., 1992; Leidenberger et al., 2012). The bladder wrack (F. vesiculosus) has a wide distribution in the Northern Hemisphere. In the Baltic, it is the only perennial, canopy-forming macroalga, forming an important habitat in the littoral zone, down to around 10 m in depth. The endemic F. radicans (narrow wrack) is thought to have recently evolved from F. vesiculosus in the BS during the last 400 years (Bergström et al., 2005; Pereyra et al., 2009). Regarding the marine isopods, among the eight European Idotea species, three – Idotea balthica (Pallas, 1772), Idotea chelipes (Pallas, 1766) and Idotea granulosa Rathke, 1843 – have successfully colonized and adapted to the BS. Whereas I. balthica has a cosmopolitan distribution, the other species are restricted to European coastlines. In the BS, Idotea spp. graze heavily on macro- and microalgae and are important prey (Leidenberger et al., 2012), for example for the three-spined stickleback, Gasterosteus aculeatus Linnaeus, 1758, a small predatory teleost living in marine and freshwater habitats in the Northern Hemisphere.
In the Baltic Proper, grazing by Idotea can lead to a dramatic decline of Fucus populations (Nilsson et al., 2004). The high grazing pressure of Idotea has driven selection for increased grazer resistance in Baltic populations of F. vesiculosus compared with populations outside the BS (Nylund et al., 2012). Idotea is also suggested to limit the southern distribution range of F. radicans in the BS through intense grazing effects (Gunnarsson & Berglund, 2012).
One level up in the trophic chain, G. aculeatus is known to be able to control benthic meso-grazers on Swedish coastlines (Eriksson et al., 2009). As a consequence of heavy commercial fishing for larger predatory fish (e.g. Gadus morhua,Esox lucius), small predatory fish, such as G. aculeatus, now dominate most of the sheltered-coast Baltic communities (Eriksson et al., 2011). Top-down and bottom-up effects on coastal ecosystems are multifaceted and become even more complex when human impacts and climate change are taken into account.
Studies using ENM to predict changes in distribution patterns of marine key species due to climate change are still rare. Many studies on changes in marine species distribution focus on invasive species (Peterson, 2003; Ba et al., 2010), or are motivated by the economic importance of fish stocks (Lenoir et al., 2011). In this study, an ENM-based approach was used to determine the potential species distribution for six species of a Baltic trophic chain. Using the PD maps obtained, temperature and/or salinity effects on the distribution pattern of Idotea spp. were analysed to answer the following questions. (1) Which environmental factors most influence the distribution range of the grazers in the BS and northern Europe? (2) What climate-induced changes are predicted for the meso-grazers, the host algae and the fish predator under a climate change scenario for 2050? (3) What might be the consequences for this food web?
Materials and methods
We used the Taverna Workflow Management System (Hull et al., 2006) to create a pipeline (workflow) of existing tools and web services. Workflows were executed using the Taverna portal (https://portal.biovel.eu/), a web interface built through the BioVeL project (http://www.biovel.eu/) that allows users to run workflows without installing the Taverna workbench (http://www.taverna.org.uk/) (accessed 25 February 2013).
For this study, we used two novel workflows: one for ENM and one for statistical analysis of potential-distribution maps (raster layers). All versions of the ENM workflows can be downloaded from the MyExperiment repository (http://purl.ox.ac.uk/workflow/myexp-3355.20; accessed 2 December 2013). Version 20 of the ENM workflow was used in our analyses. The ENM workflow uses occurrence and environmental data to model ecological niches using a web service based on openModeller, a library that provides a variety of algorithms to model species distribution patterns (http://openmodeller.sf.net/) (Muñoz et al., 2011). The second workflow, the ENM Statistical Difference Workflow (ESW DIFF) (http://purl.ox.ac.uk/workflow/myexp-3959.2; accessed 23 January 2014) allows the spatial computation of changes in PD maps by calculating the differences between two raster layers using the R statistical environment 3.0.2 (R Core Team, 2013).
Occurrence data
Occurrence data for all species were extracted from GBIF (Global Biodiversity Information Facility; http://gbif.org/) during spring 2013 (see Appendix S1 in Supporting Information) (Table1). For the Idotea spp., additional occurrence records were gathered through an extensive literature survey and manually geo-referenced (Appendix S2) as well as obtained from museum collections (FMNH Helsinki; GOM Stralsund; SMF Frankfurt; SMNH Stockholm; ZIN St. Petersburg; ZMB Berlin; ZMH Hamburg) and through our own sampling (Appendix S3). For F. vesiculosus and F. radicans, GBIF records were either concentrated in a small area of the Baltic that is not representative of the species' full distribution range, or were too few (< 50). Consequently, additional occurrence points were created by geo-referencing in the known distribution range from the literature (Bonsdorff, 2006; Schagerström, 2013) (Table1, Appendix S2). All occurrence data collected for this study have been submitted to the OBIS database (http://www.iobis.org/) (http://www.vliz.be/nl/imis?module=dataset&dasid=4607; title of data set ‘Observations of three Idotea species (I. balthica,I. chelipes and I. granulosa) in northern Europe, including the Baltic Sea’).
Table 1.
Table showing the origin of the data and the parameters used for ecological niche models (ENMs) for all study species in the Baltic Sea and their maximum distribution range. The table shows the total number of occurrence records before filtering, information on the species' biology from the literature, and the environmental layers selected. DL, distance to land (km); MD, maximum depth (m); SIC, sea ice concentration (30 arc-min); SSS, sea- surface salinity (psu); SST, sea-surface temperature (°C) (5 arc-min). All layers are mean annual values, except MD.
| Species | Total GBIF records distribution | GBIF records Baltic | Other records Baltic* | Origin | Salinity tolerance [psu] | Selected layers |
|
|---|---|---|---|---|---|---|---|
| 5 arc-min†(Bio-Oracle, Tyberghein et al., 2012) | 30 arc-min (Aqua-Maps, Kaschner et al., 2010) | ||||||
| Fucus vesiculosus | 11072 | 575 | 332 | Marine | 4–35 | SSS | (SSS), SIC, DL, MD |
| Fucus radicans | 1 | 1 | 249 | Brackish | 3–7 | SSS | (SSS), SIC, DL, MD |
| Idotea balthica | 173 | 94 | 765 | Marine | 3–35 | SSS, SST | (SSS, SST), SIC, DL, MD |
| Idotea chelipes | 518 | 44 | 376 | Marine | 3–35 | SSS, SST | (SSS, SST), SIC, DL, MD |
| Idotea granulosa | 1105 | 26 | 149 | Marine | 5–35 | SSS, SST | (SSS, SST), SIC, DL, MD |
| Gasterosteus aculeatus | 24891 | 1384 | 0 | Fresh–Marine | 0–34 | SSS, SST | (SSS, SST), SIC, DL, MD |
Data collected by S. Leidenberger, data from museum collections and literature.
Used in the present projection only; for the 2050 projection, 30 arc-min layers were used exclusively.
Environmental data
Environmental layers that are likely to affect the distribution of the species were chosen based on the literature (Table1). Global marine layers came from Bio-Oracle (http://www.bio-oracle.ugent.be/; data downloaded 14 August 2013) at a resolution of 5 arc-minutes (Tyberghein et al., 2012), and from AquaMaps (http://www.aquamaps.org/download/main.php; data downloaded 1 April 2008) at a resolution of 30 arc-minutes (Kaschner et al., 2010). Layers for mean annual sea-surface salinity (SSS) and sea-surface temperature (SST) were available at a resolution of 5 arc-minutes for the present only, so we combined them with 30 arc-minute layers from AquaMaps for sea ice concentration (SIC), mean distance to land (DL) and maximum depth (MD) (Table1). For the 2050 projection, only 30 arc-minute layers from AquaMaps were used. Present-day datasets from AquaMaps were built from long-term averages of temporally varying environmental variables (Ready et al., 2010), whereas BioOracle layers were based on monthly level-3 pre-processed satellite data from NASA (Tyberghein et al., 2012). For the PD under 2050 climate conditions, the AquaMaps layers were derived from the ECHAM5 A1B climate change scenario (Jungclaus et al., 2006; IPCC et al., 2007).
To address the question of which environmental factors mostly influence the distribution range of the grazers, we used a jackknife leave-one-out procedure (Peterson et al., 2011) based on area under the curve of a receiver operating characteristic plot (AUC) values for SIC, SST and SSS for the Baltic and the known distribution area of the species (Table2). In this procedure, for each environmental variable a model was created without it, and then model assessments were compared across the different layer sets. The most influential variable was considered the one that, when not included in the model, produced the lowest assessment value.
Table 2.
Results of the jackknife analysis (AUC values) for the sea-surface salinity (SSS), sea-surface temperature (SST) and the sea ice concentration (SIC) for Idotea spp. in the Baltic Sea (BS) and the known distribution (KD) of the species. The most influential variable is in bold.
|
Idotea balthica |
Idotea chelipes |
Idotea granulosa |
||||
|---|---|---|---|---|---|---|
| Layers/area | BS | KD | BS | KD | BS | KD |
| SIC | 0.690 | 0.940 | 0.762 | 0.937 | 0.803 | 0.943 |
| SST | 0.701 | 0.952 | 0.791 | 0.908 | 0.808 | 0.901 |
| SSS | 0.745 | 0.933 | 0.795 | 0.920 | 0.797 | 0.940 |
Occurrence point filtering
Occurrence data were filtered for environmentally unique points by running an initial BioClim workflow also based on the openModeller web service, using the same environmental layers as in the ENM workflow. This procedure avoids passing redundant information to niche modelling algorithms later. Besides filtering the points, the workflow generated a BioClim model (Busby, 1986; Nix, 1986) to calculate the environmental range for each variable (Table3). The workflow can be downloaded from http://purl.ox.ac.uk/workflow/myexp-3725.2; last accessed 2 September 2013.
Table 3.
Summary of the ranges of abiotic parameters obtained with the BioClim algorithm, ecological niche model (ENM) statistics for all study species, including AUC and omission error values for the potential distribution maps (*modelled with Baltic points only; †modelled with all distribution points) and coverage and intensity of habitat suitability (DIFF statistic %).
| Variables/Species BioClim (ranges) | Fucus vesiculosus | Fucus radicans | Idotea balthica | Idotea chelipes | Idotea granulosa | Gasterosteus aculeatus |
|---|---|---|---|---|---|---|
| Known distribution | Northern Hemisphere | Baltic Sea (endemic) | Cosmopolitan | Europe | Europe | Northern Hemisphere |
| n | 1792 | – | 485 | 316 | 508 | 1634 |
| DL [km] | 0–137 | – | 0–796 | 0–796 | 0–796 | 0–871 |
| MD [m] | 1–1773 | – | 1–4951 | 1–4500 | 2–4500 | 1–7504 |
| SIC | 0–0.27 | – | 0–0.44 | 0–0.20 | 0–0.13 | 0–0.85 |
| SST [°C] | (1.60–13.55) | – | 4.07–29.36 | 6.47–19.46 | 3.08–18.26 | –0.25–26.97 |
| SSS [psu] | 4.28–35.28 | – | 4.50–39.24 | 4.90–37.41 | 5.67–36.33 | 2.99–38.25 |
| Baltic Sea | ||||||
| n | 313 | 184 | 265 | 124 | 69 | 123 |
| DL [km] | 0–84 | 0–51 | 1–118 | 1–118 | 4–118 | 0–87 |
| MD [m] | 1–372 | 2–362 | 2–372 | 3–372 | 11–372 | 1–372 |
| SIC | 0.01–0.25 | 0.04–0.25 | 0.0007–0.22 | 0.0007–0.20 | 0.0007–0.12 | 0.055–0.29 |
| SST [°C] | (5.17–11.08) | (4.69–9.49) | 6.20–11.08 | 6.77–10.95 | 6.92–10.55 | 4.19–10.67 |
| SSS [psu] | 4.28–13.51 | 4.07–6.83 | 4.50–13.38 | 4.90–13.48 | 5.67–12.90 | 2.99–12.21 |
| ENM | ||||||
| AUC | 0.90 ± 0.03* | 0.81 ± 0.05† | 0.90 ± 0.02† | 0.93 ± 0.04† | 0.93 ± 0.05* | 0.89 ± 0.05* |
| Omission error (%) | 0.65 | 1.05 | 1.33 | 1.91 | 5.71 | 2.59 |
| DIFF statistic (%) | ||||||
| Coverage 2013 | 63.48 | 42.14 | 91.25 | 79.69 | 58.58 | 64.90 |
| Coverage 2050 | 72.05 | 46.02 | 97.85 | 92.93 | 59.84 | 70.17 |
| 2050–2013 | 8.57 | 3.88 | 6.60 | 13.24 | 1.26 | 5.27 |
| Intensity 2013 | 24.97 | 13.55 | 42.08 | 24.83 | 16.18 | 21.32 |
| Intensity 2050 | 35.43 | 15.07 | 51.54 | 32.53 | 9.86 | 15.18 |
| 2050–2013 | 10.46 | 1.52 | 9.46 | 7.70 | –6.32 | –6.14 |
Main ENM workflow
The niche modelling workflow (Fig.2) uses occurrence data as input combined with a set of environmental layers (correlative approach) and a modelling algorithm defined by the user. A manual interaction step in the workflow allows algorithm and parameter selection. In this study, we used Mahalanobis distance (Mahalanobis, 1936; Farber & Kadmon, 2003) by means of the openModeller Environmental Distance algorithm with a set of parameters indicating the centroid of the input points to be used as a reference for distance calculation, and forcing distances to be translated into chi-square probability distribution values. Although not widely used in ENM studies, Mahalanobis distance has some interesting and useful features when compared with other algorithms. Among them is the fact that model shapes produced by this algorithm are n-dimensional ellipsoids, better reflecting the principle of central tendency in niche theory (Farber & Kadmon, 2003) and matching convex representations as hypothesized by Soberón & Nakamura (2009). Additionally, only presence points are required, with no need to generate pseudo-absence or background points, therefore not requiring prior knowledge of the species' origin and dispersal ability for model calibration, according to a recent study (Barve et al., 2011). Such requirement applies to most of the other algorithms being used in ENM, as their results are clearly influenced by the choice of the region from where pseudo-absence or background points are sampled.
Figure 2.
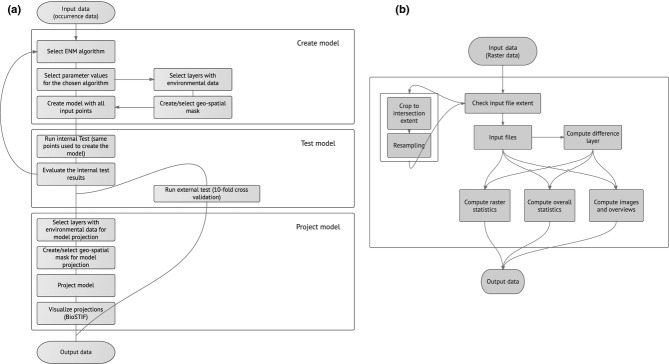
Diagrams of the workflows used in this study. (a) The ecological niche modelling (ENM) workflow takes as input a file containing species occurrence points to create a model with the openModeller web service. Algorithm, environmental layers and mask are selected during the workflow. The model is tested (internal test and optional cross validation external test) and then projected one or more times. Cross validation calculates the mean AUC and/or omission error. Model projection outputs are geotiff files with suitability values ranging from 0 to 254 (no data = 255). (b) The ENM Statistical Difference Workflow (ESW) allows the computation of the extent and intensity of change in species potential distribution through calculation of the differences between two raster layers using the R statistical environment (R Core Team, 2013). The difference file is computed from two input files (in this case present projection and 2050 projection) coming from the ecological niche modelling (ENM) workflow.
Models were created using each species' maximum distribution range and then projected into (1) the BS and (2) northern Europe. For F. vesiculosus,I. granulosa and G. aculeatus, the number of occurrence points was very unequal between the maximum distribution and the BS (i.e. there were many more points outside), which led to a weak PD in the Baltic. To handle this documented problem of semi-enclosed seas (Ready et al., 2010), ENM projections for these species were based on models generated with filtered data points from the BS only (and not on data from the whole distribution; see Table3).
For each species, we ran one model where we combined environmental layers with different resolutions (Table1), to be able to predict species distribution on a more local scale whenever possible through the generated model. The present model was also projected into a future scenario. Model performance was assessed using 10-fold cross-validation measuring the AUC and omission error. This means that for each species all points were randomly partitioned into 10 sets of equal size. For each set, a model was created using points from all other nine sets and then tested with points from the selected set. This technique is considered more robust than sub-sampling or bootstrapping, as it guarantees that all points are evenly used in both model creation and model testing. Therefore, prediction capability was assessed by means of multiple external tests, measuring the model's discriminatory power by averaging the AUC values. Because no absence data were used in this study, AUC values were calculated using the proportional area approach (Phillips et al., 2006) based on 10,000 background points randomly sampled across each mask. A model was only considered useful when the average AUC was ≥ 0.75 (Tables4). Besides the AUC, omission errors were calculated during cross-validation for each species using the lowest presence threshold (LPT). In LPT, the lowest model value across all training points is used as the suitability threshold, ensuring that all training points fall within suitable areas. This threshold criterion was chosen because all occurrence points were reviewed and considered valid before being used. The results of the ENMs are presented as maps showing the PD for each species.
Table 4.
Summary of ecological niche model (ENM) statistics for the northern Europe projections of potential distribution for the study species modelled with all distribution points and the coverage and intensity of habitat suitability (DIFF statistic %).
| Europe | Fucus vesiculosus | Idotea balthica | Idotea chelipes | Idotea granulosa | Gasterosteus aculeatus |
|---|---|---|---|---|---|
| ENM | |||||
| AUC | 0.91 ± 0.01 | 0.94 ± 0.02 | 0.92 ± 0.03 | 0.92 ± 0.02 | 0.90 ± 0.02 |
| Omission error (%) | 0.73 | 2.27 | 1.58 | 1.18 | 1.36 |
| DIFF statistic (%) | |||||
| Coverage 2013 | 16.71 | 48.17 | 33.87 | 27.71 | 60.38 |
| Coverage 2050 | 23.96 | 52.79 | 38.87 | 32.83 | 63.95 |
| 2050–2013 | 7.25 | 4.62 | 5.00 | 5.12 | 3.57 |
| Intensity 2013 | 8.11 | 13.52 | 10.01 | 8.30 | 21.38 |
| Intensity 2050 | 11.21 | 17.37 | 12.98 | 10.14 | 22.94 |
| 2050–2013 | 3.10 | 3.85 | 2.97 | 1.84 | 1.56 |
Post-processing
The ENM Statistical Difference Workflow (ESW DIFF) (Fig.2) was used to compute the extent and intensity of change in species PD by measuring the differences between two raster layers using R 3.0.2 (R Core Team, 2013). The difference file was computed from two input files, in our case the present projection (combined 5 and 30 arc-minutes) and the 2050 projection (30 arc-minutes). The difference between each corresponding raster cell value was computed and stored in the difference file, regardless of the input files' geographical extent and origin. When files had a different geographical extent and/or origin, the workflow automatically cropped them to the same extent and resampled the values using the ‘nearest neighbour’ method, resulting in a perfect cell match between the two rasters without changes in the values (Fig.2).
In the difference file the resulting value range (−254, 254) is directly associated with the range of the input files (0, 254) to capture the maximum possible variation in both directions. Difference values were categorized into five positive and five negative classes depicted in gradients from green to red (increase) and green to blue (decrease), respectively. This allows regions of change to be clearly identified for each species as a heat map, while the range (−2, 2) is kept transparent. Overall coverage, overall intensity and the difference in intensity or coverage between the two raster layers were computed. Overall coverage was computed as the percentage of raster cells with values > 0, and overall intensity was computed as the sum of all cell values divided by the number of raster cells.
Results
Environmental parameters limiting the distribution of the grazers in the Baltic
Our jackknife analysis shows that SIC (followed by SST) determines the northern distribution limit in the BS for I. balthica and I. chelipes (Table2, Fig.3). In the PD map for I. balthica using SST and SIC the area of suitability is much more restricted in the northern Baltic (Fig.3a). Idotea granulosa, however, is more restricted by SSS (Table2). Outside the BS, our jackknife results show that SST is the most important environmental factor for I. chelipes and I. granulosa, whereas for I. balthica it is not.
Figure 3.
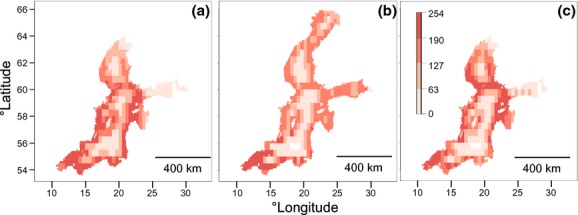
The potential distribution (PD) for Idotea balthica in the Baltic Sea using the following layers: (a) sea-surface temperature (SST), sea ice concentration (SIC), maximum depth (MD) and distance to land (DL) (AUC: 0.90, omission error: 1.34%), (b) sea-surface salinity (SSS), MD, DL (AUC: 0.91, omission error: 1.13%), and (c) SST, SIC, SSS, MD and DL (AUC: 0.90, omission error: 1.32%). The colour scale indicates habitat suitability, ranging from 0 (unsuitable, in white) to 254 (maximum suitability, in dark red).
Present potential distribution
The three marine isopods showed a widespread PD in the BS under present climate conditions (Fig.4, Table3). Whereas I. balthica has a PD deep into the Bothnian Sea (to 62° N) and into the Gulf of Finland, I. chelipes and I. granulosa are more restricted to the Baltic Proper and the Arkona Basin (Fig.4). The prediction strength was highest for I. balthica, followed by I. chelipes and I. granulosa (Table3), which coincides with their frequency and dominance in the benthic ecosystem (Leidenberger et al., 2012).
Figure 4.
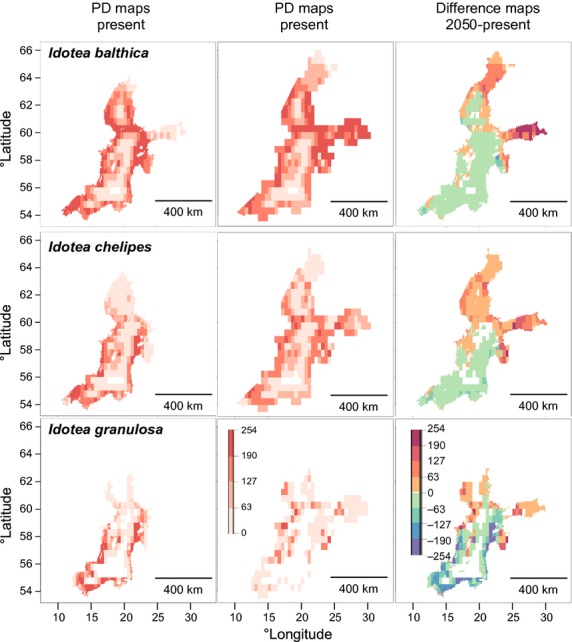
Maps showing the potential distribution for Idotea balthica,I. chelipes and I. granulosa in the Baltic Sea. The colour scale in the potential distribution (PD) maps indicates habitat suitability, ranging from 0 (unsuitable, in white) to 254 (maximum suitability, in dark red). On the difference maps (2050–present) colours from green to red indicate an increase in habitat suitability and those from green to blue indicate a decrease.
At present, both algal species are absent from the Bothnian Bay. Whereas F. vesiculosus has suitable habitats in nearly the whole BS, F. radicans is restricted to the north-east (Fig.5). The ranges of abiotic parameters (temperature, salinity) for those species were very large, showing a wide tolerance of extreme environmental conditions, with lower extremes for F. radicans (Table3). For G. aculeatus, the PD covers nearly the whole BS with its highest intensity in the Baltic Proper (Fig.5). Of all modelled species, this species has the highest tolerance of low salinity, reflecting its capacity to live even in freshwater habitats (Table3). A plot of occurrence points shows that the fish predator mostly overlaps in its habitat with the grazer I. balthica (Fig.6), whereas the endemic alga F. radicans does not overlap with the grazer.
Figure 5.
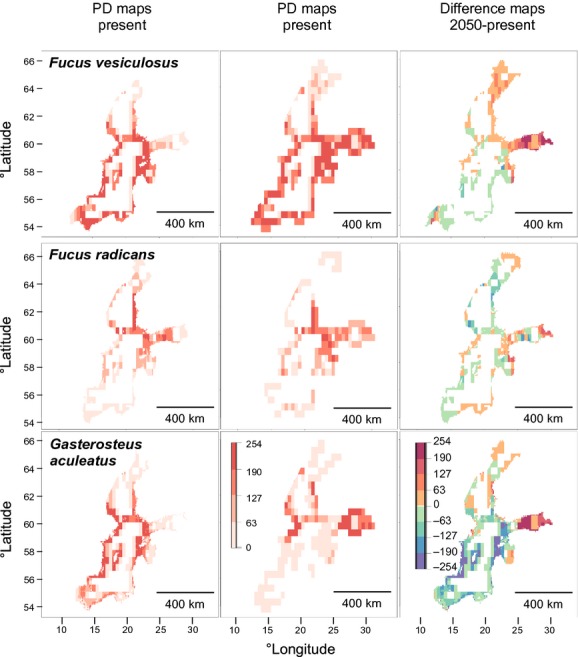
The potential distributions for Fucus vesiculosus,F. radicans and Gasterosteus aculeatus in the Baltic Sea. The colour scale in the potential distribution (PD) maps indicates habitat suitability, ranging from 0 (unsuitable, in white) to 254 (maximum suitability, in dark red). On the difference maps (2050–present) colours from green to red indicate an increase in habitat suitability and those from green to blue indicate a decrease.
Figure 6.
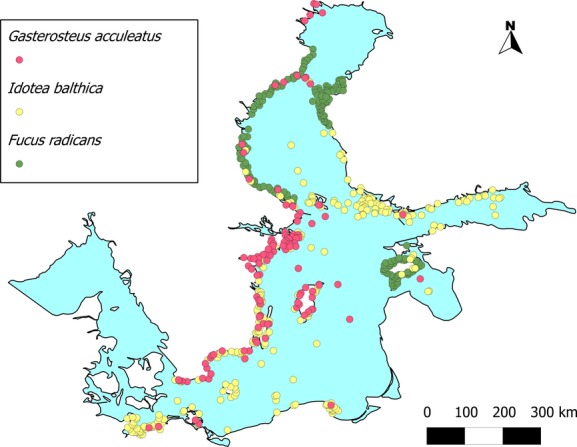
The overlap of current habitats in the Baltic Sea for the predatory fish (Gasterosteus aculeatus), the grazer (Idotea balthica) and the endemic alga (Fucus radicans), based on all the occurrence points used in this study.
PD in northern Europe (Fig.7) showed suitable habitats in the BS only for I. balthica and I. chelipes. Besides those two species, I. granulosa,F. vesiculosus and G. aculeatus showed a strong PD with high intensity along nearly all northern European coastlines.
Figure 7.
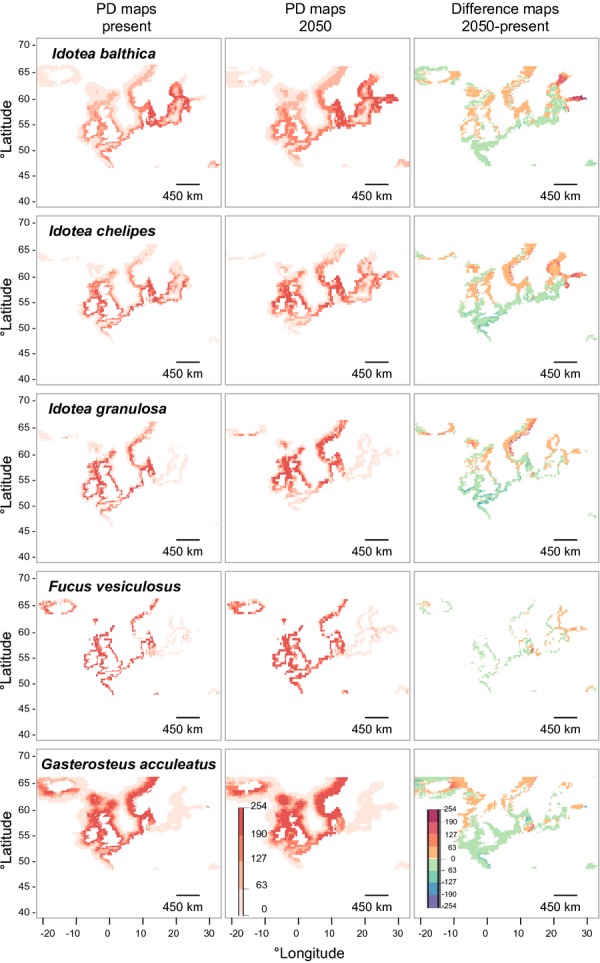
The potential distributions for Idotea balthica,I. chelipes,I. granulosa,Fucus vesiculosus and Gasterosteus aculeatus at the scale of northern Europe. The ‘enclosed sea problem’ as discussed in the main body of the text can be seen for I. granulosa,F. vesiculosus and G. aculeatus. Colour scale in the potential distribution (PD) maps indicates habitat suitability, ranging from 0 (unsuitable, in white) to 254 (maximum suitability, in dark red). On the difference maps (2050–present) colours from green to red indicate an increase and those from green to blue indicate a decrease in habitat suitability.
All statistics (AUC, omission error, coverage, intensity) for the projections are summarized in Tables2 and 3. All of the ENMs generated good to excellent predictions, as shown by their high AUC values (> 0.80). Omission errors (%) were excellent (< 5%) for all models, the only exception being the ENM for I. granulosa (5.7%).
2050 potential distribution
Under the 2050 climate scenario, the projections suggest a significant northern movement for Idotea spp., both in the BS and northern Europe (Figs4 & 7). PD in the BS shifts eastwards into the Gulf of Finland, which seems to become a more suitable habitat for all species (Figs4 & 5). Fucus radicans showed almost no difference in the 2050 PD. Coverage for I. balthica and I. chelipes was higher than for I. granulosa, and higher for F. vesiculosus than for F. radicans. Idotea balthica had the highest intensity, whereas I. granulosa and F. radicans had the lowest (Table3).
The northern shift in the Baltic coincides with the trend seen for the modelled species in northern Europe (Fig.7). Here I. granulosa and G. aculeatus show less suitable habitats in southern regions.
Changes in species distribution
The most dramatic changes in distribution were predicted for the Baltic Proper. Here, G. aculeatus showed the most significant decrease in intensity, together with the meso-grazer I. granulosa (Figs4 & 5, Table3). Even F. vesiculosus, as well as I. balthica and I. chelipes, are predicted to lose suitable habitat in this area (Figs4 & 5). For F. radicans the changes did not appear as clear as for the other species investigated, with suitable habitats decreasing or increasing slightly in different parts of the BS.
In general, all species show a slight increase in coverage in 2050. PD coverage differs between the 2050 predictions and the present most for I. chelipes and F. vesiculosus and least for I. granulosa and F. radicans. The intensity of habitat suitability (2050–2013) increased most for F. vesiculosus and I. balthica, whereas I. granulosa and G. aculeatus show a loss in intensity (Table3). In contrast, those values were not so different for species in the northern European projection (Table4). All modelled species are predicted to lose suitable habitats in the south and shift northwards (Fig.7).
Discussion
PD patterns modelled for the chosen species were only based on a few geographical and abiotic factors. It is important to note that other fundamental biotic interactions associated with resource availability can influence life-history traits and population dynamics (e.g. nutrient load for algae, predator risk or other food web interactions). These were not included in the model, as it is known that the inclusion of too many parameters can increase the uncertainty of models and the risk of multicollinearity (Lenoir et al., 2011). Moreover, it is difficult to get layers for all those parameters.
The environmental parameters were deemed to best reflect the habitat needs of the benthic Idotea spp. (Table1), as they are known to have a major influence on the species' biology (e.g. size, fertility and age at maturity) (Leidenberger, 2013); the same applies to G. aculeatus. For four Baltic macrophytes, including F. vesiculosus/radicans, Sandman et al. (2013) emphasized the importance of depth on species' distribution, and that salinity is more important in archipelagos with a strong salinity gradient than in most coastal areas of the BS, where differences were too small to be a useful predictor for habitat suitability.
Generally, the present PD of Idotea spp. (Fig.4) is in agreement with the observed distribution of the species (Leidenberger et al., 2012). Its distribution seems to be more constrained by SIC/SST than by SSS in the BS (Fig.3). All three species differ slightly in their ranges of abiotic parameters inside and outside the BS (Table3). The environmental parameters have different effects depending on the area of interest (Table2). For example, I. balthica showed the poorest tolerance of low salinities, and I. granulosa can be found at colder temperatures on North Atlantic coasts (Table3, Fig.7). The latter species is tolerant of more open exposed waters, in contrast to I. chelipes, which is a meso-grazer of lagoons and estuaries in shallow coastal areas, preferring warmer temperatures. Idotea balthica, a cosmopolitan species, is known to have a more generalist lifestyle (Leidenberger et al., 2012).
The Baltic distribution of the meso-grazer I. balthica is limited to the range of its host algae F. vesiculosus and F. radicans, which are currently both absent from the Bothnian Bay. Fucus radicans is reported only from the Swedish coast of the Bothnian Bay and north of Poori/Björneborg in Finland, as well as around Ösel island in Estonia (Bergström et al., 2005; Schagerström, 2013) (Fig.6). Schagerström (2013) explained the absence of this species on the east coast of the Bothnian Bay by intraspecific competition with F. vesiculosus. Our modelled distribution showed a potentially broader suitable area for F. radicans (Fig.5) than where it can be observed today.
Interestingly, the current distribution limits of F. radicans and I. balthica overlap only slightly on the south-eastern coast of the Bothnian Sea. The grazer is more concentrated in the south of the Bothnian Sea and F. radicans in the north (Fig.6). Habitat limitation caused by SIC/SST for Idotea might have provided a unique ecological niche for the recently evolved endemic narrow wrack in the northern Bothnian Sea. Up to now, no detailed studies on the physiology of F. radicans exist. Experimental studies have shown that F. radicans is highly sensitive to high grazing pressure, and that it was preferred as a food item by Idotea when given the choice between F. vesiculosus and F. radicans (Gunnarsson & Berglund, 2012). The overlapping habitat ranges of Idotea spp. and F. vesiculosus have forced a selection for high grazing-resistance in F. vesiculosus during colonization of the BS (Nylund et al., 2012). This seems not to be the case for F. radicans.
The estimated distribution patterns modelled for the meso-grazer guild under the 2050 scenario followed the overall trend of a shift to more northerly regions as a response to rising SST (Perry et al., 2005). This northern shift was seen in our predictions for both the BS and northern Europe projection (Figs4, 5 & 7). The eastern shift may be a consequence of the regional climate changes predicted for the Baltic.
For the BS, a regional climate model was developed [the Rossby Centre Ocean Model (RCO)], to consider the extremes of this semi-enclosed sea (Döscher et al., 2002). Meier et al. (2011) were able to show that in general circulation models (GCMs), such as the ECHAM model used in our analysis, simulations predicted warming bias of the BS due to a reduction of the ice-albedo feedback (a positive feedback climate process where a change in the area of snow-covered land or sea ice alters the albedo causing a reinforcement in the initial alteration in ice area). Different climate scenarios published in recent years resulting from the RCO, and using ECHAM (versions 4 and 5) as lateral boundary data, predict serious changes in SST, SSS, SIC and turbidity in the BS region (Meier et al., 2011, 2012). The latest RCO simulation, using ECHAM5, clearly indicated that SST would increase with time. The biggest change is predicted for the central Bothnian Bay and Bothnian Sea during summer (+4 °C), and for the Gulf of Finland in spring and winter (Meier et al., 2012). Salinity will be reduced through significantly increased runoffs into the BS. The largest decreases in SSS were predicted in the Baltic Proper (about 1.5–2 psu) (Meier et al., 2012). Effects on ecological quality indicators, such as phytoplankton concentration, Secchi depth and bottom oxygen concentration are predicted to be larger under ECHAM5 (Meier et al., 2012) than under previous scenarios (Meier et al., 2011). Secchi depth is predicted to decrease by up to 1.5 m in the Baltic Proper. The BALTEX Assessment of Climate Change for the Baltic Sea Basin (BACC) working group has already recorded numerous examples of climate-related marine biodiversity changes on all trophic levels (BACC Author Team, 2009).
Increased SST can have a direct effect on the physiology of species and may have indirect consequences through changes in food webs (Leidenberger et al., 2012), especially in Baltic fish species (Eriksson et al., 2011). If G. aculeatus coverage decreases as much as predicted in our model (Fig.5), this will have a knock-on effect on Idotea meso-grazers, which will also lead to higher grazing pressure on the Baltic algae, especially F. radicans. Therefore, climate-induced changes pose an indirect extinction risk for this endemic species, as it does not seem to have evolved protection against high grazing pressure as has F. vesiculosus. Fucus species may not be affected by SST increase in the Baltic to the same degree as was observed for populations outside the BS (Jueterbock et al., 2013; Nicastro et al., 2013), but a predicted increase in the frequency of local heat-waves can increase stress. Jueterbock et al. (2013) found a northward shift for three Fucus species, with most habitat losses south of 45° N in the North Atlantic in the near future.
The combination of serious changes in nutrient load, oxygen concentration, SSS and SST predicted for the Baltic Proper, and a northern/north-eastern shift of species, may result in genetic separation of local populations. Phylogeographical studies have indicated that Baltic populations in general (Johannesson & André, 2006), and populations of Idotea spp., F. vesiculosus and G. aculeatus in particular, have lost genetic variation in contrast to populations from the Atlantic (Tatarenkov et al., 2007; Nylund et al., 2012; DeFaveri et al., 2013; Leidenberger, 2013). A large population size with a high level of genetic variation can increase the capacity to adapt to environmental changes, in comparison with small isolated populations with low genetic diversity (Johannesson et al., 2011). Indeed, local adaptation of F. vesiculosus to the low-salinity environment of the BS is associated with reduced stress tolerance (Pearson et al., 2000) and the advent of asexual reproduction (Bergström et al., 2005). For Idotea, physiological studies on salinity and stress tolerance are still rare, but local adaptations to food algae are described inside and outside the BS (Vesakoski et al., 2009; Bell & Sotka, 2012). An experimental heat wave scenario for I. balthica significantly decreased the immune-competence of the grazer (Roth et al., 2010). A similar heat shock scenario (25 °C for 30 min) for specimens of both Baltic Fucus species indicated a higher sensitivity than for specimens from outside the BS (Lago-Lestón et al., 2010). Outside the BS, the macroalga has already experienced an 11° northward shift in distribution on the North African coast (= 1250 km) as a consequence of a significant increase in coastal SST (Nicastro et al., 2013), followed by extinction of a cryptic genetic clade. Local adaptation along an environmental gradient has also been shown for G. aculeatus (DeFaveri et al., 2013).
The evolutionary potential of the species will determine how they will be able to cope with predicted future climate changes in the BS. The outcome of our models shows that the meso-grazer guild of Idotea is likely to be affected by distribution changes under a future climate scenario leading to knock-on effects in the Baltic food web.
As our statistical analyses show, the likely winners in the BS seem to be F. vesiculosus and the grazers I. chelipes and I. balthica, whereas the losers with less habitat suitability might be the grazer I. granulosa,F. radicans and the fish G. aculeatus (Table3, Figs4 & 5). In northern Europe all species analysed are predicted to have increased habitat suitability, even if this trend is reduced for I. granulosa and G. aculeatus (Table4, Fig.7).
Uncertainties in our analyses arise from the GCM, the climate scenario itself (ECHAM5 A1B) and other limitations of ENM, such as the number and distribution of occurrence points used to create the model, although the final number of points for all species was > 50, as recommended by Farber & Kadmon (2003) for the Mahalanobis distance algorithm. Fucus radicans and I. granulosa, for example, were two species where the number of occurrence points was low (Table3), and the PD patterns (both for the present and 2050) are not as clear as for the other species modelled with higher numbers of occurrence points (Table3, Figs4 & 5). For I. chelipes, which also has relatively low numbers of occurrence points (Table3), the proportion of points inside and outside the BS was better distributed than for I. granulosa, resulting in a better model (Fig.4).
Models projected on the European scale resulted in a weak PD for I. granulosa,F. vesiculosus and G. aculeatus in the BS (Table4, Fig.7). For those species, the number of occurrence points outside the BS was much higher (up to 13 times) than in the BS (Figs4 & 5). This ‘enclosed sea problem' is known for ENM in seas with distinct environmental conditions from surrounding areas (Ready et al., 2010). For I. granulosa, occurrence points in the BS were too few, leading to a higher omission error (> 5%). More occurrence points would be needed to improve the models for this species (Table3).
Conclusions
If our modelled meso-grazer guild is not able to deal with multiple stressors resulting from decreased genetic variability, dramatic effects on Baltic coastal ecosystems may result. The capability of isolated Baltic populations to cope with future climate changes may strongly depend on their evolutionary and adaptive potential; however, the time-scales of predicted climate changes are likely to be very rapid.
In the near future, interdisciplinary research is required both in terrestrial and marine habitats, to improve our overall knowledge of the consequences of environmental changes on species' distribution ranges and their population genetics. The development of novel computational tools combining data from different sources (species occurrence data, environmental data and genetic data) is necessary to allow the rapid observation and analysis of environmental changes, which can feed into environmental management and decision-making.
Acknowledgments
This work was carried out in the Linnaeus Centre for Marine Evolutionary Biology at the University of Gothenburg (http://www.cemeb.science.gu.se/), and supported by a Linnaeus grant from the Swedish Research Councils VR and Formas, and by BioVeL. The EU's Seventh Framework Program funds BioVeL, grant no. 283359.
Biosketch
Sonja Leidenberger is a postdoctoral researcher investigating species distribution in northern Europe for the EU-funded BioVel project (http://www.biovel.eu/). Her research interests range from life-history strategies, phylogeography and species distribution modelling, to the effects of climate change on marine benthic species.
Author contributions: S.L. and S.J.B. designed the study and collected the data. A.R.W., R.G. and R.K. built the workflows. S.L., S.J.B., R.G. and R.K. performed the modelling. S.L. and S.J.B. wrote the first draft of the manuscript, and all authors contributed substantially to the revisions.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Data provider names for occurrence records downloaded from GBIF.
Appendix S2 Data references from a literature survey for all study species (a) and occurrence records manually geo-referenced by species (b–e).
Appendix S3 Occurrence records from museum material (a) and material collected by S. Leidenberger (b).
References
- Ba J, Hou Z, Platvoet D, Zhu L. Li S. Is Gammarus tigrinus (Crustacea, Amphipoda) becoming cosmopolitan through shipping? Predicting its potential invasive range using ecological niche modeling. Hydrobiologia. 2010;649:183–194. &. [Google Scholar]
- BACC Author Team. Assessment of climate change for the Baltic Sea basin. Regional Climate Studies. Berlin: Springer-Verlag; 2009. [Google Scholar]
- Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT, Soberón J. Villalobos F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling. 2011;222:1810–1819. &. [Google Scholar]
- Bell TM. Sotka EE. Local adaptation in adult feeding preference and juvenile performance in the generalist herbivore Idotea balthica. Oecologia. 2012;170:383–393. doi: 10.1007/s00442-012-2302-3. &. [DOI] [PubMed] [Google Scholar]
- Bergström L, Tatarenkov A, Johannesson K, Jönsson RB. Kautsky L. Genetic and morphological identification of Fucus radicans sp. nov. (Fucales, Phaephyceae) in the brackish Baltic Sea. Journal of Phycology. 2005;41:1025–1038. &. [Google Scholar]
- Bonsdorff E. Zoobenthic diversity-gradients in the Baltic Sea: continuous post-glacial succession in a stressed ecosystem. Journal of Experimental Marine Biology and Ecology. 2006;330:383–391. [Google Scholar]
- Busby JR. A biogeoclimatic analysis of Nothofagus cunninghamii (Hook.) Oerst. in southeastern Australia. Australian Journal of Ecology. 1986;11:1–7. [Google Scholar]
- DeFaveri J, Jonsson PR. Merilä J. Heterogeneous genomic differentiation in marine threespine sticklebacks: adaptation along an environmental gradient. Evolution. 2013;67:2530–2546. doi: 10.1111/evo.12097. &. [DOI] [PubMed] [Google Scholar]
- Döscher R, Willén U, Jones C, Rutgersson A, Meier HEM, Hansson U. Graham LP. The development of the regional coupled ocean–atmosphere model RCAO. Boreal Environment Research. 2002;7:183–192. &. [Google Scholar]
- Eriksson BK, Ljunggren L, Sandström A, Johansson G, Mattila J, Rubach A, Råberg S. Snickars M. Declines in predatory fish promote bloom-forming macroalgae. Ecological Applications. 2009;19:1975–1988. doi: 10.1890/08-0964.1. &. [DOI] [PubMed] [Google Scholar]
- Eriksson BK, Sieben K, Eklöf J, Ljunggren L, Olsson J, Casini M. Bergström U. Effects of altered offshore food webs on coastal ecosystems emphasize the need for cross-ecosystem management. Ambio. 2011;40:786–797. doi: 10.1007/s13280-011-0158-0. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber O. Kadmon R. Assessment of alternative approaches for bioclimatic modeling with special emphasis on the Mahalanobis distance. Ecological Modelling. 2003;160:115–130. &. [Google Scholar]
- Gogina M. Zettler ML. Diversity and distribution of benthic macrofauna in the Baltic Sea Data inventory and its use for species distribution modelling and predition. Journal of Sea Research. 2010;64:313–321. &. [Google Scholar]
- Guinan J, Brown C, Dolan MFJ. Grehan AJ. Ecological niche modelling of the distribution of cold-water coral habitat using underwater remote sensing data. Ecological Informatics. 2009;4:83–92. &. [Google Scholar]
- Gunnarsson K. Berglund A. The brown alga Fucus radicans suffers heavy grazing by the isopod Idotea baltica. Marine Biology Research. 2012;8:87–89. &. [Google Scholar]
- Hull D, Wolstencroft K, Stevens R, Goble C, Pocock MR, Li P. Oinn T. Taverna: a tool for building and running workflows of services. Nucleic Acids Research. 2006;34:W729–W732. doi: 10.1093/nar/gkl320. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachauri RK, Reisinger A, editors. IPCC Core Writing Team. Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: Intergovernmental Panel on Climate Change (IPCC); 2007. (ed. by the. [Google Scholar]
- Johannesson K. André C. Life on the margin: genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Molecular Ecology. 2006;15:2013–2029. doi: 10.1111/j.1365-294X.2006.02919.x. &. [DOI] [PubMed] [Google Scholar]
- Johannesson K, Smolarz K, Grahn M. André C. The future of Baltic Sea populations: local extinction or evolutionary rescue? Ambio. 2011;40:179–190. doi: 10.1007/s13280-010-0129-x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueterbock A, Tyberghein L, Verbruggen H, Coyer JA, Olsen JL. Hoarau G. Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecology and Evolution. 2013;3:1356–1373. doi: 10.1002/ece3.541. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungclaus JH, Keenlyside N, Botzet M, Haak H, Luo J-J, Latif M, Marotzke J, Mikolajewicz U. Roeckner E. Ocean circulation and tropical variability in the coupled ECHAM5/MPI-OM. Journal of Climatology. 2006;19:3952–3972. &. [Google Scholar]
- Kaschner K, Ready JS, Agbayani E, Rius J, Kesner-Reyes K, Eastwood PD, South AB, Kullander SO, Rees T, Close CH, Watson R, Pauly D. Froese R. Editors AquaMaps Environmental Dataset: Half-Degree Cells Authority File (HCAF) 2010. &. Available at: http://aquamaps.org/envtdata/main.php. [Google Scholar]
- Kautsky H, Kautsky L, Kautsky N. Lindblad C. Studies on the Fucus vesiculosus community in the Baltic Sea. Acta Phytogeographica Suecica. 1992;78:33–48. &. [Google Scholar]
- Kulhanek SA, Leung B. Ricciardi A. Using ecological niche models to predict the abundance and impact of invasive species: application to the common carp. Ecological Applications. 2011;21:203–213. doi: 10.1890/09-1639.1. &. [DOI] [PubMed] [Google Scholar]
- Lago-Lestón A, Mota C, Kautsky H. Person GA. Functional divergence in heat shock response following rapid speciation of Fucus spp. in the Baltic Sea. Marine Biology. 2010;157:683–688. &. [Google Scholar]
- Leidenberger S. 2013. Göteborg University of Gothenburg Adaptation to the Baltic Sea – the case of isopod genus Idotea. PhD Thesis,
- Leidenberger S, Harding K. Jonsson PR. Ecology and distribution of the isopod genus Idotea in the Baltic Sea: key species in a changing environment. Journal of Crustacean Biology. 2012;33:359–381. &. [Google Scholar]
- Lenoir S, Beaugrand G. Lecuyer E. Modeled spatial distribution of marine fish and projected modifications in the North Atlantic Ocean. Global Change Biology. 2011;17:115–129. &. [Google Scholar]
- Mahalanobis PC. On the generalized distance in statistics. Proceedings of the National Institute of Science of India. 1936;12:49–55. [Google Scholar]
- Meier HEM, Eilola K. Almroth E. Climate-related changes in marine ecosystems simulated with a 3-dimensional coupled physical–biogeochemical model of the Baltic Sea. Climate Research. 2011;48:31–35. &. [Google Scholar]
- Meier HEM, Hordoir R, Andersson HC, Dietrich C, Eilola K, Gustafsson BG, Höglund A. Schimanke S. Modeling the combined impact of changing climate and changing nutrient loads on the Baltic Sea environment in an ensemble of transient simulations for 1961–2099. Climate Dynamics. 2012;39:2421–2441. &. [Google Scholar]
- Muñoz MES, Giovanni R, Siqueira MF, Sutton T, Brewer P, Pereira RS, Canhos DAL. Canhos VP. openModeller: a generic approach to species' potential distribution modelling. Geoinformatica. 2011;15:111–135. &. [Google Scholar]
- Nicastro KR, Zardi GL, Teixeira S, Neiva J, Serrao EA. Pearson GA. Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biology. 2013;11:1–13. doi: 10.1186/1741-7007-11-6. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Engkvist R. Persson L-E. Long-term decline and recent recovery of Fucus populations along the rocky shores of southeast Sweden, Baltic Sea. Aquatic Ecology. 2004;38:587–598. &. [Google Scholar]
- Nix HA. A biogeographic analysis of Australian elapid snakes. In: Longmore R, editor. Atlas of Australian elapid snakes. Canberra: Australian Government Publishing Service; 1986. pp. 4–15. (ed. by ). Australian Flora and Fauna Series 7, [Google Scholar]
- Nylund GM, Pereyra RT, Wood HL, Johannesson K. Pavia H. Increased resistance towards generalist herbivory in the new range of a habitat-forming seaweed. Ecosphere. 2012;3:125. &. [Google Scholar]
- Paavola M, Olenin S. Leppakoski E. Are invasive species most successful in habitats of low native species richness across European brackish water seas? Estuarine Coastal and Shelf Science. 2005;64:738–750. &. [Google Scholar]
- Pearson GA, Kautsky H. Serrao EA. Recent evolution in Baltic Fucus vesiculosus: reduced tolerance to emersion stresses compared to intertidal (North Sea) populations. Marine Ecology Progress Series. 2000;202:67–79. &. [Google Scholar]
- Pereyra RT, Bergström L, Kautsky L. Johannesson K. Rapid speciation in a newly opened postglacial marine environment, the Baltic Sea. BMC Evolutionary Biology. 2009;9:70. doi: 10.1186/1471-2148-9-70. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AL, Low PJ, Ellis JR. Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. &. [DOI] [PubMed] [Google Scholar]
- Peterson AT. Predicting the geography of species' invasions via ecological niche modelling. The Quarterly Review of Biology. 2003;78:419–433. doi: 10.1086/378926. [DOI] [PubMed] [Google Scholar]
- Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M. Araújo MB. Ecological niches and geographic distributions. Princeton, NJ: Princeton University Press; 2011. &. Monographs in Population Biology, no. 49. [Google Scholar]
- Phillips SJ, Anderson RP. Schapire RE. Maximum entropy modelling of species geographic distributions. Ecological Modelling. 2006;190:231–259. &. [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Ready J, Kaschner K, South AB, Eastwood PD, Rees T, Rius J, Agbayani E, Kullander S. Froese R. Predicting the distributions of marine organisms at the global scale. Ecological Modelling. 2010;221:467–478. &. [Google Scholar]
- Roth O, Kurtz J. Reusch TBH. A summer heat wave decreases the immunocompetence of the mesograzer, Idotea balthica. Marine Biology. 2010;157:1605–1655. &. [Google Scholar]
- Sandman AN, Wikström SA, Blomqvist M, Kautsky H. Isaeus H. Scale-dependent influence of environmental variables on species distribution: a case study on five coastal benthic species in the Baltic Sea. Ecography. 2013;36:354–363. &. [Google Scholar]
- Schagerström E. Fucus radicans – reproduction, adaptation and distribution patterns. Plants & Ecology. 2013;2013/2:1–22. . Available at: http://www.diva-portal.org/smash/get/diva2:606901/FULLTEXT01. [Google Scholar]
- Soberón J. Nakamura M. Niches and distributional areas: concepts, methods, and assumptions. Proceedings of the National Academy of Sciences USA. 2009;106:19644–19650. doi: 10.1073/pnas.0901637106. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipa T. Vepsäläinen J. The fragile climatological niche of the Baltic Sea. Boreal Environment Research. 2002;7:335–342. &. [Google Scholar]
- Sunday MJ, Bates AE. Dulvy NK. Thermal tolerance and the global redistribution of animals. Nature Climate Change. 2012;2:686–690. &. [Google Scholar]
- Tatarenkov A, Jönsson RB, Kautsky L. Johannesson K. Genetic structure in populations of Fucus vesiculosus (Phaeophyceae) over spatial scales from 10 m to 800 km. Journal of Phycology. 2007;43:675–685. &. [Google Scholar]
- Tyberghein L, Verbruggen H, Pauly K, Troupin C, Mineur F. De Clerck O. Bio-Oracle: a global environmental dataset for marine species distribution modelling. Global Ecology and Biogeography. 2012;21:272–281. &. [Google Scholar]
- Vesakoski O, Rautanen J, Jormalainen V. Ramsay T. Divergence in host use ability of a marine herbivore from two habitat types. Journal of Evolutionary Biology. 2009;22:1545–1555. doi: 10.1111/j.1420-9101.2009.01767.x. &. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data provider names for occurrence records downloaded from GBIF.
Appendix S2 Data references from a literature survey for all study species (a) and occurrence records manually geo-referenced by species (b–e).
Appendix S3 Occurrence records from museum material (a) and material collected by S. Leidenberger (b).


