Abstract
Background
The significance of thrombocytopenia to the morbidity and mortality of malaria is poorly defined. We compared the platelet counts and clinical correlates of patients with and those without malaria in southern Papua, Indonesia.
Methods
Data were collated on patients presenting to a referral hospital between April 2004 and December 2012.
Results
Platelet measurements were available in 215 479 patients (23.4%), 66 421 (30.8%) of whom had clinical malaria. Patients with Plasmodium falciparum monoinfection had the lowest platelet counts and greatest risk of severe thrombocytopenia (platelet count, <50 000 platelets/µL), compared with those without malaria (adjusted odds ratio [OR], 6.03; 95% confidence interval [CI], 5.77–6.30]). The corresponding risks were 5.4 (95% CI, 5.02–5.80) for mixed infections, 3.73 (95% CI, 3.51–3.97) for Plasmodium vivax infection, and 2.16 (95% CI, 1.78–2.63) for Plasmodium malariae infection (P < .001). In total, 1.3% of patients (2701 of 215 479) died. Patients with severe malarial anemia alone (hemoglobin level, <5 g/dL) had an adjusted OR for death of 4.93 (95% CI, 3.79–6.42), those with severe malarial thrombocytopenia alone had an adjusted OR of 2.77 (95% CI, 2.20–3.48), and those with both risk factors had an adjusted OR of 13.76 (95% CI, 10.22–18.54; P < .001).
Conclusions
Severe thrombocytopenia identifies both children and adults at increased risk of death from falciparum or vivax malaria, particularly in those with concurrent severe anemia.
Keywords: malaria, Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, thrombocytopenia, Indonesia, platelets
Malaria remains an important threat to public health, particularly in communities with poor resources. Although Plasmodium falciparum accounts for the majority of severe malarial disease in sub-Saharan Africa, outside of Africa, nonfalciparum malarias are responsible for an increasing proportion of infections [1]. Anemia is a common manifestation of Plasmodium infection and is responsible for substantial morbidity, as well as for direct and indirect mortality [2, 3]. Thrombocytopenia is also a common feature of malaria due to all Plasmodium species [4–6], but in the absence of significant bleeding it is not regarded as a defining clinical manifestation of severe malaria [7]. In most cases, thrombocytopenia is not associated with bleeding and requires no treatment, with the platelet count rapidly returning to normal after successful treatment of the malarial episode. Pregnant women and infants appear to be at increased risk of thrombocytopenia, although the adverse consequences of this are unclear [8, 9]. Severe thrombocytopenia (defined as a platelet count of <50 000 platelets/μL) is reported in both P. falciparum and Plasmodium vivax infections, and although it has been associated with bleeding [10, 11] and disseminated intravascular coagulation [12–14], hemorrhagic manifestations are unusual. More recently, low platelet counts have been associated with mortality in patients with P. falciparum [15] and P. vivax infections [16, 17]. However, other studies have not demonstrated an association between thrombocytopenia and significant clinical risk [18, 19].
The present study is part of a prospective surveillance of clinical and laboratory data from Mitra Masyarakat Hospital in southern Papua, Indonesia, an area where 4 species of malaria are coendemic. This analysis was conducted to establish the comparative platelet counts of patients infected by the different Plasmodium species and to define the associated risks of morbidity and mortality.
METHODS
Study Site
Mimika district lies in south-central Papua, the easternmost province of Indonesia. Its geography, climate, and demographic characteristics have been described elsewhere [3, 20]. In brief, malaria transmission occurs throughout the year but is limited to the lowland areas. The average annual incidence of parasitemia is estimated to be 876 episodes per 1000 people, with most cases due to P. falciparum. The prevalence of asexual parasitemia in 2005 was estimated to be 7.5% for P. falciparum, 6.4% for P. vivax, 1.9% for mixed infection, and 0.6% for Plasmodium malariae [20]. Until November 2008, Rumah Sakit Mitra Masyarakat (RSMM) was the only referral hospital in the district, and since 2008 approximately 80% of patients with malaria attending an inpatient facility in the district have been treated there. RSMM has a capacity of 110 beds, with a high-dependency unit, a 24-hour emergency department, and an outpatient department that reviews approximately 300 patients per day, 6 days per week. The age distribution of all patient presentations peaks in infancy, with a second peak among individuals aged in their late 20s [3], whereas the absolute number of patient presentations with malaria peaks during the second year of life. Vivax malaria is the dominant cause of malaria in patients <3 years of age in both the outpatient and inpatient setting [9], and thereafter, P. falciparum is the most common malaria parasite [3, 20].
Laboratory and Data Collection Procedures
Hospital protocols recommend that all patients presenting to the outpatient department with a fever and that all inpatients, regardless of diagnosis, should have a blood film performed for detection of malaria parasites. Microbiological diagnosis of malaria is based on a thick blood film examination, with confirmatory thin blood films and rapid diagnostic tests for P. falciparum also performed in some cases. Microscopy quality control of the hospital laboratory suggests >90% accuracy [21].
On their first presentation to RSMM, every patient is assigned a unique hospital record number, and this is used to link all clinical and laboratory data from all presentations. Demographic and administrative information is recorded by hospital clerks, along with the diagnosis from the attending physician (classified according to the International Classification of Diseases) and any deaths. For the purposes of analyses, ethnicity was categorized as Highland Papuan, Lowland Papuan, or non-Papuan, based on location of the clans' village(s). Complete blood counts are ordered according to clinical indication and are generated by coulter counter (JT Coulter, Ramsey, Minnesota).
Data Merging and Statistical Analyses
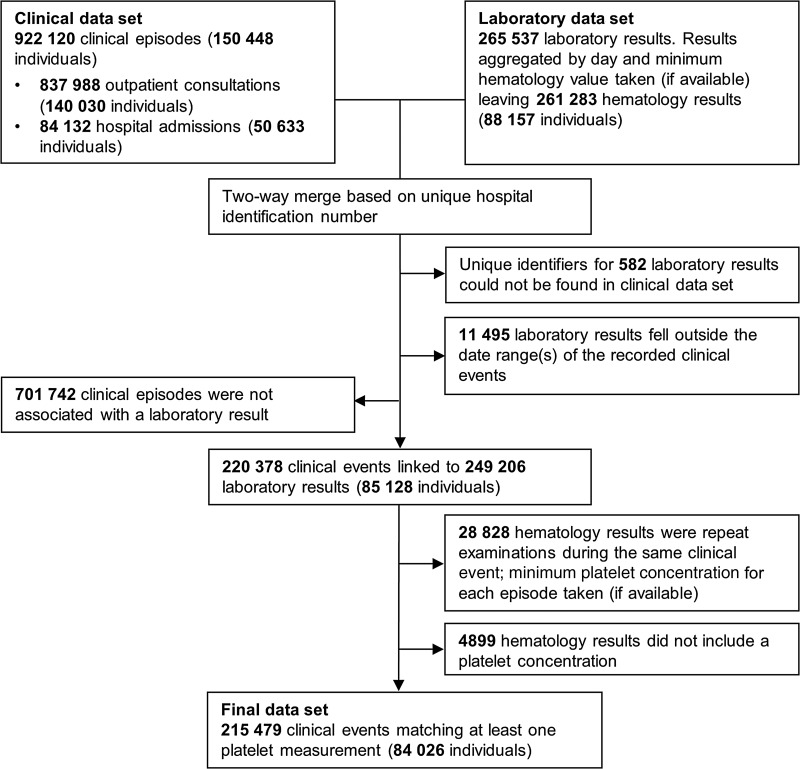
All statistical analyses were done in Stata, version 12.1. Clinical and hematology data were merged using the unique hospital record number and date of presentation. If >1 laboratory measurement was available for a single presentation, the minimum platelet count was taken (Figure 1). The primary outcome in this study was the mean number of platelets per microliter associated with infection for each Plasmodium species, compared with patients without malaria. Secondary measures included the risk of severe thrombocytopenia, the population attributable fraction (PAR) of severe thrombocytopenia associated with infection by the different Plasmodium species, and all-cause mortality. Thrombocytopenia was defined as severe if the platelet count was <50 000 platelets/μL (approximately the fifth percentile) and very severe if the count was <20 000 platelets/μL (approximately the first percentile; Supplementary Figure 1). Severe anemia was defined as a hemoglobin concentration of <5 g/dL. Continuous data were analyzed using linear regression, and binary data (such as severe thrombocytopenia and death) were analyzed using logistic regression. Since some patients appeared in the database multiple times, robust standard errors were calculated using the clustered sandwich estimator.
Figure 1.
Flow diagram of the data merging process. Individual patients could present on multiple occasions and, in some cases, with multiple platelet measurements within each clinical episode. To highlight the degree of multiple sampling, the number of individuals who contributed to these presentations is provided in parentheses.
For the purposes of these analyses, mixed infections were defined as concomitant infection with any combination of Plasmodium species. Univariable and multivariable analyses were performed for each of the following variables: infecting Plasmodium species (negative, P. falciparum, P. vivax, P. malariae, P. ovale, or mixed species), sex, ethnicity (non-Papuan, Highland Papuan, or Lowland Papuan), age group (<1 year, 1 to <5 years, 5 to <15 years, and ≥15 years), year of presentation (2004–2012), department (outpatient vs inpatient), and number of presentations with malaria in the preceding 2 months. Fractional polynomials were used to define the nonlinear relationship between age and the mean platelet count and risk of severe thrombocytopenia [22], but to maintain the stability of these models the following patients were excluded: patients with platelet counts of <5000 or >1 000 000 platelets/μL (338 [0.16%]), infants <1 week of age (1073 [0.50%]), and adults >63 years of age (the 99th percentile; 2205 [1.02%]).
Adjusted PAFs of severe thrombocytopenia were calculated from multivariable logistic regression models, using the punaf module for Stata, which derives PAFs by means of the formulae provided in Greenland and Dreschler [23]. Because of very small numbers, data from patients with P. ovale infections (30 [0.01%]) are included in the baseline values and the univariable analyses but excluded from the multivariable analyses.
Ethics Approval
Ethics approval for this study was obtained from the Health Research Ethics Committees of the University of Gadjah Mada, Indonesia, and the Menzies School of Health Research, Darwin, Australia. Since data were gathered from routine hospital surveillance, informed consent was not requested from participants. However, all records were anonymized, to ensure patient confidentiality.
RESULTS
Of the 922 120 patient presentations to the Mitra Masyarakat Hospital between April 2004 and December 2012, 837 989 (90.9%) were to the outpatient department alone, and 84 131 (9.1%) resulted in hospital admission (Table 1). Microscopically confirmed malaria was diagnosed in 18.3% of patient presentations (168 525), with P. falciparum accounting for 53.3% of monoinfections, P. vivax for 32.3%, P. malariae for 2.7%, and P. ovale for 0.07%. Mixed-species infections were detected in 19 569 presentations (11.6%), which, in 18 489 (94.5%) cases, were mixed P. falciparum and P. vivax infections (Table 1).
Table 1.
Distribution of Clinical and Laboratory Data and Thrombocytopenia Status, by Clinical and Demographic Group
| Variable | Clinical Events, No. | Patients, No. | Distribution of Clinical and Laboratory Data |
Platelet Data |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OP Events, No. |
OP Events With Platelet Count, No. (%)a |
IP Events, No. | IP Events With Platelet Count, No. (%)a |
Mean Platelet Count (per 1000) |
P Valueb | < 50 000 Platelets/μL, No. (%) |
P Valueb | |||
| Infecting Plasmodium species | ||||||||||
| None (negative test results) | 753 595 | 135 229 | 695 051 | 105 432 (15.2) | 58 544 | 43 626 (74.5) | 256.37 ± 141.38 | Ref | 4084 (2.7) | Ref |
| P. falciparum | 89 748 | 44 171 | 73 392 | 21 828 (29.7) | 16 356 | 14 931 (91.3) | 129.03 ± 100.47 | <.001 | 5722 (15.6) | <.001 |
| P. vivax | 54 495 | 28 841 | 49 041 | 14 512 (29.6) | 5454 | 5040 (92.4) | 165.42 ± 121.37 | <.001 | 1650 (8.4) | <.001 |
| >1 (mixed infection) | 19 569 | 14 206 | 16 210 | 5411 (33.4) | 3359 | 3089 (92) | 128.98 ± 96.19 | <.001 | 1148 (13.5) | .203 |
| P. malariae | 4598 | 4045 | 4190 | 1198 (28.6) | 408 | 382 (93.6) | 142.66 ± 98.15 | <.001 | 116 (7.3) | <.001 |
| P. ovale | 115 | 110 | 105 | 22 (21) | 10 | 8 (80) | 146.2 ± 79.97 | <.001 | 2 (6.7) | <.001 |
| Sex | ||||||||||
| Female | 506 055 | 70 493 | 458 599 | 78 552 (17.1) | 47 456 | 38 562 (81.3) | 219.24 ± 136.35 | Ref | 6051 (5.2) | Ref |
| Male | 416 065 | 80 186 | 379 390 | 69 851 (18.4) | 36 675 | 28 514 (77.7) | 222.03 ± 149.37 | .008 | 6671 (6.8) | <.001 |
| Ethnic group | ||||||||||
| Non-Papuan | 149 017 | 44 196 | 136 604 | 22 851 (16.7) | 12 413 | 9146 (73.7) | 247.13 ± 123.31 | Ref | 822 (2.6) | Ref |
| Highland Papuan | 644 073 | 79 443 | 586 388 | 105 757 (18) | 57 685 | 47 318 (82) | 203.73 ± 137.81 | <.001 | 11 235 (7.3) | <.001 |
| Lowland Papuan | 127 385 | 26 413 | 113 440 | 19 589 (17.3) | 13 945 | 10 555 (75.7) | 277.5 ± 164.07 | <.001 | 654 (2.2) | .003 |
| Age, y | ||||||||||
| <1 | 70 221 | 20 086 | 55 359 | 12 998 (23.5) | 14 862 | 6595 (44.4) | 323.49 ± 177.22 | <.001 | 651 (3.3) | <.001 |
| 1 to <5 | 139 033 | 23 596 | 124 985 | 29 337 (23.5) | 14 048 | 11 230 (79.9) | 279.93 ± 170.61 | <.001 | 1403 (3.5) | <.001 |
| 5 to <15 | 107 163 | 23 318 | 100 319 | 19 341 (19.3) | 6844 | 6177 (90.3) | 214.15 ± 136.73 | <.001 | 1478 (5.8) | <.001 |
| ≥15 | 605 588 | 101 728 | 557 250 | 86 659 (15.6) | 48 338 | 43 046 (89.1) | 187.57 ± 111.13 | Ref | 9188 (7.1) | Ref |
| Malaria presentations in the past 2 mo, no. | ||||||||||
| 0 | 799 988 | 150 448 | 724 429 | 126 653 (17.5) | 75 559 | 59 237 (78.4) | 221.41 ± 142.98 | Ref | 11 268 (6.1) | Ref |
| ≥1 | 122 132 | 31 080 | 113 560 | 21 750 (19.2) | 8572 | 7839 (91.4) | 214.90 ± 138.89 | <.001 | 1454 (4.9) | <.001 |
| Year | ||||||||||
| 2004 | 62 985 | 25 864 | 56 227 | 8316 (14.8) | 6758 | 4495 (66.5) | 205.66 ± 149.48 | Ref | 1045 (8.2) | Ref |
| 2005 | 88 400 | 30 950 | 78 398 | 16 676 (21.3) | 10 002 | 8498 (85) | 219.91 ± 153.23 | <.001 | 1688 (6.7) | <.001 |
| 2006 | 96 086 | 34 260 | 86 288 | 12 667 (14.7) | 9798 | 8312 (84.8) | 243.51 ± 160.02 | <.001 | 1.025 (4.9) | <.001 |
| 2007 | 106 046 | 36 914 | 95 272 | 12 872 (13.5) | 10 774 | 8469 (78.6) | 220.4 ± 148.08 | <.001 | 1533 (7.2) | .002 |
| 2008 | 98 074 | 34 127 | 88 967 | 11 184 (12.6) | 9107 | 7504 (82.4) | 211.83 ± 134.54 | .001 | 1245 (6.7) | <.001 |
| 2009 | 110 796 | 34 341 | 100 756 | 20 165 (20) | 10 040 | 8188 (81.6) | 212.5 ± 133.49 | <.001 | 1716 (6.1) | <.001 |
| 2010 | 112 566 | 35 041 | 103 263 | 22 152 (21.5) | 9303 | 7525 (80.9) | 214.67 ± 136.52 | <.001 | 1735 (5.8) | <.001 |
| 2011 | 115 683 | 37 055 | 106 640 | 18 840 (17.7) | 9043 | 6654 (73.6) | 225.24 ± 133.65 | <.001 | 1230 (4.8) | <.001 |
| 2012 | 131 484 | 41 495 | 122 178 | 25 531 (20.9) | 9306 | 7431 (79.9) | 225.64 ± 136.95 | <.001 | 1505 (4.6) | <.001 |
| Overall | 922 120 | 150 448 | 837 989 | 148 403 (17.7) | 84 131 | 67 076 (79.7) | 220.52 ± 142.44 | 12 722 (5.9) | ||
Abbreviations: IP, inpatient; OP, outpatient; Ref, reference.
Events in which the platelet count was measured.
Based on univariable linear regression with correction of the variance-covariance matrix for within-patient correlation.
Availability of Platelet Data
Overall, 215 479 presentations (23.4%) could be matched with at least 1 platelet count measurement (Figure 1). A greater proportion of outpatient visits were linked to a platelet count measurement if the patient had malaria rather than no malaria (30.1% vs 15.2%, respectively; P < .001). The corresponding figures for patients admitted to the wards were (91.6% vs 74.5%; P < .001). Patients who had a platelet measurement had a median age of 21.0 years, compared with 23.0 years among those without a measurement. Measurement of platelets was also slightly more common in males, compared with females (23.6% vs 23.1%), and in Papuans, compared with non-Papuans (23.7% vs 21.5%).
Reduction in Platelet Concentration Associated With Malaria
The mean platelet count was significantly lower in Highland Papuans (204 × 103 platelets/μL), compared with that for Lowland Papuans (278 × 103 platelets/μL) and non-Papuans (247 × 103 platelets/μL; P < .001 for both comparisons). Whereas the overall risk of severe thrombocytopenia with each presentation fell to <2% from early childhood in non-Papuans and lowlanders, highlanders remained at significantly greater risk throughout adulthood (P < .001; Supplementary Figure 2).
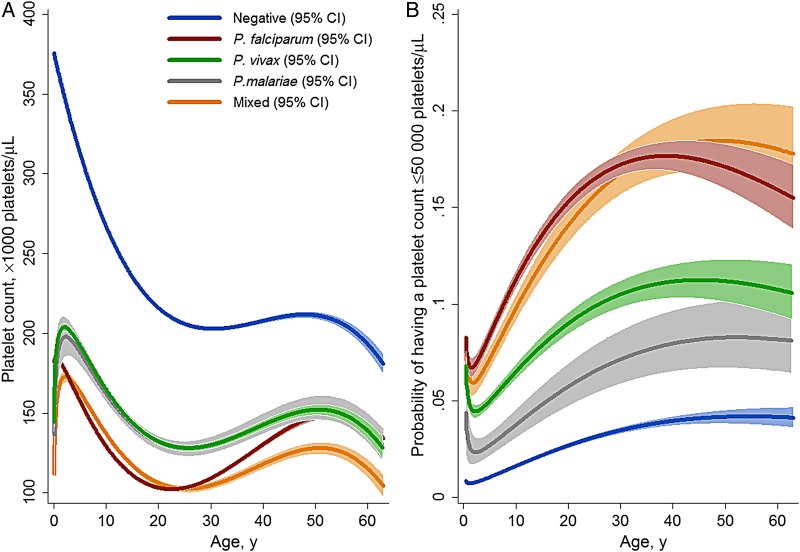
The mean platelet concentration and prevalence of severe thrombocytopenia varied significantly with Plasmodium species (Table 1). Overall, P. falciparum, alone or as part of a mixed infection, was associated with the greatest difference in mean platelet counts, compared with counts for individuals without malaria (−127 × 103 platelets/μL [95% confidence interval [CI], −126 to −129 × 103 platelets/μL]; P < .001). After adjustment for confounding factors, patients presenting to hospital with malaria had lower mean platelet counts and higher odds of severe thrombocytopenia than patients without malaria at all ages (Figure 2). Patients with P. falciparum (alone or as part of mixed infections) without a history of presentation to the hospital with malaria within the preceding 2 months had significantly lower platelet counts (127 × 103 platelets/μL [95% CI, 126 × 103–128 × 103 platelets/μL]), compared with those with a single recent episode of malaria (140 × 103 platelets/μL [95% CI, 138 × 103–143 × 103 platelets/μL]) and those with ≥2 episodes (149 × 103 platelets/μL [95% CI, 143 to 155 × 103 platelets/μL]). The influence of recent history of malaria was not apparent in patients presenting without malaria or in those with P. vivax monoinfection.
Figure 2.
Estimated mean platelet count among hospital attendees (A) and estimated probability of severe thrombocytopenia (platelet concentration, <50 000 platelets/µL; B), by Plasmodium species. Figures were generated by multiple fractional polynomial regression analyses with the following covariables: Plasmodium species, by age, sex, ethnic group, and year. Bands represent 95% CIs. Abbreviation: CI, confidence interval.
Risk of Severe Thrombocytopenia
In total, 75 029 (34.8%) of the 215 479 presentations were associated with a platelet count of <150 × 103 platelets/μL, 12 722 (5.9%) had counts of <50 × 103 platelets/μL, and 2001 (0.9%) had counts of <20 × 103 platelets/μL. Compared with patients without malaria, patients with P. falciparum infection were at the greatest risk of severe thrombocytopenia (adjusted odds ratio [OR], 6.03 [95% CI, 5.77–6.30]), followed by those with mixed infections (adjusted OR, 5.40 [95% CI, 5.02–5.80]), those with P. vivax infection (adjusted OR, 3.73 [95% CI, 3.51–3.97]), and those with P. malariae infection (adjusted OR, 2.16 [95% CI, 1.78–2.63]); P < .001 for all comparisons; Table 2).
Table 2.
Risk Factors for Severe Thrombocytopenia
| Risk Factor | Univariable Analysis |
Multivariable Analysis |
|||
|---|---|---|---|---|---|
| Crude OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Overall PAF, % (95% CI) | |
| Infecting Plasmodium species | |||||
| None (negative test results) | Reference | Reference | |||
| P. falciparum | 6.54 (6.28–6.83) | <.001 | 6.03 (5.77–6.30) | <.001 | 35.88 (34.92–36.83) |
| P. vivax | 3.27 (3.08–3.47) | <.001 | 3.73 (3.51–3.97) | <.001 | 9.14 (8.55–9.72) |
| P. malariae | 2.81 (2.32–3.41) | <.001 | 2.16 (1.78–2.63) | <.001 | 0.47 (.31–0.62) |
| >1 (mixed infection) | 5.54 (5.17–5.94) | <.001 | 5.40 (5.02–5.80) | <.001 | 7.04 (6.58–7.51) |
| Age, y | |||||
| ≥15 | Reference | Reference | |||
| <1 | 0.45 (.42–.49) | <.001 | 0.47 (.43–.51) | <.001 | <0 |
| 1 to <5 | 0.47 (.44–.50) | <.001 | 0.32 (.30–.34) | <.001 | <0 |
| 5 to <15 | 0.81 (.76–.85) | <.001 | 0.50 (.47–.53) | <.001 | <0 |
| Sex | |||||
| Female | Reference | Reference | |||
| Male | 1.34 (1.29–1.38) | <.001 | 1.39 (1.34–1.44) | <.001 | 12.99 (11.49–14.46) |
| Ethnicity | |||||
| Non-Papuan | Reference | Reference | |||
| Highland Papuan | 3.00 (2.80–3.23) | <.001 | 2.75 (2.56–2.97) | <.001 | 52.71 (49.92–55.34) |
| Lowland Papuan | 0.84 (.76–.93) | .001 | 0.96 (.86–1.07) | .430 | <0 |
| Hb level <5 g/dLa | |||||
| No | Reference | Reference | |||
| Yes | 4.02 (3.79–4.27) | <.001 | 3.16 (2.96–3.37) | <.001 | 7.00 (6.49–7.51) |
| Yearb | |||||
| 2004 | Reference | Reference | … | ||
| 2005 | 0.81 (.75–.88) | <.001 | 0.86 (.79–.94) | <.001 | … |
| 2006 | 0.58 (.53–.63) | <.001 | 0.65 (.59–.71) | <.001 | … |
| 2007 | 0.87 (.80–.95) | .001 | 0.98 (.90–1.07) | .590 | … |
| 2008 | 0.80 (.74–.88) | <.001 | 1.08 (.98–1.18) | .110 | … |
| 2009 | 0.73 (.67–.79) | <.001 | 0.97 (.89–1.06) | .516 | … |
| 2010 | 0.70 (.65–.76) | <.001 | 0.96 (.88–1.05) | .375 | … |
| 2011 | 0.57 (.52–.62) | <.001 | 0.83 (.76–.91) | <.001 | … |
| 2012 | 0.54 (.50–.58) | <.001 | 0.72 (.66–.79) | <.001 | … |
| Departmentc | |||||
| Outpatient | Reference | Excluded | … | ||
| Inpatient | 2.38 (2.30–2.47) | <.001 | … | … | |
| Malaria presentations in the past 2 mo, no. | |||||
| 0 | Reference | Reference | … | ||
| ≥1 | 0.80 (.76–.85) | <.001 | 0.71 (.67–0.75) | <.001 | <0 |
Severe thrombocytopenia was defined as a platelet count of <50 000 platelets/μL.
Abbreviations: CI, confidence interval; Hb, hemoglobin; OR, odds ratio; PAF, population attributable fraction.
Criterion for anemia.
Included in the model for calculation of overall PAFs, but PAFs are not presented.
Not included in the multivariable model because admission was deemed to be a consequence of severe disease, rather than a confounder.
Patients with a recent presentation to the hospital with malaria due to any Plasmodium species were at a lower risk of severe thrombocytopenia, compared with those with no recent malaria (adjusted OR, 0.71 [95% CI, .67–.75]; P < .001; Table 2). The effect of recent malaria was most noticeable in patients presenting with P. falciparum infection (adjusted OR, 0.50 [95% CI, .45–.55]; P < .001), compared with those without malaria (adjusted OR, 0.89 [95% CI, .80–.98]; P = .017) or those with P. vivax (adjusted OR, 0.96 [95% CI, .84–1.09]; P = .510).
In total, 215 044 (99.8%) of patients with platelet counts available also had hemoglobin levels measured; of these, 3.7% (7931) had severe anemia. The risk of severe thrombocytopenia was 18.7% (1484 of 7931) among patients with severe anemia, compared with 5.4% (11 221 of 207 113) among those without severe anemia (adjusted OR, 3.16 [95% CI, 2.96–3.37]; P < .001). The overall adjusted population fraction of severe thrombocytopenia attributable to P. falciparum infection was 35.9% (95% CI, 34.9%–36.8%), with 9.1% (95% CI, 8.6%–9.7%) attributable to P. vivax, 0.5% (95% CI, 0.3%–0.6%) attributable to P. malariae, and 7.0% (95% CI, 6.6%–7.5%) attributable to mixed species infections (Table 2).
Consequences of Severe Thrombocytopenia
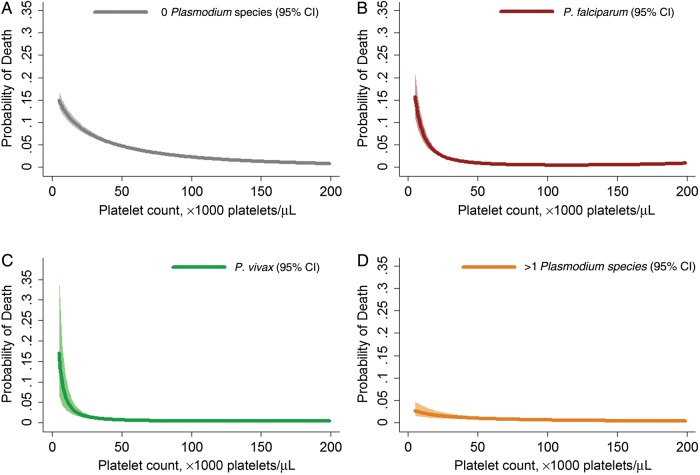
Admission for inpatient care was required in 49.6% of patients (6196 12 499) initially presenting to the outpatient department with severe thrombocytopenia, compared with 29.0% (57 966 of 200 066) among patients without severe thrombocytopenia (OR, 2.41 [95% CI, 2.32–2.50]; P < .001). Overall, 1.3% of patients (2701 of 215 479) with a platelet measurement died, with the risk of death rising exponentially as the platelet count fell (Figure 3). The mortality risk among patients with severe thrombocytopenia (<50 000 platelets/μL) was 7.9% (324 of 4084) among those without malaria, 2.1% (120 of 5722) among those with P. falciparum infection, 1.5% (25 of 1650) among those with P. vivax infection, 1.7% (2 of 114) among those with P. malariae infection, and 1.7% (19 of 1148) among those with mixed infections. When platelet counts fell to <20 000 platelets/μL, the risk of death increased to 11% (108 of 978) among patients without malaria, 5.6% (40 of 708) among those with P. falciparum infection, 3.6% (6 of 168) among those with P. vivax infection, 0% (0 of 15) among those with P. malariae infection, and 3.1% (4 of 131) among those with mixed infections.
Figure 3.
Estimated probability of mortality, by platelet count. Figures were generated by multiple fractional polynomial regression analyses (with adjustment for age, sex, ethnic group, and year), according to infecting Plasmodium species, as follows: 0 Plasmodium species (negative test results; A), P. falciparum (B), P. vivax (C), and >1 Plasmodium species (mixed infection; D). Abbreviation: CI, confidence interval.
Overall, compared with patients with neither severe anemia nor thrombocytopenia, the adjusted ORs for death were 5.21 (95% CI, 4.53–5.98) among those with severe anemia alone, 4.65 (95% CI, 4.10–5.28) among those with severe thrombocytopenia alone, and 16.44 (95% CI, 13.70–19.74) among those with both (Table 3). This relationship was apparent in both children and adults with malaria and in P. falciparum, P. vivax, and mixed infections (Table 4).
Table 3.
Univariable and Multivariable Analyses of the Risk Factors for Death Among 215 449 Patients
| Variable | Univariable Analysis |
Multivariable Analysis |
|||
|---|---|---|---|---|---|
| Crude OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | PAF, % (95% CI) | |
| Platelet count, Hb levela | |||||
| ≥50 000 platelets/μL | |||||
| ≥5 g/dL | Reference | Reference | … | ||
| <5 g/dL | 3.92 (3.43–4.49) | <.001 | 5.21 (4.53–5.98) | <.001 | 7.26 (6.17–8.33) |
| <50 000 platelets/μL | |||||
| ≥5 g/dL | 3.07 (2.73–3.45) | <.001 | 4.65 (4.10–5.28) | <.001 | 9.71 (8.43–10.97) |
| <5 g/dL | 11.28 (9.49–13.42) | <.001 | 16.44 (13.70–19.74) | <.001 | 5.24 (4.42–6.04) |
| Infecting Plasmodium species | |||||
| None (negative test results) | Reference | Reference | |||
| P. falciparum | 0.61 (.54–0.69) | <.001 | 0.43 (.38–.49) | <.001 | <0 |
| P. vivax | 0.38 (.31–.46) | <.001 | 0.32 (.26–.39) | <.001 | <0 |
| P. malariae | 0.56 (.32–.96) | .036 | 0.50 (.29–.87) | .014 | <0 |
| >1 (mixed infection) | 0.49 (.38–.63) | <.001 | 0.36 (.28–.47) | <.001 | <0 |
| Age, y | |||||
| ≥15 | Reference | Reference | … | ||
| <1 | 1.37 (1.22–1.53) | <.001 | 1.47 (1.30–1.65) | <.001 | 4.24 (2.78–5.67) |
| 1 to <5 | 0.60 (.53–.67) | <.001 | 0.72 (.64–.82) | <.001 | <0 |
| 5 to <15 | 0.56 (.48–.65) | <.001 | 0.65 (.56–.76) | <.001 | <0 |
| Sex | |||||
| Female | Reference | Reference | … | ||
| Male | 1.58 (1.46–1.71) | <.001 | 1.63 (1.51–1.77) | <.001 | 21.56 (18.10–24.87) |
| Ethnicity | |||||
| Non-Papuan | Reference | Reference | … | ||
| Highland Papuan | 0.61 (.56–.67) | <.001 | 0.60 (.54–.66) | <.001 | <0 |
| Lowland Papuan | 0.93 (.83–1.05) | .267 | 0.96 (.85–1.09) | .540 | <0 |
| Yearb | |||||
| 2004 | Reference | Reference | … | ||
| 2005 | 0.98 (.80–1.20) | .867 | 1.11 (.91–1.36) | .316 | … |
| 2006 | 1.41 (1.16–1.71) | .001 | 1.55 (1.27–1.89) | <.001 | … |
| 2007 | 1.57 (1.29–1.90) | <.001 | 1.55 (1.28–1.89) | <.001 | … |
| 2008 | 1.84 (1.52–2.22) | <.001 | 1.82 (1.50–2.21) | <.001 | … |
| 2009 | 0.92 (.75–1.12) | .401 | 1.04 (.85–1.27) | .687 | … |
| 2010 | 0.76 (.62–0.93) | .009 | 0.87 (.71–1.07) | .201 | … |
| 2011 | 0.93 (.76–1.14) | .497 | 1.07 (.87–1.31) | .513 | … |
| 2012 | 0.87 (.71–1.06) | .158 | 1.03 (.85–1.26) | .748 | … |
| Malaria presentations in the past 2 mo, no. | |||||
| 0 | Reference | Reference | … | ||
| ≥1 | 0.63 (.55–.72) | <.001 | 0.80 (.69–.91) | .001 | <0 |
Abbreviations: CI, confidence interval; Hb, hemoglobin; OR, odds ratio; PAF, population attributable fraction.
Severe thrombocytopenia was defined as a platelet count of <50 000 platelets/μL, and anemia was defined as a hemoglobin (Hb) level of <5 g/dL.
Included in the model for calculation of overall PAFs, but PAFs are not presented.
Table 4.
Risk of Mortality According to Severe Thrombocytopenia, Severe Anemia, and Malaria Status
| Variable | Platelet Count ≥50 000 Platelets/μL, Hb Level <5 g/dL |
Platelet Count <50 000 Platelets/µL, Hb Level ≥5 g/dL |
Platelet Count <50 000 Platelets/µL, Hb Level <5 g/dL |
|||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI)a | P Value | Adjusted OR (95% CI)a | P Value | Adjusted OR (95% CI)a | P Value | |
| Infecting Plasmodium speciesa | ||||||
| None (negative test results) | 5.10 (4.32–6.01) | <.001 | 6.21 (5.37–7.20) | <.001 | 17.14 (13.61–21.58) | <.001 |
| P. falciparum | 5.05 (3.64–7.00) | <.001 | 2.71 (2.06–3.55) | <.001 | 16.97 (11.81–24.38) | <.001 |
| P. vivax | 4.04 (2.22–7.33) | <.001 | 2.67 (1.55–4.60) | <.001 | 9.21 (4.53–18.73) | <.001 |
| >1 (mixed infection) | 5.29 (2.69–10.43) | <.001 | 2.52 (1.26–5.05) | .009 | 9.77 (4.39–21.73) | <.001 |
| Any, overall | 4.93 (3.79–6.42) | <.001 | 2.77 (2.20–3.48) | <.001 | 13.76 (10.22–18.54) | <.001 |
| Age among malarial patients, yb | ||||||
| <1 | 4.36 (1.80–10.56) | .001 | 3.96 (1.55–10.09) | .004 | 7.17 (2.60–19.79) | <.001 |
| 1 to <5 | 3.23 (1.92–5.43) | <.001 | 1.77 (.84–3.71) | .133 | 11.89 (6.52–21.66) | <.001 |
| 5 to <15 | 6.35 (3.24–12.46) | <.001 | 2.40 (1.14–5.04) | .021 | 5.98 (1.41–25.40) | .015 |
| ≥15 | 6.18 (4.28–8.92) | <.001 | 2.87 (2.19–3.77) | <.001 | 20.05 (13.57–29.62) | <.001 |
Abbreviations: CI, confidence interval; Hb, hemoglobin; OR, odds ratio.
Model presents risk with respect to the reference group of patients without severe anemia or thrombocytopenia (severe thrombocytopenia was defined as a platelet count of <50 000 platelets/μL, and anemia was defined as a Hb level of <5 g/dL). Adjusted for age group, sex, ethnicity, year, and recent malaria presentations in the past 2 months.
Model presents risk in patients with malaria, with respect to the reference group of patients without severe anemia or thrombocytopenia (severe thrombocytopenia was defined as a platelet count of <50 000 platelets/μL, and anemia was defined as a Hb level of <5 g/dL). Adjusted for species, sex, ethnicity, year, and recent malaria presentations in the past 2 months.
In the absence of severe anemia, the greatest risk of mortality associated with severe thrombocytopenia was among patients without malaria (adjusted OR, 6.21 [95% CI, 5.37–7.20]; P < .001), whereas the risk among patients with malaria was 2.77 (95% CI, 2.20 to 3.480; P < .001), with no difference between infecting species (Table 4). The overall PAF of death associated with severe thrombocytopenia was 14.6% (95% CI, 13.1%–16.0%); the full multivariable model for mortality is presented in Table 3. There was no significant difference in the risk of bleeding recorded in patients with (4.3% [21 of 490]) and those without (5.7% [126 of 2211]) severe thrombocytopenia (P = .228).
DISCUSSION
In this very large hospital-based surveillance study, almost two thirds of patients with acute malaria had thrombocytopenia (platelet count, <150 000 platelets/μL), with 13% of patients presenting with platelet counts of <50 000 platelets/μL. The greatest risk of severe thrombocytopenia was in patients infected with P. falciparum, either alone or mixed (OR, 5.4–6.1), accounting for >40% of observed cases. Severe thrombocytopenia was associated with a 2.4-fold greater risk of admission to hospital and a 4.7-fold increased risk of death, rising to 16-fold when both severe anemia and severe thrombocytopenia were present (Table 3). Similar relationships between the risk of death and severe thrombocytopenia were seen in both children and adults with malaria and in cases of P. falciparum and P. vivax infections.
Malaria causes a variety of hematological insults arising from hemolysis, host inflammatory response, hematopoietic suppression, and splenic pooling [24, 25]. Severe anemia is an important prognostic indicator of fatal outcome, particularly in young children [3, 26]. While thrombocytopenia is also extremely common, its contribution to morbidity and mortality has been less clear. In patients with falciparum malaria, severe disease and mortality are increased with severe thrombocytopenia [15, 27], and more recently this has also been observed in patients with severe vivax malaria [16]. Many previous studies examining predictors of malaria mortality have not included platelet counts [7]. Other studies showing no relationship between malarial thrombocytopenia and mortality have been smaller and may have been underpowered [28, 29].
Previous studies have shown a consistent inverse correlation between parasitemia at presentation and the platelet count [17], but our study did not record the peripheral parasite count routinely, and hence we were unable to explore this. In Papua, we have shown that peripheral parasitemia is considerably higher in symptomatic patients with P. falciparum infections, compared with patients infected with P. vivax [20, 30]. This may have contributed to the greater risk of severe thrombocytopenia in patients with P. falciparum infection (OR, 6.1), compared with those with P. vivax (OR, 3.7).
We have shown previously that the risk of anemia in this population is greatest in young patients, highlanders, and those presenting with recurrent episodes of malaria [3]. In contrast, in the current analysis, the risk of severe thrombocytopenia was significantly lower in patients with malaria who had had a prior episode of malaria within the preceding 2 months (OR, 0.8); this attenuation was most apparent in patients presenting with P. falciparum but not in those presenting with P. vivax monoinfection or without malaria. Furthermore, after the first year of life, lowland and non-Papuan patients had a low risk of severe thrombocytopenia. The risk of thrombocytopenia was significantly higher in Highland Papuans, and this was sustained throughout adulthood. Highlanders constitute an ethnic group originating from non–malaria-endemic regions who have not been under genetic selection pressure from malaria parasite infections. In the last decade, many highlanders have migrated at all ages to the lowland areas, where they have been exposed to malaria, often getting their first episodes of malaria in later life. Our findings are consistent with lowland ethnicity or recent malaria resulting in a reduction of the host inflammatory response to acute malaria and decreased platelet activation and consumption.
The pathogenic mechanisms by which platelets mediate disease severity remain to be delineated. However, clinical, autopsy, ex vivo, and in vitro studies have shown that platelets are involved in parasite sequestration [31], as well as in clumping and/or agglutination of infected and uninfected erythrocytes [32, 33]. Platelets express Toll-like receptors (TLRs), which, on recognition of P. falciparum molecular patterns, release prepackaged inflammatory mediators [34]. This could partially explain the attenuation with repeat exposure, as repeated stimulation of TLRs leads to decreased signaling and decreased inflammatory responses [35]. Nitric oxide (NO) is also a key mediator of platelet homeostasis, and the decreased NO bioavailability found in both children [36] and adults [37] with severe and fatal malaria may contribute to increased platelet activation and consumption.
Our large-scale observational study has a number of limitations. First, platelet counts were only available in 26% of all presentations, and so there may be a degree of residual confounding in our multivariable analyses. However, the available hematology data rose to 80% in patients requiring admission. Although the risk of thrombocytopenia was greater in inpatients than outpatients, the magnitude of the other risk factors remained similar in both departments. Second, the surveillance program did not document the presence of all severe manifestations of malaria in these patients, so it is not possible in this data set to determine whether the presence of severe thrombocytopenia would have identified patients at risk of death in whom other World Health Organization (WHO) criteria for severe disease were not apparent [7]. Previous studies have used multivariate analysis to identify biomarkers predictive of poor outcome, but most have not included platelet counts [7]. Hence, it is possible that the mortality risk associated with severe thrombocytopenia may be better represented by other clinical biochemical and inflammatory markers. However, platelet counts are readily available from an automated blood count, a routine laboratory test that is widely accessible even in referral inpatient facilities and even some more remote health posts and that is more accessible than other recognized laboratory predictors of mortality in WHO severity criteria (such lactate and bicarbonate levels or creatinine level) [7]. Our study, the largest to date that examined relationships between severe thrombocytopenia and malaria mortality, highlights that severe thrombocytopenia should serve as a warning sign of poor outcome, particularly when coexisting with severe anemia. We believe that severe thrombocytopenia may be useful in guiding the need for referral or triage to a ward where a higher level of care is provided. Our analysis focused on applying a threshold of 50 000 platelets/μL, which was associated with an overall mortality of 3.9%, a PAF of 14.5% and sufficient power to determine other relevant confounding factors. However, the mortality risk rose to 7.9% in patients with a platelet count of <20 000 platelets/μL (5.6% in falciparum malaria and 3.6% in vivax malaria). We propose that a platelet count of ≤20 000 platelets/μL should be included as a defining severity criterion for both falciparum and vivax malaria. Prospective studies are warranted to evaluate the prognostic value of using platelet counts in conjunction with hemoglobin concentrations to define medical interventions and to determine the underlying processes by which thrombocytopenia contributes to the pathology of malaria.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Prof Yati Soenarto and Dr Yodi Mahendradhata, from the University of Gadjah Mada, for their support and facilitation of this study; and Lembaga Pengembangan Masyarakat Amungme Kamoro and the staff of Rumah Sakit Mitra Masyarakat.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Wellcome Trust (senior fellowship in Clinical Science 091625 to R. N. P.), the National Health and Medical Reearch Council (practitioner fellowship 1042072 to N. M. A. and program grant 1037304), and AusAID (to the Timika Research Facility and Papuan Community Health Foundation).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gething PW, Elyazar IR, Moyes CL, et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calis JC, Phiri KS, Faragher EB, et al. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–99. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 3.Douglas NM, Lampah DA, Kenangalem E, et al. Major burden of severe anemia from non-falciparum malaria species in southern papua: a hospital-based surveillance study. PLoS Med. 2013;10:e1001575. doi: 10.1371/journal.pmed.1001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacerda MV, Mourao MP, Coelho HC, Santos JB. Thrombocytopenia in malaria: who cares? Mem Inst Oswaldo Cruz. 2011;106(suppl 1):52–63. doi: 10.1590/s0074-02762011000900007. [DOI] [PubMed] [Google Scholar]
- 5.Horstmann RD, Dietrich M, Bienzle U, Rasche H. Malaria-induced thrombocytopenia. Blut. 1981;42:157–64. doi: 10.1007/BF01026385. [DOI] [PubMed] [Google Scholar]
- 6.Barber BE, William T, Grigg MJ, et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis. 2013;56:383–97. doi: 10.1093/cid/cis902. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. 4th ed. Trop Med Int Health; 2014. Severe and complicated malaria. In press. [Google Scholar]
- 8.Tan SO, McGready R, Zwang J, et al. Thrombocytopaenia in pregnant women with malaria on the Thai-Burmese border. Malar J. 2008;7:209. doi: 10.1186/1475-2875-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poespoprodjo JR, Fobia W, Kenangalem E, et al. Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis. 2009;48:1704–12. doi: 10.1086/599041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thapa R, Biswas B, Mallick D, Sardar S, Modak S. Childhood Plasmodium vivax malaria with severe thrombocytopenia and bleeding manifestations. J Pediatr Hematol/Oncol. 2009;31:758–9. doi: 10.1097/MPH.0b013e3181b7eb12. [DOI] [PubMed] [Google Scholar]
- 11.Stone WJ, Hanchett JE, Knepshield JH. Acute renal insufficiency due to falciparum malaria. Review of 42 cases. Arch Int Med. 1972;129:620–8. doi: 10.1001/archinte.129.4.620. [DOI] [PubMed] [Google Scholar]
- 12.Phillips RE, Warrell DA. The pathophysiology of severe falciparum malaria. Parasitol Today. 1986;2:271–82. doi: 10.1016/0169-4758(86)90136-5. [DOI] [PubMed] [Google Scholar]
- 13.Punyagupta S, Srichaikul T, Nitiyanant P, Petchclai B. Acute pulmonary insufficiency in falciparum malaria: summary of 12 cases with evidence of disseminated intravascular coagulation. Am J Trop Med Hyg. 1974;23:551–9. doi: 10.4269/ajtmh.1974.23.551. [DOI] [PubMed] [Google Scholar]
- 14.Song JY, Park CW, Jo YM, et al. Two cases of Plasmodium vivax malaria with the clinical picture resembling toxic shock. Am J Trop Med Hyg. 2007;77:609–11. [PubMed] [Google Scholar]
- 15.Gerardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, Imbert P. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–91. doi: 10.4269/ajtmh.2002.66.686. [DOI] [PubMed] [Google Scholar]
- 16.Kochar DK, Das A, Kochar A, et al. Thrombocytopenia in Plasmodium falciparum, Plasmodium vivax and mixed infection malaria: a study from Bikaner (Northwestern India) Platelets. 2010;21:623–7. doi: 10.3109/09537104.2010.505308. [DOI] [PubMed] [Google Scholar]
- 17.Leal-Santos FA, Silva SB, Crepaldi NP, et al. Altered platelet indices as potential markers of severe and complicated malaria caused by Plasmodium vivax: a cross-sectional descriptive study. Malar J. 2013;12:462. doi: 10.1186/1475-2875-12-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimalizeni Y, Kawaza K, Taylor T, Molyneux M. The platelet count in cerebral malaria, is it useful to the clinician? Am J Trop Med Hyg. 2010;83:48–50. doi: 10.4269/ajtmh.2010.09-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119:839–47. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 20.Karyana M, Burdarm L, Yeung S, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. doi: 10.1186/1475-2875-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tjitra E, Anstey NM, Sugiarto P, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Statist. 1994;43:429–67. [Google Scholar]
- 23.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–72. [PubMed] [Google Scholar]
- 24.Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitol Today. 2000;16:469–76. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 25.Douglas NM, Anstey NM, Buffet PA, et al. The anaemia of Plasmodium vivax malaria. Malar J. 2012;11:135. doi: 10.1186/1475-2875-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 27.Pain A, Ferguson DJ, Kai O, et al. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci U S A. 2001;98:1805–10. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladhani S, Newton CR. Plasmodium falciparum malaria in a hypoendemic region in Senegal. Am J Trop Med Hyg. 2003;68:379–80. author reply 80–1. [PubMed] [Google Scholar]
- 29.Moulin F, Lesage F, Legros AH, et al. Thrombocytopenia and Plasmodium falciparum malaria in children with different exposures. Arch Dis Child. 2003;88:540–1. doi: 10.1136/adc.88.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratcliff A, Siswantoro H, Kenangalem E, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–65. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bridges DJ, Bunn J, van Mourik JA, et al. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood. 2010;115:1472–4. doi: 10.1182/blood-2009-07-235150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grau GE, Mackenzie CD, Carr RA, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–6. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- 33.Chotivanich K, Sritabal J, Udomsangpetch R, et al. Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J Infect Dis. 2004;189:1052–5. doi: 10.1086/381900. [DOI] [PubMed] [Google Scholar]
- 34.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–67. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutlis CS, Yeo TW, Anstey NM. Malaria tolerance--for whom the cell tolls? Trends Parasitol. 2006;22:371–7. doi: 10.1016/j.pt.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.