Abstract
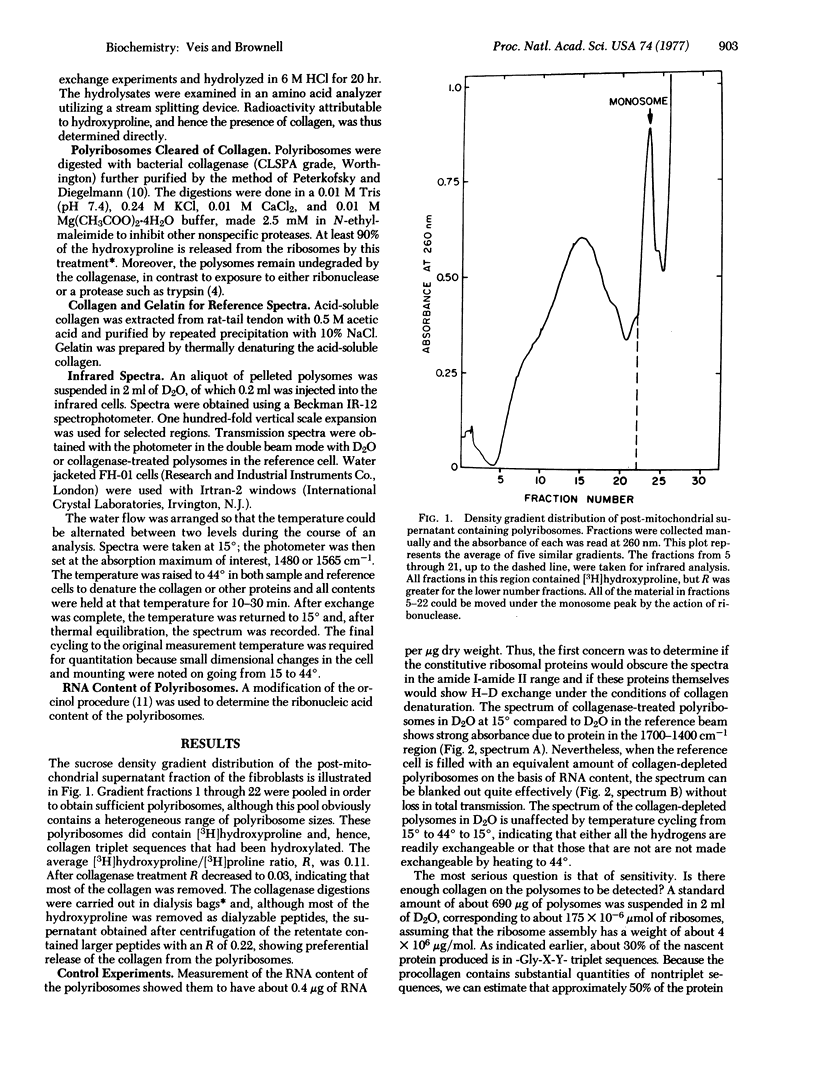
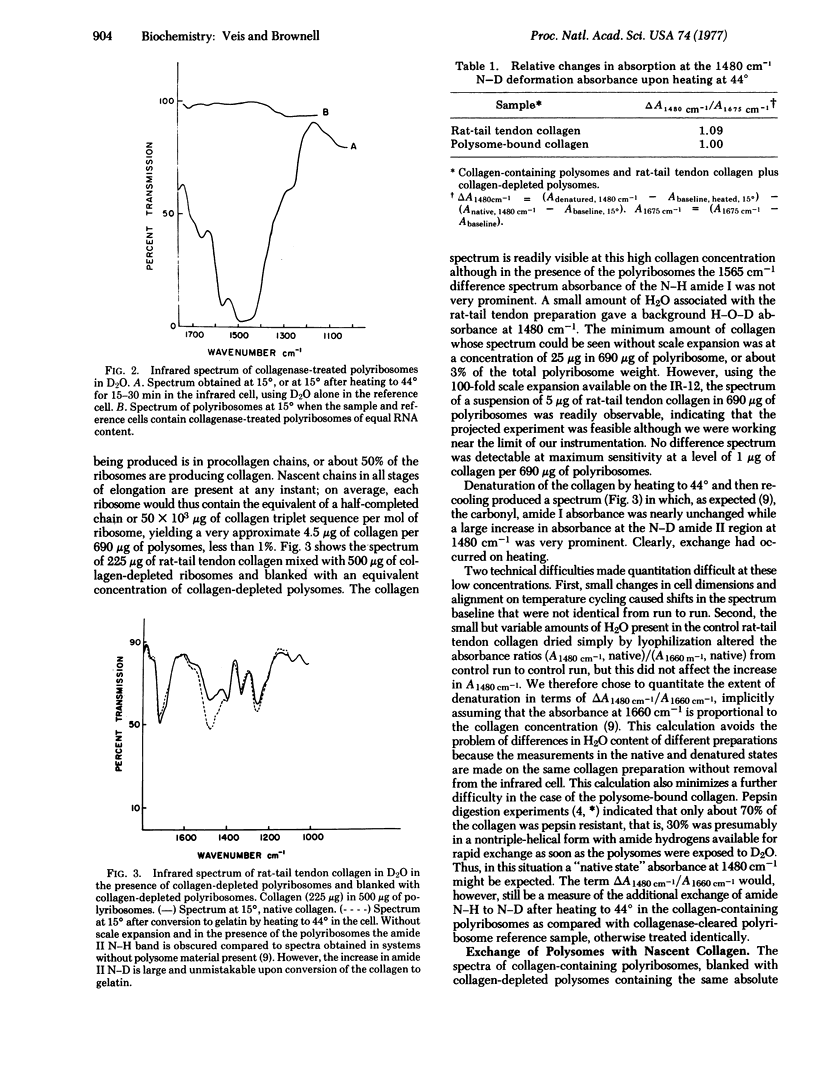
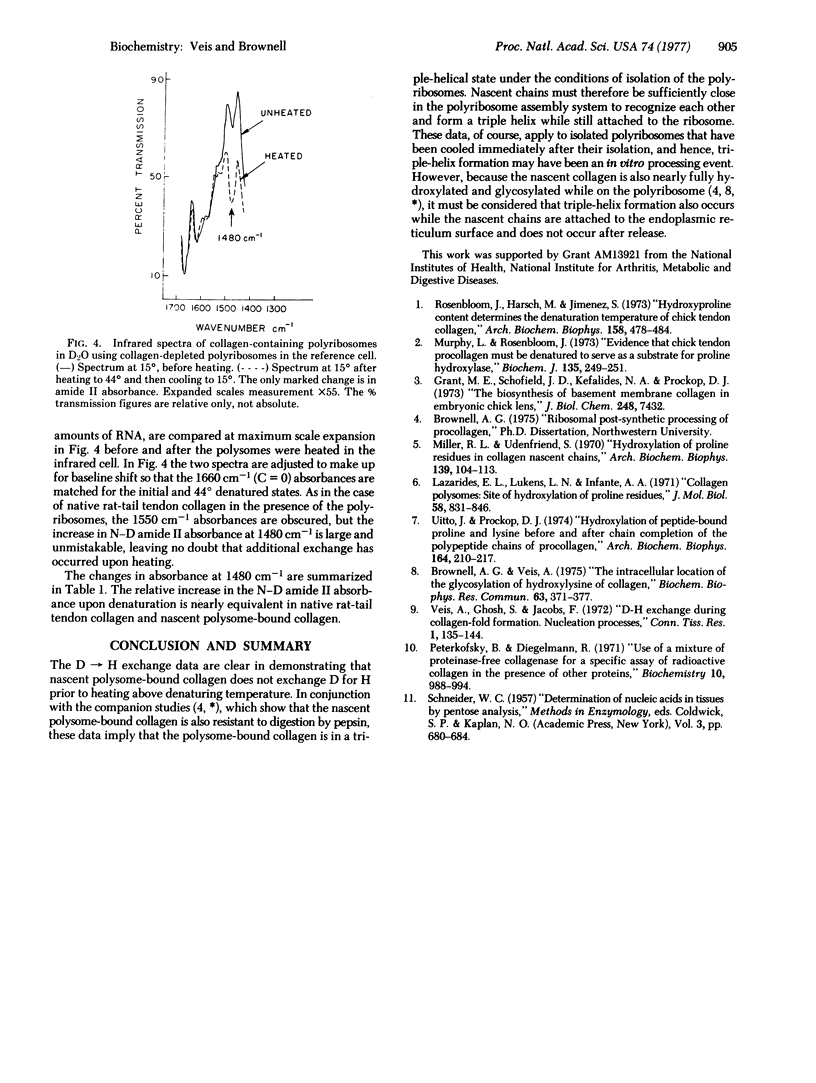
Polyribosomes containing nascent [3H]proline-labeled collagen chains were isolated from chick embryo fibroblasts in culture. These nascent chains were nearly completely hydroxylated, as indicated by the presence of [3H]hydroxyproline and high hydroxyproline/proline ratios. The polyribosomes were suspended in D2O at 15 degrees and the infrared spectrum was determined using a reference cell containing collagen-depleted polyribosomes in D2O, matched to equal RNA content. The amide I and amide II bands were observed. When the polyribosomes were heated in D2O at 44 degrees in the infrared cells, the N--D amide II absorbance at 1480 cm-1 increased markedly, indicating that H leads to D exchange had occurred. Collagen-depleted polyribosomes showed no such changes in absorbance at 1450-1480 cm-1 upon heating. Polyribosomes recovered from the infrared cells after treatment at 44 degrees and cooling still contained collagen, as indicated by their [3H]hydroxyproline content. These data indicate that nascent collagen bound to the polyribosomes can assume a hydrogen-bonded structure. Taken with prior data showing that the nascent collagen was also resistant to pepsin digestion, it is suggested that the collagen examined is in triple-helix conformation. Because the nascent polyribosome-bound collagen is nearly fully hydroxylated, it must be considered that triple-helix formation can occur between nascent chains while they are attached to the endoplasmic reticulum surface and that chain association and triple-helix formation in vivo may well occur before rather than after release.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownell A. G., Veis A. The intracellular location of the glycosylation of hydroxylysine of collagen. Biochem Biophys Res Commun. 1975 Mar 17;63(2):371–377. doi: 10.1016/0006-291x(75)90698-1. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Schofield J. D., Kefalides N. A., Prockop D. J. The biosynthesis of basement membrane collagen in embryonic chick lens. 3. Intracellular formation of the triple helix and the formation of aggregates through disulfide bonds. J Biol Chem. 1973 Nov 10;248(21):7432–7437. [PubMed] [Google Scholar]
- Lazarides E. L., Lukens L. N., Infante A. A. Collagen polysomes: site of hydroxylation of proline residues. J Mol Biol. 1971 Jun 28;58(3):831–846. doi: 10.1016/0022-2836(71)90043-x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Udenfriend S. Hydroxylation of proline residues in collagen nascent chains. Arch Biochem Biophys. 1970 Jul;139(1):104–113. doi: 10.1016/0003-9861(70)90051-2. [DOI] [PubMed] [Google Scholar]
- Murphy L., Rosenbloom J. Evidence that chick tendon procollagen must be denatured to serve as substrate for proline hydroxylase. Biochem J. 1973 Sep;135(1):249–251. doi: 10.1042/bj1350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Harsch M., Jimenez S. Hydroxyproline content determines the denaturation temperature of chick tendon collagen. Arch Biochem Biophys. 1973 Oct;158(2):478–484. doi: 10.1016/0003-9861(73)90539-0. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Hydroxylation of peptide-bound proline and lysine before and after chain completion of the polypeptide chains of procollagen. Arch Biochem Biophys. 1974 Sep;164(1):210–217. doi: 10.1016/0003-9861(74)90024-1. [DOI] [PubMed] [Google Scholar]


