Abstract
We propose for the first time to divide histone proteolysis into “histone degradation” and the epigenetically connoted “histone clipping”. Our initial observation is that these two different classes are very hard to distinguish both experimentally and biologically, because they can both be mediated by the same enzymes. Since the first report decades ago, proteolysis has been found in a broad spectrum of eukaryotic organisms. However, the authors often not clearly distinguish or determine whether degradation or clipping was studied. Given the importance of histone modifications in epigenetic regulation we further elaborate on the different ways in which histone proteolysis could play a role in epigenetics. Finally, unanticipated histone proteolysis has probably left a mark on many studies of histones in the past. In conclusion, we emphasize the significance of reviving the study of histone proteolysis both from a biological and an experimental perspective.
Keywords: epigenetics, histone clipping, histone degradation, histone proteolysis
Introduction
Histone proteolysis has a long history of disregard
Proteolysis associated with nucleosomes was described about 50 years ago, even before histones received their current nomenclature 1. Not surprisingly, many subsequent reports have alluded to the theoretical transcriptional implications of such enzymatic reaction because of the central role that histones play in DNA packaging and epigenetic regulation. While evidence of such epigenetic potential is only now gradually emerging, it is increasingly becoming clear that histones are also being degraded at much higher rates than was initially anticipated. Together this has created a very confusing amalgam of recent reports in which findings with often little biological coherence are continuously being cross-referenced.
A brief history of histone proteolysis shows that it has consistently been treated in stepmotherly fashion by the scientific community. As the first papers started to suggest that histone truncation might greatly impact transcription, a sudden surge of biochemical studies on nuclear histone-degrading enzymes occurred during the 70s and 80s (partially reviewed in 2). While histone proteolysis nearly disappeared from the publication record during the 90s and early 2000 s, a second wave of papers seemed to be upon us by around 2010, with the publication of the first evidence of the epigenetic potential of histone clipping in mouse and yeast 3,4. Strangely, however, this promise was again not fulfilled. At the very least it is surprising that especially in light of its function as a posttranslational modification (PTM) – which can sweep away en masse all other modifications 5 – histone clipping is not being picked up by the broader scientific community at a time when over 5,000 papers are published on histones every year. A first step in tackling this apparent lack of interest is to recognize the difficulty of studying proteolysis in a well-defined biological context.
Here, we have categorized earlier reports into different classes, each of which contributes to the biological picture in its own way (Table1). In doing so, it became increasingly clear that even the main two classes, which we call here “histone degradation” and “histone clipping”, are far from straightforward to discriminate. In part this is due to the fact that (i) the same enzymes apparently can mediate both histone degradation and histone clipping, (ii) many reports may potentially have been based on in vitro side effects, blurring coherence of the biological context, (iii) many reports only briefly mention detecting histone fragments, but never investigated their origin, because it was outside the scope of the authors at the time, and (iv) even the most well-documented reports struggle to completely elucidate the biological significance of degradation and clipping, maybe in part because of redundancy.
Table 1.
Structural overview of the different classes of histone proteolysis in 100 references
| Enzyme | Substrate (specificity) | Organism | Tissue | References | ||||
|---|---|---|---|---|---|---|---|---|
| Histone degradation | Biologically unclassified | Early reports | Neutral serine protease, Trypsin-like | Mainly H1/H3 degradation | Rat/Calf | Liver/thymus | 1,2,6–23 | |
| In vitro assays | Trypsin | H1>H3>H2A>H4>H2B | 24–26 | |||||
| Other enzymes | H3R26/H2AR11/H2BK20/H4R19 | 27 | ||||||
| Enzyme panel screening | E.g. Cathepsin D is “H2A-specific” | Rat | Liver/skin | 28 | ||||
| Direct expression of truncated histones | H3K27/H4K10&K20/H2A13-117/H2B24-122 | 29–31 | ||||||
| Biologically classified | Developmental | Spermatogenesis | Inhibited by Leupeptin and TLCK | Histones, not protamines | Trout/rat/mouse | 32,33 | ||
| Proteasome (PA200) | Degradation | Yeast/mouse | Testis | 34 | ||||
| Embryogenesis | SpH = Cathepsin L | Protamines (H1/H2B SPKK motif) | Sea Urchin | Embryo | 35–41 | |||
| Macronuclei | H3A21/H2BK14/H4G13/H2AZG18/H1 proteolysis (H1αβγδ) | Tetrahymena | Macronuclear degradation | 42,43 | ||||
| Pathogen | EUO gene | H1/H5, not calf histones | Chlamydia | 44 | ||||
| Continuous | Lysosmal | Cathepsin L | H3A21 (H3cs.1 Ab) + H3 C-term | Human | Cell lines | 45 | ||
| Proteasomal | Proteasome (PA200) | Degradation | Yeast/mouse | Testes | 34 | |||
| Immunological | NET formation | Azurophilic enzymes | All histones | Neutrophils | 46–48 | |||
| Induced apoptosis | GranzymeA | Mainly H1 | Human | Cells targeted by T-cells | 49 | |||
| Histone clipping | Clipped histone expression | N-tail | H4δ4-28/H3δ4-30/H2Aδ4-20/H2Bδ3-32 | Yeast | 50–55 | |||
| H4δ2-26/H3δ1-20/H3δ1-28 | Yeast | 56 | ||||||
| C-tail | H2AE121 | Human | Embryonic kidney cell line | 57,58 | ||||
| H2AV114/H2AS122 | Human | Embryonic kidney cell line | 59 | |||||
| H2A-specific | H2AspV114 | H2Asp = neutrophil elastase | H2AV114 | Calf/human/mouse | Thymus/haematopietic cells | 60–73 | ||
| H2AspE91 | Neutral aspartate protease | H2AE91 | Chicken | Liver | 19,74–76 | |||
| Buforins (AMP) | Pepsin/Cathepsin D | H2AS19/H2AY39 | Trout/amphibia | Mucosa | 77,78 | |||
| H3-specific | Developmental | Micronuclei | H3T6 (=H3F) | Tetrahymena | Micronuclei | 79–83 | ||
| Pathogens | Protease 3C | H3L20 | FMVD | BHK cells | 84–86 | |||
| H3 C-terminus | HIV | T-cells | 87 | |||||
| H1 | mengovirus | Ehrlich ascites tumor | 88 | |||||
| Differentiation | Cathepsin L/serine protease | H3A21-H3A31 | Mouse/human/rat | ESC and hepato/myo genisis | 3,89–92 | |||
| Mammary involution | Cathepsin D | H3K23 | Mouse | Mammary gland | 93 | |||
| Sporulation | PRB1 | H3A21 | Yeast | Sporulation/starvation | 4,94,95 | |||
| Other | Glutamate dehydrogenase | H3R18/H3K23/H3R26/H3K27 | Quail/chicken | Liver | 96–99 | |||
| Legumain | cH3 (12 kDa) | Human | Colorectal cancer | 100 |
Histone proteolysis
The initial impetus for classification
An appreciable number of the reports on histone proteolysis mentioned here have recently been subsumed for the first time in a review 101. However, this very informative review perpetuates the main message found in the discussion of many of these records: Histone proteolysis could play a very important role in epigenetics. While supporting such notion ourselves, our findings on both histone H2A and histone H3 proteolysis 70,71,92 did not straightforwardly corroborate earlier findings and hypotheses. This encouraged us to extrapolate the current epigenetically biased view by more clearly categorizing earlier reports into different functional classes of (biologically regulated) processes. In summary of the text, Table1 structurally bundles the references in their respective categories. By no means is this a complete overview, as many papers only briefly mention a histone truncation event.
Histone degradation
Biologically unclassified degradation
Did early reports simply lack experimental precautionary measures?
Many of the early reports on histone proteolysis focused primarily on rat liver and calf thymus (partially reviewed in the introduction of 2). They describe either total degradation or a susceptibility of histone H1 and histone H3 to a neutral serine protease with a trypsin-like specificity. Intensive effort was made to avoid cytoplasmic contamination and the proteolytical activity was consistently hard to detach from the chromatin. Furthermore, some reports incubated the proteases with substrates other than histones to validate their specificity. This “neutral protease” activity was later attributed to different enzyme activities in different tissues and animals 14,20,21. At least three different proteases were pursued in search of the “neutral protease”, one of which cleaves a histone H3K23 22 and one that was attributed to cytoplasmic contamination 19. One report mentions a high molecular weight (HMW) “protease A” that could be converted into a low and an intermediate MW protease by high pH or NaCl concentrations 17,23. Most claims of epigenetic potential of these protease activities were made on the basis of their apparent chromatin association and the impact of DNA or nucleotides on enzyme activity. Yet, because no convincing evidence of true histone clipping was provided in these early reports they are categorized here.
These reports might be considered by many to be “outdated”. However, apart from confirming the most susceptible histone sequence stretches, they can also provide important insights into which tissues are specifically prone to enzymatic degradation.
In vitro histone truncation assays provide important structural insights
Primarily with a view to structural study, many groups have incubated chromatin, whole nucleosomes and separate histones with enzymes. Only a small selection is shown in Table1. Although most of these reports have no direct biological significance, they have provided several crucial insights that should be taken into account when considering histone proteolysis:
The nucleosome retains its globular structure, even when the tails are removed 27. Tails do play an essential role in the higher order solenoid formation of chromatin 24 and removing them from the nucleosome makes DNA increasingly susceptible to degradation by DNAse enzymes 26.
Not surprisingly, the linker Histone H1 is the most susceptible to histone proteolysis, followed by H3, which has the largest N-tail protruding from the nucleosome. The order in which histones are attacked by enzymes has thus been recognized to be H1>H3>H2A>H4>H2B, and some reports caution that for this reason it is hard to study H1 and H3 proteolysis in vivo 25. Bohm et al. have even suggested that H3A21 truncation, described in more detail below under histone clipping, is the result of autolysis.
Truncated histones have also been recombinantly expressed for structural studies, leading to similar conclusions as those found by enzymatic treatment 29–31. For example, a recent FRET study structurally reinterpreted increased susceptibility to DNAse degradation as a more “breathing” conformation of truncated nucleosomes 31.
These basic structural insights can significantly help in the search for new histone proteolytical events as well as in explaining earlier findings. Moreover, they can serve as a first framework for the potential biological consequences of a histone proteolysis event.
Biologically classified degradation
Histone degradation occurs predominantly during important developmental transitions
Increasingly it is becoming clear that the once presumed extremely stable histones, are in fact degraded during different developmental stages in diverse organisms. Here again, not many studies conclusively contribute to the elucidation of this process (for a review see 32). Here, we have classified these developmentally related histone degradation events into four different groups:
During spermatogenesis, histones are largely replaced transiently by transition proteins and subsequently by protamines in postmeiotic cells 33. These histones thus need to be degraded, a process that was initially described to be mediated by a serine-type enzyme that is specific for histones, does not degrade protamines, and is associated primarily with oligonucleosomes in mouse. To the best of our knowledge, this enzyme has not yet been annotated 32. Apart from enzymatic degradation, the proteasome can also degrade histones during spermatogenesis. Some reports hint at a ubiquitin-mediated degradation, but it was shown recently that the proteasome can also degrade histones based on their acetylation status 34.
During early embryogenesis, protamines and the remaining sperm histones again need to be degraded. This was first attributed to a cystein protease named SpH in sea urchin, where this process has been studied most extensively 36. This SpH enzyme was recently found to be a nuclear Cathepsin L (CATL) 39.
Histone degradation in earlier Eukaryotes has also been described during specific stages of development, such as macronucleus degradation in, for example, Tetrahymena 43. Remarkably, the most abundant fragment of histone H3 is H3A21, which will be discussed in greater detail under histone clipping.
Some intracellular pathogens such as Chlamydia seem to be able to selectively degrade their own histones upon infection 44.
Taken together, developmental histone degradation can be expected to be an omnipresent phenomenon in eukaryotic organisms, especially during reproduction. The lack of studies focusing on these histone turnover events greatly hampers insight into its relation to histone clipping, for the latter process seems to be associated mainly with differentiation, which equally is an important developmental transition.
Continuous degradation is mediated by lysosomes and the proteasome
Apart from specific developmental stages, histones are also being replaced during normal cell growth at much higher rates than was previously assumed 102. To our knowledge, only little is thus far known about what happens to these evicted histones, but at least two different pathways by which subsequent histone degradation could be mediated are becoming evident:
Lysosome-mediated processing, for example, such as found in cytoplasmic chromatin fragments (CCF) that are budded off the nucleus and degraded in the autophagy pathway, a process that seems to be increased by senescence 45. Maybe not surprisingly, an important part of the degradation is mediated by CATL, and the specific H3A21 fragment mentioned under histone clipping is particularly abundant in these CCF.
Proteasome-mediated degradation: Just as for the process of histone degradation during spermatogenesis, acetylation also precedes histone eviction at double stranded DNA breaks 34.
Continuous degradation might be the hardest process to experimentally isolate from epigenetic histone clipping, as there is no experimental setting where it is known to be absent, and it will prove very hard to isolate every stage of CCF formation from histones in chromatin.
Histone degradation also plays a role in immunology
Recent reports on histone proteolysis events in samples from blood seem to suggest such truncated species might predominantly have an immunological origin.
Neutrophil extracellular traps (NET) have evolved in neutrophils and other cells as a way to eliminate invading pathogens 46. These NETs are generated via a specific form of cell death in which chromatin decondenses and binds to granular and cytoplasmic antimicrobial proteins such as myeloperoxidase and neutrophil elastase (NE). These structures are then released into the milieu. The entangled enzymes proteolyze histones in NETs at very specific sites, generating specific band patterns on Western blot 47. Of note, the most abundant lower band of histone H2A has a similar molecular weight to that of the H2AV114 fragment discussed under histone clipping. Of note, selective translocation of active NE to the nucleus is a conditio sine qua non to initiate NET formation 47, and is regulated at least in part by histone citrullination 48.
In vitro and after cell loading with perforin, GranzymeA completely degrades histone H1 and cleaves core histones into ∼16-kDa fragments 49. Histone digestion provides a mechanism for unfolding compacted chromatin and facilitating endogenous DNase access to DNA during T cell and natural killer cell granule-mediated apoptosis.
Blood cells might thus prove to be a very hard target in which to find the potential role of histone clipping in myeloid cell differentiation as described below.
Histone clipping
We have separately classified histone clipping as the category comprising very specific enzymatic cleavage events that have been regarded to be of potential epigenetic importance. The amino-terminal tail of the histones protrudes from the nucleosome and can become modified by many different PTMs. With the onset of the use of mass spectrometry in this field, a new wave of PTM discovery is currently ongoing 103. A striking example of this was the discovery of a staggering 67 new histone marks in 2011 104. It is thus surprising that histone clipping as a potential epigenetic modification continues to escape the attention of the broader scientific community, while it too can be identified by mass spectrometry 3,70,71,92.
Direct expression of clipped histone forms in cells does not compromise viability
Apart from the recombinantly expressed truncated histone forms that have contributed greatly to the understanding of the structural role of tails in nucleosome formation, specific truncated histone forms have also been directly expressed in living cells. These studies have provided important biological insights into how these truncated forms might exert their epigenetical influence and into how “stretchable” these truncation events are in terms of cell viability.
N-tail truncation: All core histones have been expressed in yeast without their respective N-tails 50–52,54. It was thus shown that all four N-termini are dispensable for viability in yeast. The sequence near the helical core of each histone seems to play an important role in the repression of basal transcription 55.
C-tail truncation: To our knowledge, only the H2A C-tail has been expressed in a truncated form in cells, as this is the only histone to have a truly protruding C-tail from the nucleosome 57,59. At all three different locations expressed in human cell lines (V114, E121, and S122) it was found that both chromatin compaction and transcription were impacted by this truncation event. Of note, the H2AV114 form is also generated by NE-mediated H2A clipping discussed below. For completeness, we here also mention that nickel(II) treatment induces a H2A C-tail truncation as well, making this another potential model to study the molecular outcome of tail truncation in living cells 58.
The viability of cells that express truncated histone forms not only argues in favor of the plausibility of clipping actually occurring in vivo; it also is a first and very important reminder of the potential pitfalls in studying histone proteolysis from an epigenetic perspective. As these tails can be discarded genome-wide, while they can also be modified by such a complex network of PTMs, the most obvious mechanism by which such cells survive seems to be through redundancy of regulation, which would be in line with the evolutionary conservation of histones in all eukaryotic organisms.
H2A-specific clipping still lacks proof of epigenetic significance
So far, only H3 N-tail- and H2A C-tail-specific histone clipping have been described in considerable detail. Figure 1 summarizes these clipping events in their interplay with the Polycomb group (PcG) proteins. Historically, H2A specific proteolysis was described first, and we thus commence by summarizing the reports hereon.
Figure 1.
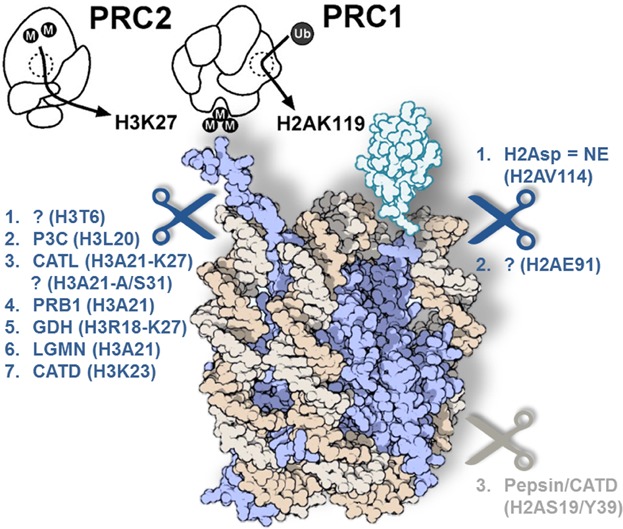
Model for a potential interplay between the Polycomb group (PcG) – Tritorax Group (Trx) axis and histone clipping. The PcG (depicted in black line drawing) comprises almost 20 different proteins, which are generally divided into three subgroups: The PRC1, PRC2, and PhoRC. In the hierarchical recruitment model, the PRC2 trimethylation of K27 on the N-terminus of histone H3 (H3K27) forms an anchor point for the PRC1 proteins to bind and ubiquitinate K119 (transparent blue) on the C-terminus of histone H2A (H2AK119), pausing RNA polymerase II and repressing transcription. This repressive PcG group of proteins antagonizes the activating Trx, which di- and trimethylate K4 of the H3 N-tail at bivalent genes (not shown here). When occurring in vivo, histone-clipping events would thus interfere with this PcG-Trx balanced epigenetic control and with other PTMs on these tails. Histones are shown in purple, DNA in yellow. Enzymes are ordered and abbreviated as discussed in detail under “Histone Clipping” (Left: H3-specific clipping; right: H2A specific clipping). Question marks represent unannotated enzymes. Figure adapted from http://www.pdb.org/ 134,135.
In 1976 a paper was published describing for the first time a chromatin-bound proteolytic activity in calf thymus with unique specificity, for histone H2A 64. Under high ionic strength a pentadecapeptide is cleaved from the C-tail of histone H2A isolated from thymus, making valine 114 (V114) its new carboxy-terminal residue. In the following 10 years this enzyme was further studied and was named the ‘H2A specific protease’ (H2Asp) 60,62,63,72,73. This fragment was also found in histone extracts from several myeloid and lymphatic leukemia cells 67,69. More recently the epigenetic potential of this clipping event gained interest when it was found that retinoic acid (RA)-induced differentiation of THP-1 promonocytes into macrophages is briefly accompanied by histone H2AV114 clipping 65,71. While pursuing this cleavage in our samples of Chronic Lymphatic Leukemia B-cells, we have recently found strong evidence that the H2Asp actually might be neutrophil elastase (NE) 70,71. Up until now all references made to the H2AV114 fragment were epigenetically inspired, because such truncation also removes the K119 ubiquitinylation site (Fig. 1). However, with NE now part of the story, experimentally uncoupling such epigenetic promise from, for example, NET formation will most probably prove very challenging.
Very recently, a second “H2A-specific protease” was described in chicken liver that clips H2A near its globular domain, at H2AE91 74. To the best of our knowledge, the identity of this enzyme is still unknown. However, based on the classification of previous reports on avian cells, CATD (light chain) surfaces as a potential candidate 19,28,74–76. Of note, if this link is correct, at least one earlier report has attributed this specific enzyme activity to cytoplasmic contamination 19.
A completely different kind of histone H2A clipping has been described in the context of host defense. Some amphibians and fish are able to cleave off N-terminal parts of histone H2A by pepsin- and cathepsin D mediated proteolysis to generate anti-microbial peptides (AMP) called buforins (reviewed in 78). These buforins have also been detected in gastric fluid of pigs, cattle and humans. When NET were first described, the authors also referred to the antimicrobial properties of histones and their derived peptides 46, but to the best of our knowledge these have not been extensively studied to date.
Despite its long history, H2A-specific clipping is nothing near a coherent biological framework. Many challenges remain, especially now that one of the H2A-specific proteases turns out to be NE. Experimentally resolving H2A clipping during myeloid differentiation from NET formation will require stringent controls.
H3-specific clipping: The only epigenetic footing?
Compared to histone H2A, H3 has been studied more extensively as an epigenetic template in general. In the same way the role of histone H3 clipping as a PTM has been more elaborately studied.
Two electrophoretically distinct forms of histone H3 were described in 1980 to be selectively present in micronuclei of Tetrahymena thermophila, the faster species of which was generated by the removal of six amino acids from the N-terminal tail 79,80. This modification was later speculated to be a demethylation mechanism 83, and it has since been regarded to be a different modification from the abovementioned degradation of histones in the transcriptionally active macronucleus of T. thermophila 43.
In a completely different biological setting, foot-and-mouth disease virus expresses the so-called protease 3C (P3C) in host cells, which mediates clipping of host histone H3 at leucine 20 84–86,105. It is thus tempting to speculate that this virus might have evolved a mechanism to manipulate the epigenetic tools of its host.
In 2008 enzymatic truncation of histones resurfaced: The H3 N-terminus is clipped from A21 to K27 by CATL in differentiating mouse embryonic stem cells (mESC) 3. Recently, we too found comparable clipping events from A21 to A/S31 during differentiation of human ESC, but our data point towards a serine protease activity 92, and we emphasize the influence of the culture conditions used. The report on mESC was actually the first ever to specifically investigate the potential epigenetic implications of such clipping events. The authors found that cleaved H3 showed altered affinity for Cbx7, another epigenetic mediator. Also, certain modifications inhibited the clipping at H3A21 when synthetic peptide substrates with different modifications were tested. Importantly, a later in vitro enzyme-substrate study on similar H3-derived peptide substrates has put the impact of PTMs on CATL clipping efficiency into perspective 89. This apparent contradicting importance of PTMs on the tail is most probably due to the effect that PTMs have on the quaternary structure of the nucleosome and the surrounding DNA. Note, we also classify the additional H3 band seen on western blot in Drosophilla polytenes here because the authors of this manuscript interpreted it as a differentiation-related event 106.
Almost simultaneously with the publication of the CATL mediated H3 truncation in differentiating mESC, a similar histone clipping event mediated by a serine protease was found in sporulating yeast cells 4. Santos-Rosas et al. extended the epigenetic implications by showing that H3 truncation precedes H3 eviction from induced promotors and that abrogation of H3 tail clipping impairs gene induction. The occurrence of a proteolytic fragment of H3 beginning at amino acid 23 in yeast cells (actually reported 15 years earlier) might also be functionally related to this clipping event 94. Despite the fascinating epigenetic implications and the parallel to the differentiating mESC it took five years for the first yeast candidate enzyme to appear in literature: Cerevisin (PRB1 or vacuolar protease B) is now at least considered to be capable of H3 clipping in starving yeast 95.
A surprising H3 N-tail specificity for the H3R18-K27 sequence stretch was reported recently for glutamate dehydrogenase (GDH) found in chicken liver tissue 97,98. When browsing in earlier reports it is tempting to speculate that the histone H3 truncation seen in senescent quail liver much earlier might be due to the same enzymatic interaction 96,99. Whether the C-terminal H3 proteolysis in hepatocytes from mice deprived of spermidine should be categorized here is even more speculative, however 107.
Nuclear localized cysteine protease legumain (LGMN) has emerged as a new enzyme to be able to clip the histone H3 N-tail. In human colorectal cancer cell lines legumain probably clips the same sequence stretch that is truncated by CATL 100.
The latest addition to the list of histone H3 clipping enzymes is CATD, which migrates to the nucleus to clip H3.3 between K23 and R24 in involuting mammary glands 93.
Based on the overview of histone H3 clipping events, we agree with the notion that H3 clipping could well represent a common feature of differentiation 90. Taken together, these papers for the first time are starting to provide increasingly strong evidence for epigenetic regulation of histone clipping (reviewed in 5,108), but evidence is still not conclusive. Uncoupling it from continuous histone degradation, which is mediated by the same enzymes, will prove the biggest challenge.
Can histone clipping still be brushed aside?
The lack of corroborating evidence for transcriptional implications of histone clipping calls for prudence. When we first came across the H2AV114 clipping event in leukemia samples 71 we started to pursue this truncation in a context of potential epigenetic effects, based on the literature at hand. However, the finding that the “H2A-specific protease” actually is NE for the first time suggested that histone degradation explains the presence of H2AV114 more accurately 70. Still, the fact that NE can be active in the nucleus, can be expressed by non-myeloid cell types and can even be actively taken up by others 109–111, emphasizes the importance of elucidating its nuclear biology. Similarly, the interaction of GDH, CATL, LGMN, P3C, PRB1, and CATD with histones should be studied in light of this broader perspective.
Thus, although we recognize that reflecting on the clipping functionality of all these different enzymes could be considered somewhat preliminary, we briefly extend here the review by Duncan and Allis 112 in which they summarize different mechanisms that could be at play when CATL engages in regulated histone clipping. Here we broaden this view to all candidate histone clippers by trying to answer the caption question from different points of view.
The enzymes' point of view: Different enzymes appear in similar biological stories
One comparison in the literature frames CATL and NE together in a specifically appealing epigenetic picture: Retinoic acid (RA)-induced differentiation of THP-1 promonocytes into macrophages is briefly accompanied by histone H2AV114 clipping 65,71, just as H3 clipping by CATL is induced while mESC and hESC are differentiated with RA 3,92. Although in our hands both these events showed considerable amounts of variability, their combined suggestive importance led us to mine the literature on these enzymes for their reported role in cell cycle regulation and especially differentiation. Indeed both enzymes can process and hyperactivate the same transcription factor CDP/CUX during G1/S transition 113–116. While CATL has been classified into the so-called “differentiation module” in an in silico gene co-expression network derived from expression analysis of differentiating ESC 117, mutations in ELA2, the gene encoding NE, cause a neutrophil differentiation disease called cyclic neutropenia 118,119. Also, a hitherto undefined substrate of NE in the nucleus promotes leukemogenesis in an acute myeloid leukemia mouse model 120. While the substrate in all these reports was never considered to be the histones themselves, active enzymes in the nucleus, including GDH, LGMN, P3C, and PRB1, might very well clip histone tails and thus impact the cell cycle or regulate differentiation in this way.
The substrates' point of view: Clipping could impact transcription in many ways
Clipping occurs mainly at the epigenetic hub of nucleosomes
The N-terminus of H3 and the C-terminus of H2A protrude from the nucleosome at the entry and exit points of the DNA 121 (Fig. 1). Histone H2A is the only core histone that contains an additional flexible C-terminal extension besides the N-terminal tail. These tails are thus very important substrates in epigenetics, and target to a plethora of different epigenetic regulators. One such example is the PcG of proteins, which specifically and sequentially targets the H3 N-tail and the H2A C-tail in what is called the hierarchical recruitment model leading to transcriptional inhibition 122. This repressive signal is counterbalanced by the activating Trithorax (Trx) Proteins that trimethylate H3K4, a process best known in the context of bivalent gene regulation during differentiation. One tempting model for epigenetic regulation by histone clipping would thus be the direct proteolytical interference with the hierarchical recruitment model or any other PTM cascade centered around the histone tails.
Clipping influences chromatin compaction
Apart from directly interfering with other PTMs, the impact of histone clipping on chromatin compaction can be considered a separate but complementary mechanism for explaining potential transcriptional consequences. In vitro both enzyme incubation essays and direct expression of truncated histones have hinted at increased “breathability” of chromatin when tails are lacking. However, only by expressing truncated histone forms in cells was it convincingly illustrated that indeed chromatin dynamics and expression patterns could both be influenced by histone clipping events, without viability being compromised.
Clipping might precede histone eviction
As mentioned above, histone degradation and histone clipping might be very hard to distinguish in some cases. This is best illustrated when considering histone eviction, where histones are either degraded or re-inserted into the chromatin after transcription. To the best of our knowledge Santos-Rosa 4 provided the only evidence of a histone-clipping event preceding histone eviction for gene transcription. They consider both an active and a passive role of H3 truncation in eviction: Either by occluding repressors of this process or by recruiting a protein complex necessary for eviction.
Potential other outcomes of clipping
By directly expressing several N-terminally truncated forms of H3, Psathas et al. 56 show that the removal of the H3 N-tail also interferes with intratail regulatory mechanisms. Adding yet another layer of complexity, the liberated N-terminal tail peptide of histone H3 may also directly bind the H3 mRNA 123. In this way, H3 could regulate its own translation.
The PTM's point of view: Removing a histone tail might not be so drastic
If indeed it is an important transcriptional regulator, histone clipping is arguably a very drastic PTM. Shortly after the discovery of H3 N-tail processing in both mouse and yeast, Osley 5 for the first time questioned how these histones are replaced. Only in 2010 it was shown that nucleosomes at regulatory elements in Drosophila S2 cells were reconstituted from new histones multiple times during one cell cycle with some regions showing histone turnover rates as fast as 1 h 102. The authors suggest that epigenetic information could be based on regulated nucleosome turnover, inasmuch as histone PTM and secondary effector proteins collectively dictate the intrinsic stability of a given nucleosome as well as its propensity to be remodeled. In this view, clipping a histone tail is not so different from any other PTM.
Histone proteolysis calls for experimental precautionary measures
Many more histone proteolysis events have most probably been encountered in the past but remained outside the scope of the authors at the time. One very remarkable recent case shows additional histone H3 bands in HIV latency-infected cell lines (NCHA1 and NCHA2) 87. These bands are strongly reminiscent of the H3 clipping patterns as described earlier during footh-and-mouth disease virus infection, and could be interpreted as supporting evidence for a viral hijacking of the histone epigenome of the host 84–86,105. But until such findings are made the focus of separate research, this tempting hypothesis will remain untested.
CATL as well as NE were initially described in cytoplasmic vesicles as proteinases that degrade protein substrates with broad specificity and that both have well-defined elastinolytic and collagenolytic activity 109,124–127. PRB1 in yeast also has long been associated mainly with vacuolar degradation 128, and GDH is known mainly as a metabolic enzyme. Only over the past decade was convincing evidence found for these enzymes as to their ability to migrate to the nucleus where they maintain enzymatic activity 95,98,111,113,114,116,120,129. It is important always to keep an open mind towards alternative functions of known proteins, as is beautifully reviewed in 130.
While this review focuses predominantly on the biological picture of histone proteolysis, it is equally important to realize that any experimental approach that targets histone tails and their modifications is prone to the effects of both in vitro and in vivo histone proteolysis. This is where the abovementioned “early reports” could greatly contribute to awareness of the extent of efforts that might be needed to avoid involuntary histone proteolysis. As we and others have described before, inhibiting these enzymatic reactions can prove surprisingly difficult, especially if they strongly associate with chromatin 70,131. Epigenetic screening techniques such as antibody detection of modifications or immunoprecipitation of modified histone tails are entirely blind to these effects. If one only considers the intensity of the cleaved fragment of histone H3 in differentiating ESC from both mouse and human it is fair to state that up to half of all histone H3 can be clipped at certain time points in ESC differentiation 3,92. According to our knowledge, researchers have rarely specifically taken care to avoid this technical pitfall 132.
Conclusions
The urgency of a better understanding of histone proteolysis is patent. However, the difficulties of specifying the in vivo versus in vitro origin of this PTM, the fact that the same enzymes mediate both histone degradation and clipping, and the complexity and redundancy of the histone code, all contribute to the surprising shortage of reports on the biology of this potentially far-reaching PTM. This review for the first time suggests that future reports on histone proteolysis should be more explicitly classified into “histone degradation” and “histone clipping”.
One technique that holds the promise of detecting histone fragments more readily is (top-down) mass spectrometry, where all proteoforms in the sample can theoretically be monitored simultaneously 133. But if push comes to shove, isolating the histones in the form that they were present inside the chromatin of the living cell is the true challenge for the field of histone epigenetics. Only by avoiding isolation all together can the presence of and the correlation between histone clipping and degradation be studied in vivo. One way this could be attained is by directly staining truncated histone in fixed cells in time-lapse experiments.
For now, trying to understand histone proteolysis still is a fuzzy art.
Glossary
- CATL
Cathepsin L
- CATD
Cathepsin D
- CCF
cytoplasmic chromatin fragments
- GDH
glutamate dehydrogenase
- H2Asp
H2A specific protease
- LGMN
legumain
- NE
neutrophil elastase
- NET
neutrophil extracellular traps
- P3C
protease 3C
- PcG
polycomb group
- PRB1
vacuolar protease B (cerevisin)
- PTM
posttranslational modification
- Trx
tritorax group
References
- Reid BR, Cole RD. Biosynthesis of a Lysine-rich histone in isolated calf thymus nuclei. Proc Natl Acad Sci USA. 1964;51:1044–50. doi: 10.1073/pnas.51.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DB, Efird PH, Chae CB. Chromatin-bound proteases and their inhibitors. Methods Cell Biol. 1978;19:175–90. doi: 10.1016/s0091-679x(08)60023-0. [DOI] [PubMed] [Google Scholar]
- Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135:284–94. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H, Kirmizis A, Nelson C, Bartke T. Histone H3 tail clipping regulates gene expression. Nat Struct Mol Biol. 2009;16:17–22. doi: 10.1038/nsmb.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley MA. Epigenetics: How to lose a tail. Nature. 2008;456:885–6. doi: 10.1038/456885a. [DOI] [PubMed] [Google Scholar]
- Carter DB, Chae CB. Chromatin-bound protease: Degradation of chromosomal proteins under chromatin dissociation conditions. Biochemistry. 1976;15:180–5. doi: 10.1021/bi00646a028. [DOI] [PubMed] [Google Scholar]
- Carter DB, Ross DA, Ishaq KS, Suarez GM. The inhibition of rat liver chromatin protease by congeners of the phenylboronic acids. Biochim Biophys Acta. 1977;484:103–8. doi: 10.1016/0005-2744(77)90116-4. [DOI] [PubMed] [Google Scholar]
- Dyson M, Walker JM. Chromatin associated protease from calf thymus. Int J Pept Prot Res. 1984;24:201–7. doi: 10.1111/j.1399-3011.1984.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Furlan M, Jericijo M. Protein catabolism in thymus nuclei. II. Binding of histone-splitting nuclear proteases to deoxyribonucleic acid. Biochim Biophys Acta. 1967;147:145–53. [PubMed] [Google Scholar]
- Furlan M, Jericijo M. Effect of whole-body gamma-irradiation on the subcellular distribution of proteinases in rat thymus. Strahlentherapie. 1967;133:262–7. [PubMed] [Google Scholar]
- Gaziev AI, Kutsyi MP. Gamma-irradiated DNA activates histone H1-specific proteinase of rat liver nuclei. Int J Radiat Biol. 1992;61:169–74. doi: 10.1080/09553009214550781. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Miyazaki K, Matuo Y, Yamashita J. Novel protease bound with chromatins in normal and tumorous tissues of rats. Biochem Biophys Res Commun. 1980;94:988–95. doi: 10.1016/0006-291x(80)91332-7. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Miyazaki K, Matuo Y, Yamashita J. Purification and characterization of alkaline protease and neutral protease from chromatin of rats. Biochim Biophys Acta. 1981;660:73–82. doi: 10.1016/0005-2744(81)90110-8. [DOI] [PubMed] [Google Scholar]
- Kurecki T, Kowalska-Loth B, Toczko K, Chmielewska I. Evidence that neutral protease from calf thymus chromatin is a serine type enzyme. FEBS Lett. 1975;53:313–5. doi: 10.1016/0014-5793(75)80044-5. [DOI] [PubMed] [Google Scholar]
- Miki BL, Neelin JM. Comparison of the histones from fish erythrocytes. Can J Biochem. 1977;55:1220–7. doi: 10.1139/o77-182. [DOI] [PubMed] [Google Scholar]
- Surowy CS, Berger NA. Nucleotide-stimulated proteolysis of histone H1. Proc Natl Acad Sci USA. 1983;80:5510–4. doi: 10.1073/pnas.80.18.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugi K, Ogata K. Studies on the serine proteases associated with rat liver chromatin. J Biochem. 1982;92:1369–81. doi: 10.1093/oxfordjournals.jbchem.a134061. [DOI] [PubMed] [Google Scholar]
- Tsurugi K, Ogata K. Effects of DNA and urea on the specificity for H1 histone of the neutral protease B partially-purified from rat liver chromatin. J Biochem. 1986;99:237–41. doi: 10.1093/oxfordjournals.jbchem.a135464. [DOI] [PubMed] [Google Scholar]
- Lindsey G, Thompson P, Von Holt C. Lysosomal origin of chicken erythrocyte nuclear protease. FEBS Lett. 1981;135:81–5. doi: 10.1016/0014-5793(81)80948-9. [DOI] [PubMed] [Google Scholar]
- Bartley J, Chalkley R. Further studies of a thymus nucleohistone-associated protease. J Biol Chem. 1970;245:4286–92. [PubMed] [Google Scholar]
- Chong MT, Garrard WT, Bonner J. Purification and properties of a neutral protease from rat liver chromatin. Biochemistry. 1974;13:5128–34. doi: 10.1021/bi00722a012. [DOI] [PubMed] [Google Scholar]
- Brandt WF, Bohm L, Von Holt C. Proteolytic degradation of histones and site of cleavage in histone F2al and F3. FEBS Lett. 1975;51:88–93. doi: 10.1016/0014-5793(75)80860-x. [DOI] [PubMed] [Google Scholar]
- Motizuki M, Mitsui K, Endo Y, Tsurugi K. Detection and partial characterization of the chromatin-associated proteases of yeast Saccharomyces cerevisiae. Eur J Biochem. 1986;158:345–50. doi: 10.1111/j.1432-1033.1986.tb09757.x. [DOI] [PubMed] [Google Scholar]
- Allan J, Harborne N, Rau DC, Gould H. Participation of core histone “tails” in the stabilization of the chromatin solenoid. J Cell Biol. 1982;93:285–97. doi: 10.1083/jcb.93.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm L, Briand G, Sautiere P, Crane-Robinson C. Proteolytic digestion studies of chromatin core-histone structure. Identification of the limit peptides of histones H3 and H4. Eur J Biochem. 1981;119:67–74. doi: 10.1111/j.1432-1033.1981.tb05577.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci USA. 1974;71:4249–53. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis-Kervabon A, Encontre I, Etienne G, Jauregui-Adell J. A chromatin core particle obtained by selective cleavage of histones by clostripain. EMBO J. 1986;5:1735–42. doi: 10.1002/j.1460-2075.1986.tb04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvima RJ, Yabe K, Fraki JE, Fukuyama K. Hydrolysis of histones by proteinases. Biochem J. 1988;250:859–64. doi: 10.1042/bj2500859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, Durand D, Renouard M, Livolant F. H2A and H2B tails are essential to properly reconstitute nucleosome core particles. Eur Biophys J. 2007;36:1083–94. doi: 10.1007/s00249-007-0212-9. [DOI] [PubMed] [Google Scholar]
- Bertin A, Renouard M, Pedersen JS, Livolant F. H3 and H4 histone tails play a central role in the interactions of recombinant NCPs. Biophys J. 2007;92:2633–45. doi: 10.1529/biophysj.106.093815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse NP, Jimenez-Useche I, Smith IT, Yuan C. Clipping of flexible tails of histones H3 and H4 affects the structure and dynamics of the nucleosome. Biophys J. 2013;104:1081–8. doi: 10.1016/j.bpj.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher J, Reynoird N, Montellier E, Boussouar F. From meiosis to postmeiotic events: The secrets of histone disappearance. FEBS J. 2010;277:599–604. doi: 10.1111/j.1742-4658.2009.07504.x. [DOI] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian MX, Pang Y, Liu CH, Haratake K. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell. 2013;153:1012–24. doi: 10.1016/j.cell.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha C, Monardes A, Even Y, Morin V. Inhibition of cysteine protease activity disturbs DNA replication and prevents mitosis in the early mitotic cell cycles of sea urchin embryos. J Cell Physiol. 2005;204:693–703. doi: 10.1002/jcp.20338. [DOI] [PubMed] [Google Scholar]
- Imschenetzky M, Diaz F, Montecino M, Sierra F. Identification of a cysteine protease responsible for degradation of sperm histones during male pronucleus remodeling in sea urchins. J Cell Biochem. 1997;67:304–15. [PubMed] [Google Scholar]
- Morin V, Acuna P, Diaz F, Inostroza D. Phosphorylation protects sperm-specific histones H1 and H2B from proteolysis after fertilization. J Cell Biochem. 1999;76:173–80. doi: 10.1002/(sici)1097-4644(20000201)76:2<173::aid-jcb1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Morin V, Sanchez A, Quinones K, Huidobro JG. Cathepsin L inhibitor I blocks mitotic chromosomes decondensation during cleavage cell cycles of sea urchin embryos. J Cell Physiol. 2008;216:790–5. doi: 10.1002/jcp.21459. [DOI] [PubMed] [Google Scholar]
- Morin V, Sanchez-Rubio A, Aze A, Iribarren C. The protease degrading sperm histones post-fertilization in sea urchin eggs is a nuclear cathepsin L that is further required for embryo development. PLoS One. 2012;7:e46850. doi: 10.1371/journal.pone.0046850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchi M, Garcia-Huidobro J, Cordova C, Aguilar R. A new nuclear protease with cathepsin L properties is present in Hela and Caco-2 cells. J Cell Biochem. 2010;111:1099–106. doi: 10.1002/jcb.22712. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sugiura M, Ebashi S. Sea urchin protease specific to the SPKK motif in histone. J Biochem. 1990;108:347–55. doi: 10.1093/oxfordjournals.jbchem.a123205. [DOI] [PubMed] [Google Scholar]
- Allis CD, Allen RL, Wiggins JC, Chicoine LG. Proteolytic processing of h1-like histones in chromatin: A physiologically and developmentally regulated event in Tetrahymena micronuclei. J Cell Biol. 1984;99:1669–77. doi: 10.1083/jcb.99.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Cook RG, Allis CD. Proteolytic removal of core histone amino termini and dephosphorylation of histone H1 correlate with the formation of condensed chromatin and transcriptional silencing during Tetrahymena macronuclear development. Genes Dev. 1991;5:1601–10. doi: 10.1101/gad.5.9.1601. [DOI] [PubMed] [Google Scholar]
- Kaul R, Hoang A, Yau P, Bradbury EM. The chlamydial EUO gene encodes a histone H1-specific protease. J Bacteriol. 1997;179:5928–34. doi: 10.1128/jb.179.18.5928-5934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pawlikowski J, Manoharan I, van Tuyn J. Lysosome-mediated processing of chromatin in senescence. J Cell Biol. 2013;202:129–43. doi: 10.1083/jcb.201212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li M, Stadler S, Correll S. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Pasternack MS, Beresford PJ, Wagner L. Induction of rapid histone degradation by the cytotoxic T lymphocyte protease Granzyme A. J Biol Chem. 2001;276:3683–90. doi: 10.1074/jbc.M005390200. [DOI] [PubMed] [Google Scholar]
- Kayne PS, Kim UJ, Han M, Mullen JR. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell. 1988;55:27–39. doi: 10.1016/0092-8674(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Durrin LK, Mann RK, Kayne PS, Grunstein M. Yeast histone H4 N-terminal sequence is required for promoter activation in vivo. Cell. 1991;65:1023–31. doi: 10.1016/0092-8674(91)90554-c. [DOI] [PubMed] [Google Scholar]
- Schuster T, Han M, Grunstein M. Yeast histone H2A and H2B amino termini have interchangeable functions. Cell. 1986;45:445–51. doi: 10.1016/0092-8674(86)90330-2. [DOI] [PubMed] [Google Scholar]
- Hirschhorn JN, Bortvin AL, Ricupero-Hovasse SL, Winston F. A new class of histone H2A mutations in Saccharomyces cerevisiae causes specific transcriptional defects in vivo. Mol Cell Biol. 1995;15:1999–2009. doi: 10.1128/mcb.15.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JW, Rykowski M, Grunstein M. Yeast histone H2B containing large amino terminus deletions can function in vivo. Cell. 1983;35:711–9. doi: 10.1016/0092-8674(83)90104-6. [DOI] [PubMed] [Google Scholar]
- Lenfant F, Mann RK, Thomsen B, Ling X. All four core histone N-termini contain sequences required for the repression of basal transcription in yeast. EMBO J. 1996;15:3974–85. [PMC free article] [PubMed] [Google Scholar]
- Psathas JN, Zheng S, Tan S, Reese JC. Set2-dependent K36 methylation is regulated by novel intratail interactions within H3. Mol Cell Biol. 2009;29:6413–26. doi: 10.1128/MCB.00876-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaczyn AA, Cheng RY, Buzard GS, Hartley J. Truncation of histone H2A's C-terminal tail, as is typical for Ni(II)-assisted specific peptide bond hydrolysis, has gene expression altering effects. Ann Clin Lab Sci. 2009;39:251–62. [PMC free article] [PubMed] [Google Scholar]
- Karaczyn AA, Bal W, North SL, Bare RM. The octapeptidic end of the C-terminal tail of histone H2A is cleaved off in cells exposed to carcinogenic nickel(II) Chem Res Toxicol. 2003;16:1555–9. doi: 10.1021/tx0300277. [DOI] [PubMed] [Google Scholar]
- Vogler C, Huber C, Waldmann T, Ettig R. Histone H2A C-terminus regulates chromatin dynamics, remodeling, and histone H1 binding. PLoS Genet. 2010;6:e1001234. doi: 10.1371/journal.pgen.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JR, Numerow L, Delcuve GP. The nonhistone chromosomal protein, H2A-specific protease, is selectively associated with nucleosomes containing histone H1. J Biol Chem. 1986;261:10410–6. [PubMed] [Google Scholar]
- Davie JR, Saunders CA. Chemical composition of nucleosomes among domains of calf thymus chromatin differing in micrococcal nuclease accessibility and solubility properties. J Biol Chem. 1981;256:12574–80. [PubMed] [Google Scholar]
- Eickbush TH, Godfrey JE, Elia MC, Moudrianakis EN. H2a-specific proteolysis as a unique probe in the analysis of the histone octamer. J Biol Chem. 1988;263:18972–8. [PubMed] [Google Scholar]
- Eickbush TH, Moudrianakis EN. The histone core complex: An octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978;17:4955–64. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Eickbush TH, Watson DK, Moudrianakis EN. A chromatin-bound proteolytic activity with unique specificity for histone H2A. Cell. 1976;9:785–92. doi: 10.1016/0092-8674(76)90141-0. [DOI] [PubMed] [Google Scholar]
- Minami J, Takada K, Aoki K, Shimada Y. Purification and characterization of C-terminal truncated forms of histone H2A in monocytic THP-1 cells. Int J Biochem Cell Biol. 2007;39:171–80. doi: 10.1016/j.biocel.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Okawa Y, Takada K, Minami J, Aoki K. Purification of N-terminally truncated histone H2A-monoubiquitin conjugates from leukemic cell nuclei: Probable proteolytic products of ubiquitinated H2A. Int J Biochem Cell Biol. 2003;35:1588–600. doi: 10.1016/s1357-2725(03)00140-7. [DOI] [PubMed] [Google Scholar]
- Pantazis P, Sarin PS, Gallo RC. Detection of the histone-2A related polypeptide in differentiated human myeloid cells (HL-60) and its distribution in human acute leukemia. Int J Cancer. 1981;27:585–92. doi: 10.1002/ijc.2910270504. [DOI] [PubMed] [Google Scholar]
- Rubin RL, Moudrianakis EN. The F3-F2a1 complex as a unit in the self-assembly of nucleoproteins. Biochemistry. 1975;14:1718–26. doi: 10.1021/bi00679a026. [DOI] [PubMed] [Google Scholar]
- Simpkins H, Mahon K. The histone content of chromatin preparations from leukaemic cells. Br J Haematol. 1977;37:467–73. doi: 10.1111/j.1365-2141.1977.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Dhaenens M, Glibert P, Lambrecht S, Vossaert L. Neutrophil Elastase in the capacity of the “H2A-specific protease. Int J Biochem Cell Biol. 2014;51:39–44. doi: 10.1016/j.biocel.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Glibert P, Vossaert L, Van Steendam K, Lambrecht S. Quantitative proteomics to characterize specific histone H2A proteolysis in chronic lymphocytic leukemia and the myeloid THP-1 cell line. Int J Mol Sci. 2014;15:9407–21. doi: 10.3390/ijms15069407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia MC, Moudrianakis EN. Regulation of H2a-specific proteolysis by the histone H3: H4 tetramer. J Biol Chem. 1988;263:9958–64. [PubMed] [Google Scholar]
- Watson DK, Moudrianakis EN. Histone-dependent reconstitution and nucleosomal localization of a nonhistone chromosomal protein: The H2A-specific protease. Biochemistry. 1982;21:248–56. doi: 10.1021/bi00531a008. [DOI] [PubMed] [Google Scholar]
- Panda P, Chaturvedi MM, Panda AK, Suar M. Purification and characterization of a novel histone H2A specific protease (H2Asp) from chicken liver nuclear extract. Gene. 2013;512:47–54. doi: 10.1016/j.gene.2012.09.098. [DOI] [PubMed] [Google Scholar]
- Appels R, Wells JR. Synthesis and turnover of DNA-bound histone during maturation of avian red blood cells. J Mol Biol. 1972;70:425–34. doi: 10.1016/0022-2836(72)90550-5. [DOI] [PubMed] [Google Scholar]
- Harlow R, Wells JR. Histone proteases of avian erythroid cells. J Cell Sci. 1975;18:217–25. doi: 10.1242/jcs.18.2.217. [DOI] [PubMed] [Google Scholar]
- Cho JH, Park IY, Kim HS, Lee WT. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J. 2002;16:429–31. doi: 10.1096/fj.01-0736fje. [DOI] [PubMed] [Google Scholar]
- Cho JH, Sung BH, Kim SC. Buforins: Histone H2A-derived antimicrobial peptides from toad stomach. Biochim Biophys Acta. 2009;1788:1564–9. doi: 10.1016/j.bbamem.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Allis CD, Bowen JK, Abraham GN, Glover CV. Proteolytic processing of histone H3 in chromatin: A physiologically regulated event in Tetrahymena micronuclei. Cell. 1980;20:55–64. doi: 10.1016/0092-8674(80)90234-2. [DOI] [PubMed] [Google Scholar]
- Allis CD, Glover CV, Gorovsky MA. Micronuclei of Tetrahymena contain two types of histone H3. Proc Natl Acad Sci USA. 1979;76:4857–61. doi: 10.1073/pnas.76.10.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis CD, Wiggins JC. Proteolytic processing of micronuclear H3 and histone phosphorylation during conjugation in Tetrahymena thermophila. Exp Cell Res. 1984;153:287–98. doi: 10.1016/0014-4827(84)90601-3. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Schneider R, Kouzarides T. Histone methylation: Dynamic or static. Cell. 2002;109:801–6. doi: 10.1016/s0092-8674(02)00798-5. [DOI] [PubMed] [Google Scholar]
- Capozzo AV, Burke DJ, Fox JW, Bergmann IE. Expression of foot and mouth disease virus non-structural polypeptide 3ABC induces histone H3 cleavage in BHK21 cells. Virus Res. 2002;90:91–9. doi: 10.1016/s0168-1702(02)00140-5. [DOI] [PubMed] [Google Scholar]
- Falk MM, Grigera PR, Bergmann IE, Zibert A. Foot-and-mouth disease virus protease 3C induces specific proteolytic cleavage of host cell histone H3. J Virol. 1990;64:748–56. doi: 10.1128/jvi.64.2.748-756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigera PR, Tisminetzky SG. Histone H3 modification in BHK cells infected with foot-and-mouth disease virus. Virology. 1984;136:10–9. doi: 10.1016/0042-6822(84)90243-5. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kim KC, Roh TY, Park J. Gene silencing in HIV-1 latency by polycomb repressive group. Virol J. 2011;8:179. doi: 10.1186/1743-422X-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub U, Traub P. Changes in the microheterogeneity of histone H1 after mengovirus infection of Ehrlich ascites tumor cells. Hoppe Seylers Z Physiol Chem. 1978;359:581–9. doi: 10.1515/bchm.1978.359.1.581. [DOI] [PubMed] [Google Scholar]
- Adams-Cioaba MA, Krupa JC, Xu C, Mort JS. Structural basis for the recognition and cleavage of histone H3 by cathepsin L. Nat Commun. 2011;2:197. doi: 10.1038/ncomms1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp P, Blum R, Vethantham V, Parisi F. PNAS Plus: Genome-wide remodeling of the epigenetic landscape during myogenic differentiation. Proc Natl Acad Sci USA. 2011;108:E149–58. doi: 10.1073/pnas.1102223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugi K, Ogata K. Presence of a thiol protease in regenerating rat-liver nuclei. Partial purification and some properties. Eur J Biochem. 1980;109:9–15. doi: 10.1111/j.1432-1033.1980.tb04761.x. [DOI] [PubMed] [Google Scholar]
- Vossaert L, Meert P, Scheerlinck E, Glibert P. Identification of histone H3 clipping activity in human embryonic stem cells. Stem Cell Res. 2014;13:123–34. doi: 10.1016/j.scr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Khalkhali-Ellis Z, Goossens W, Margaryan NV, Hendrix MJ. Cleavage of Histone 3 by Cathepsin D in the involuting mammary gland. PLoS One. 2014;9:e103230. doi: 10.1371/journal.pone.0103230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortvin A, Winston F. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science. 1996;272:1473–6. doi: 10.1126/science.272.5267.1473. [DOI] [PubMed] [Google Scholar]
- Xue Y, Vashisht AA, Tan Y, Su T. PRB1 is required for clipping of the histone H3 N terminal tail in Saccharomyces cerevisiae. PLoS One. 2014;9:e90496. doi: 10.1371/journal.pone.0090496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendra G, Gupta S, Kanungo MS. Effect of 17beta estradiol and progesterone on the conformation of the chromatin of the liver of female Japanese quail during aging. Arch Gerontol Geriatr. 1999;28:149–58. doi: 10.1016/s0167-4943(99)00002-3. [DOI] [PubMed] [Google Scholar]
- Mandal P, Azad GK, Tomar RS. Identification of a novel histone H3 specific protease activity in nuclei of chicken liver. Biochem Biophys Res Commun. 2012;421:261–7. doi: 10.1016/j.bbrc.2012.03.149. [DOI] [PubMed] [Google Scholar]
- Mandal P, Verma N, Chauhan S, Tomar RS. Unexpected histone H3 tail-clipping activity of glutamate dehydrogenase. J Biol Chem. 2013;288:18743–57. doi: 10.1074/jbc.M113.462531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra RN, Kanungo MS. Alterations in histones of the liver and oviduct of Japanese quail during aging. Mol Biol Rep. 1994;20:15–8. doi: 10.1007/BF00999850. [DOI] [PubMed] [Google Scholar]
- Haugen MH, Johansen HT, Pettersen SJ, Solberg R. Nuclear legumain activity in colorectal cancer. PLoS One. 2013;8:e52980. doi: 10.1371/journal.pone.0052980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad GK, Tomar RS. Proteolytic clipping of histone tails: The emerging role of histone proteases in regulation of various biological processes. Mol Biol Rep. 2014;41:2717–30. doi: 10.1007/s11033-014-3181-y. [DOI] [PubMed] [Google Scholar]
- Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010;328:1161–4. doi: 10.1126/science.1186777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton LM, Gonzales-Cope M, Zee BM, Garcia BA. Breaking the histone code with quantitative mass spectrometry. Expert Rev Proteomics. 2011;8:631–43. doi: 10.1586/epr.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Luo H, Lee S, Jin F. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–28. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar M, Marquardt O. Foot-and-mouth disease virus protease 3C inhibits cellular transcription and mediates cleavage of histone H3. Virology. 1990;174:364–74. doi: 10.1016/0042-6822(90)90090-e. [DOI] [PubMed] [Google Scholar]
- Pauli A, van Bemmel JG, Oliveira RA, Itoh T. A direct role for cohesin in gene regulation and ecdysone response in Drosophila salivary glands. Curr Biol. 2010;20:1787–98. doi: 10.1016/j.cub.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Buttner S. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Suganuma T, Workman JL. Crosstalk among histone modifications. Cell. 2008;135:604–7. doi: 10.1016/j.cell.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: Physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–42. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–23. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AA, Ley TJ. Neutrophil elastase cleaves PML-RARalpha and is important for the development of acute promyelocytic leukemia in mice. Cell. 2003;115:305–18. doi: 10.1016/s0092-8674(03)00852-3. [DOI] [PubMed] [Google Scholar]
- Duncan EM, Allis CD. Errors in erasure: Links between histone lysine methylation removal and disease. Prog Drug Res. 2011;67:69–90. doi: 10.1007/978-3-7643-8989-5_4. [DOI] [PubMed] [Google Scholar]
- Goulet B, Baruch A, Moon NS, Poirier M. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol Cell. 2004;14:207–19. doi: 10.1016/s1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- Goulet B, Sansregret L, Leduy L, Bogyo M. Increased expression and activity of nuclear cathepsin L in cancer cells suggests a novel mechanism of cell transformation. Mol Cancer Res. 2007;5:899–907. doi: 10.1158/1541-7786.MCR-07-0160. [DOI] [PubMed] [Google Scholar]
- Mellgren RL. Evidence for participation of a calpain-like cysteine protease in cell cycle progression through late G1 phase. Biochem Biophys Res Commun. 1997;236:555–8. doi: 10.1006/bbrc.1997.7003. [DOI] [PubMed] [Google Scholar]
- Goulet B, Markovic Y, Leduy L, Nepveu A. Proteolytic processing of cut homeobox 1 by neutrophil elastase in the MV4;11 myeloid leukemia cell line. Mol Cancer Res. 2008;6:644–53. doi: 10.1158/1541-7786.MCR-07-0268. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Fan G, Plath K, Zhou Q. Signed weighted gene co-expression network analysis of transcriptional regulation in murine embryonic stem cells. BMC Genomics. 2009;10:327. doi: 10.1186/1471-2164-10-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MS, Duan Z, Korkmaz B, Lee HH. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood. 2007;109:1817–24. doi: 10.1182/blood-2006-08-019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Roth W, Wong P, Nelson A. Cathepsin L: Critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–3. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- Uy GL, Lane AA, Welch JS, Grieselhuber NR. A protease-resistant PML-RAR{alpha} has increased leukemogenic potential in a murine model of acute promyelocytic leukemia. Blood. 2010;116:3604–10. doi: 10.1182/blood-2008-11-189282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–60. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Stock JK, Giadrossi S, Casanova M, Brookes E. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–35. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee NJ, Hyun S, Park YK. Histone H3 N-terminal peptide binds directly to its own mRNA: A possible mode of feedback inhibition to control translation. ChemBioChem. 2009;10:1313–6. doi: 10.1002/cbic.200900154. [DOI] [PubMed] [Google Scholar]
- Kirschke H, Langner J, Wiederanders B, Ansorge S. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977;74:293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Janoff A, Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968;128:1137–55. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H, Kembhavi AA, Bohley P, Barrett AJ. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982;201:367–72. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RW, Johnson DA, Barrett AJ, Chapman HA. Elastinolytic activity of human cathepsin L. Biochem J. 1986;233:925–7. doi: 10.1042/bj2330925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Jones EW. Protein degradation, meiosis and sporulation in proteinase-deficient mutants of Saccharomyces cerevisiae. Genetics. 1981;97:45–64. doi: 10.1093/genetics/97.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K, Vreemann A, Qu H, Brix K. Release of endo-lysosomal cathepsins B, D, and L from IEC6 cells in a cell culture model mimicking intestinal manipulation. Biol Chem. 2009;390:471–80. doi: 10.1515/BC.2009.047. [DOI] [PubMed] [Google Scholar]
- Butler GS, Overall CM. Proteomic identification of multitasking proteins in unexpected locations complicates drug targeting. Nat Rev Drug Discov. 2009;8:935–48. doi: 10.1038/nrd2945. [DOI] [PubMed] [Google Scholar]
- Groth I, Alban S. Elastase inhibition assay with peptide substrates—an example for the limited comparability of in vitro results. Planta Med. 2008;74:852–8. doi: 10.1055/s-2008-1074549. [DOI] [PubMed] [Google Scholar]
- Arico JK, Katz DJ, van der Vlag J, Kelly WG. Epigenetic patterns maintained in early Caenorhabditis elegans embryos can be established by gene activity in the parental germ cells. PLoS Genet. 2011;7:e1001391. doi: 10.1371/journal.pgen.1001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaudo AM, Garcia BA. Proteomic characterization of novel histone post-translational modifications. Epigenetics Chromatin. 2013;6:24. doi: 10.1186/1756-8935-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PW, Bi C, Bluhm WF, Christie CH. The RCSB Protein Data Bank: New resources for research and education. Nucleic Acids Res. 2013;41:D475–82. doi: 10.1093/nar/gks1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski IJ, Ritchie ME, Phipson B, Corbin J. Opposing roles of polycomb repressive complexes in hematopoietic stem and progenitor cells. Blood. 2010;116:731–9. doi: 10.1182/blood-2009-12-260760. [DOI] [PubMed] [Google Scholar]


