SUMMARY
Commensal gut microflora and dietary fiber protect against colonic inflammation and colon cancer through unknown targets. Butyrate, a bacterial product from fermentation of dietary fiber in the colon, has been implicated in this process. GPR109A (encoded by Niacr1) is a receptor for butyrate in the colon. GPR109A is also a receptor for niacin, which is also produced by gut microbiota and suppresses intestinal inflammation. Here we showed that Gpr109a signaling promoted anti-inflammatory properties in colonic macrophages and dendritic cells and enabled them to induce differentiation of Treg cells and IL-10-producing T cells. Moreover, Gpr109a was essential for butyrate-mediated induction of IL-18 in colonic epithelium. Consequently, Niacr1−/− mice were susceptible to development of colonic inflammation and colon cancer. Niacin, a pharmacological Gpr109a agonist, suppressed colitis and colon cancer in a Gpr109a-dependent manner. Thus, Gpr10a has an essential role in mediating the beneficial effects of gut microbiota and dietary fiber in colon.
INTRODUCTION
Commensal microbiota in the gut have profound effects on human health (Backhed et al., 2005; Honda and Littman, 2012). Germ-free and antibiotic-treated mice are more susceptible for dextran sulfate sodium (DSS)-induced colonic inflammation (Maslowski et al., 2009; Rakoff-Nahoum et al., 2004). Bacteroides fragilis and Clostridium species protects against trinitrobenzenesulfonic acid- or DSS-induced colitis (Atarashi et al., 2011; Mazmanian et al., 2008). Multiple intestinal neoplasia (Min, Apcmin/+) mice carry a germline-truncating mutation in one copy of Apc gene and spontaneoulsy develop adenomas throughout the intestinal tract. Lactobacillus acidophilus and certain gut microbial metabolites such as conjugated linoleic acids decrease intestinal tumorigenesis in Apcmin/+ mice (Davis and Milner, 2009; Urbanska et al., 2009). In contrast, depletion of microbiota ameliorates intestinal inflammation and cancer in mouse models of spontaneous colitis (Il10−/−, Tbx21−/−Rag2−/−, or Apcmin+/−) (Garrett et al., 2009; Grivennikov et al., 2012; Li et al., 2012; Uronis et al., 2009). Bacteroides fragilis toxin (BFT) and Bacteroides vulgatus increases inflammation and colon cancer in Il10−/− and Apcmin+/− mice, respectively (Uronis et al., 2009; Wu et al., 2009). Thus, commensal bacteria promote as well as suppress colonic inflammation and colon cancer in a context-dependent manner.
One of the mechanisms by which gut microbiota promote colonic health is through production of the short-chain fatty acids (SCFAs) acetate, propionate and butyrate by fermentation of dietary fiber. Among SCFAs, butyrate has received most attention for its effects on colonic health (Hamer et al., 2008). The functions of butyrate in promoting colonic health range from being energy source for colonocytes to being a key mediator of anti-inflammatory and anti-tumorigenic effects. Gut microbiome analysis has revealed a significant decrease in the number of butyrate-producing bacteria in colon of patients with ulcerative colitis and colon cancer (Frank et al., 2007; Wang et al., 2012). Colonic irrigation with butyrate suppresses inflammation during ulcerative colitis (Hamer et al., 2008).
IL-10 deficiency leads to spontaneous colitis (Huber et al., 2011; Izcue et al., 2009; Rubtsov et al., 2008). Polymorphisms in the genes that encode IL-10 or IL-10 receptor are linked to increased incidence of ulcerative colitis and inflammatory bowel disease (Franke et al., 2008; Glocker et al., 2009). Human monocyte-derived dendritic cells (DCs), when matured in the presence of butyrate, have increased expression of IL-10 and decreased production of IL-6 (Millard et al., 2002; Wang et al., 2008). IL-18 plays an essential role in suppression of colonic inflammation and inflammation-associated cancers (Chen et al., 2011; Dupaul-Chicoine et al., 2010; Elinav et al., 2011; Salcedo et al., 2010; Zaki et al., 2010). Moreover, an IL18 gene promoter polymorphism leading to decreased expression is found at higher frequency in patients with ulcerative colitis (Takagawa et al., 2005). Butyrate induces expression of IL18 in colonic epithelium (Kalina et al., 2002). In addition, the G-protein-coupled receptor 43 (Gpr43) mediates proliferation of colonic regulatory T (Treg) cells in response to exogenously SCFAs but not under steady-state conditions (Smith et al., 2013). Although these studies demonstrate that SCFAs serve as anti-inflammatory agents in the colon, the underlying molecular mechanisms remain poorly understood.
The most widely studied function of butyrate is its ability to inhibit histone deacetylases. However, cell-surface receptors have been identified for butyrate; these receptors, GPR43 and GPR109A (also known as hydroxycarboxylic acid receptor 2 or HCA2), are G-protein-coupled and are expressed in colonic epithelium, adipose tissue and immune cells (Blad et al., 2012; Ganapathy et al., 2013). GPR43-deficient mice undergo severe colonic inflammation and colitis in DSS-induced colitis model and the GPR43 agonist acetate protects germ-free mice from DSS-induced colitis (Maslowski et al., 2009). While GPR43 is activated by all three SCFAs, GPR109A (encoded by Niacr1) is activated only by butyrate (Blad et al., 2012; Taggart et al., 2005). GPR109A is also activated by niacin (vitamin B3) (Blad et al., 2012; Ganapathy et al., 2013). In colonic lumen, butyrate is generated at high concentrations (10–20 mM) by gut microbiota and serves as an endogenous agonist for GPR109A (Thangaraju et al., 2009). We have shown that Gpr109a expression in colon is induced by gut microbiota and is down-regulated in colon cancer (Cresci et al., 2010; Thangaraju et al., 2009). Gpr109a in immune cells plays a non-redundant function in niacin-mediated suppression of inflammation and atherosclerosis (Lukasova et al., 2011). Gut microbiota also produce niacin. Niacin deficiency in humans results in pellagra, characterized by intestinal inflammation, diarrhea, dermatitis and dementia (Hegyi et al., 2004). It is of great clinical relevance that lower abundance of GPR109A ligands niacin and butyrate in gut is associated with colonic inflammation.
Here we demonstrate an anti-inflammatory and anti-cancer function for Gpr109a in colon. Gpr109a signaling imposed anti-inflammatory properties in colonic antigen-presenting cells, which in turn induced differentiation of Treg cells and IL-10-producing T cells. Gpr109a was also required for the expression of IL-18. Niacr1−/− mice showed enhanced susceptibility to colitis and colon cancer. Depletion of gut microbiota or dietary fiber increased the risk for colitis and cancer, which is effectively suppressed by niacin in a Gpr109a-dependent manner.
RESULTS
Gpr109a signaling regulates Treg and IL-10-producing CD4+ T cell frequency in the colon
We hypothesized that GPR109A has an anti-inflammatory role in the colon. Because Treg cells are potent anti-inflammatory cells, we studied the status of Treg cells in colons of mice lacking Gpr109a. Colonic lamina propria (LP) of Niacr1−/− mice harbor significantly less (~40%) frequency and number of Foxp3+ (Treg) cells among CD4+ cells than those of WT mice (Figures 1A,B and S1A). In contrast, a similar frequency of Treg cells was present among splenic and small intestinal CD4+ T cells from both WT and Niacr1−/− mice, suggesting that reduction in Treg cells is specific to colon in Niacr1−/− mice (Figures 1A,B and S1B,C). CD4+ T cells producing IL-10 were also significantly reduced in colonic LP of Niacr1−/− mice compared to those from WT mice (Figures 1C,D). In contrast, the frequency and number of CD4+ T cells producing inflammatory cytokine IL-17 were elevated in colonic LP of Niacr1−/− mice compared to that of WT mice (Figures 1E,F and S1D). Similar fractions of splenic CD4+ T cells produced IL-17 or IL-10 in both WT and Niacr1−/− mice, suggesting that proinflammatory phenotype of CD4+ T cells in Niacr1−/− mice was specific to colonic LP.
Figure 1. Pro-inflammatory phenotype of colonic CD4+T cells from Niacr1−/− mice.
(A) Foxp3 expression by CD4+ T cells from colonic lamina propria (LP) and spleens of WT and Niacr1−/− mice.
(B) Percent of Foxp3+ in CD4+ populations from colonic LP and spleens of WT and Niacr1−/− mice (n=3).
(C and E) IL-10 and IL-17 expression by (PMA+ionomycin)-stimulated colonic LP and splenic CD4+ T cells from WT and Niacr1−/− mice.
(D and F) Quantification of IL-10 and IL-17 producing CD4+ T cells shown in C and E respectively (n=3).
The numbers in A, C and E represent percentage of positive cells in indicated area. Error bars represent standard deviation (SD) of mean. *p<0.002. A representative of 3 independent experiments is shown.
Colonic DCs and macrophages from Niacr1−/− mice are defective in inducing differentiation of Treg and IL-10-producing CD4+ T cells
To gain insight into the mechanism of reduced Treg and IL-10-producing CD4+ T cells in colons of Niacr1−/− mice, first we evaluated the expression of Gpr109a by various immune cells. Niacr1 mRNA is expressed by adipocytes, and innate immune cells (Gille et al., 2008). To determine its expression at the protein level, human peripheral blood mononuclear cells were stained with an antibody specific to human GPR109A was expressed at highest levels in monocytes (Figure S2A). Human blood DCs expressed lower but substantial amounts of GPR109A. In contrast, T, B and NK cells did not exhibit detectable amounts of GPR109A. qPCR revealed similar pattern of Niacr1 expression on mouse splenic T cells, B cells, DCs and macrophages (Figure S2B). Similarly, colonic DCs, macrophages as well as epithelium also expressed Gpr109a (Figure S2B).
Because macrophages and DCs expressed Gpr109a, and colonic DCs are critical in maintaining the balance between Treg, and IL-10- and IL-17-producing CD4+ T cells (Coombes et al., 2007; Manicassamy et al., 2010; Sun et al., 2007), we reasoned that the proinflammatory phenotype of colonic CD4+ T cells in Niacr1−/− mice is dependent on colonic DCs. Therefore, we tested the ability of macrophages (CD45+I-Ab+CD11b+) and DCs (CD45+I-Ab+CD11c+) isolated from colons of WT and Niacr1−/− mice to promote differentiation of naïve CD4+ T cells from OT-II mice into Treg or Th17 cell lineage. Colonic DCs and macrophages from Niacr1−/− mice were defective in inducing differentiation of naïve OT II CD4+ T cells into Treg cells (Figure 2A). In line with this, CD4+ T cells primed with colonic DCs and macrophages from Niacr1−/− mice produced reduced amount of IL-10 compared to those primed with WT counterparts (Figure 2B). In contrast, colonic DCs and macrophages from Gp109a−/− mice induced differentiation of CD4+ T cells into cells producing higher amounts of the inflammatory cytokine IL-17 compared to WT mice (Figure 2B). CD103+ intestinal DCs express aldehyde dehydrogenase (Aldh1a) and induce conversion of naïve T cells into Treg cells (Coombes et al., 2007; Sun et al., 2007). Similar frequency of CD103+ cells was present among colonic DCs from both WT and Niacr1−/− mice (data not shown), ruling out the possibility that reduced ability of Gpr109a-deficient colonic DCs to induce conversion of naïve T cells into Treg cells was due to fewer number of CD103+ DCs. These data indicate that colonic DCs from Niacr1−/− mice are defective in inducing differentiation of naïve T cells into Treg and IL-10-producing T cells.
Figure 2. Butyrate and niacin induce anti-inflammatory properties in DCs and macrophages in a Gpr109a-dependent manner.
(A) Foxp3 expression by OT-II CD4+ T cell differentiated in the presence of TGFβ1, IL-2 and cognate peptide with colonic LP CD11c+ (CD45+I-Ab+CD11c+) and CD11b+ (CD45+I-Ab+CD11b+) cells from WT or Niacr1−/− mice.
(B) OT-II CD4+ T cell differentiated as described in A were restimulated with anti-CD3 and anti-CD28 antibodies. One day later, IL-10 and IL-17 in culture supernatants were quantified by ELISA.
(C) Expression of Il10, Il6 and Aldh1a1 by colonic LP DCs and macrophages isolated from WT and Niacr1−/− mice were quantified by qPCR.
(D) Sorted splenic DCs were cultured with butyrate or niacin. Two days later, cells were harvested, and expression of Il10 and Aldh1a1 was measured by qPCR.
(E) Naïve OT-II CD4+ T cells were differentiated with butyrate- or niacin-treated splenic DCs from indicated mice as described in A. Shown is the FoxP3 expression by differentiated CD4+ T cells.
(F) WT and Niacr1−/− mice were treated with antibiotics in the presence or absence of niacin in drinking water. Four weeks later, colons were harvested and FoxP3 expression by colonic CD4+ T cell was analyzed.
The numbers in A, E and F represent percentage of positive cells in indicated area. Error bars represent SD of mean (n=2–3). A representative of at least 2 independent experiments is shown.
Butyrate- or niacin-GPR109a signaling imparts anti-inflammatory phenotype on DCs and macrophages
Intestinal DCs and macrophages express anti-inflammatory molecules such as IL-10 and Aldh1a that enable them to preferentially favor differentiation of naïve T cells into Treg cells and suppress development of Th17 cells (Coombes et al., 2007; Manicassamy et al., 2010; Sun et al., 2007). Niacr1−/− colonic DCs and macrophages were defective in expression of Aldh1a1 and IL-10 (Figure 2C). In contrast, expression of the proinflammatory cytokine IL-6, which induces differentiation of naïve CD4+ T cells into proinflammatory Th17 cells, was higher in colonic APCs from Niacr1−/− mice than from WT counterparts (Figure 2C).
We hypothesized that butyrate, which is produced in colon by gut microbiota and is an agonist for GPR109A, induces expression of IL-10 and Aldh1a1 in macrophages and DCs in a Gpr109a-dependent manner. For this, we cultured splenic CD11c+ (DCs) and CD11b+ (macrophages) cells in the presence or absence of butyrate and niacin, and then analyzed the expression of Il10 and Aldh1a1. Both butyrate and niacin induced expression of Il10 and Aldh1a1 by WT splenic DCs and macrophages (Figures 2D and S2C). In contrast, butyrate and niacin failed to influence the expression levels of Il10 and Aldh1a1 in Niacr1−/− splenic DCs and macrophages. Consistent with this, WT splenic DCs and macrophages treated with either butyrate or niacin showed superior capability to induce differentiation of naïve into Treg cells than untreated counterparts (Figures 2E and S2D). In addition, CD4+ T cells primed with butyrate- or niacin-treated WT DCs or macrophages produced higher amounts of IL-10 and reduced amounts of IL-17 (Figure S2E and S2F). In contrast, treatment with butyrate or niacin failed to affect the ability of Niacr1−/− splenic DCs and macrophages to influence T cell differentiation (Figures 2E and S2D–F).
Antibiotic treatment reduces Treg cells number in colon (Atarashi et al., 2011; Smith et al., 2013). We tested whether niacin induces Treg cells in antibiotic-treated mice in vivo. Compared to control mice, antibiotic-treated WT mice had fewer Treg cells in colon (Figure 2F and S2G). Niacin administration restored the Treg cell number in antibiotic-treated WT mice, but niacin was ineffective in influencing the Treg cell number in Niacr1−/− mice. To test the role of niacin and Gpr109a in antigen-specific Treg cell induction in vivo, OTII T cell were transferred into WT or Niacr1−/− mice that were treated with antibiotics in the presence or absence of niacin, and the mice were fed the cognate antigen ovalbumin in drinking water. Antibiotic treatment reduced FoxP3+ T cells among OTII CD4+ T cell population in WT mice. Niacin increased Treg cells in adoptively transferred OTII CD4+ T cells in colons of antibiotic-treated WT mice, whereas the effect of niacin to increase Treg cells in adoptively transferred OTII CD4+ T cells was blunted in colons of Niacr1−/− mice (Figure S2H). Colonic macrophages and DCs promote Treg cell differentiation via retinoic acid (RA); hence, RA receptor (RAR) antagonists inhibit intestinal macrophages- or DC-induced conversion of naïve T cells into Treg cells. RAR antagonist LE135, completely abrogated the butyrate- or niacin-mediated enhancement of Treg conversion by WT DCs (Figure S2I), indicating that butyrate or niacin instructs splenic DCs to acquire anti-inflammatory properties.
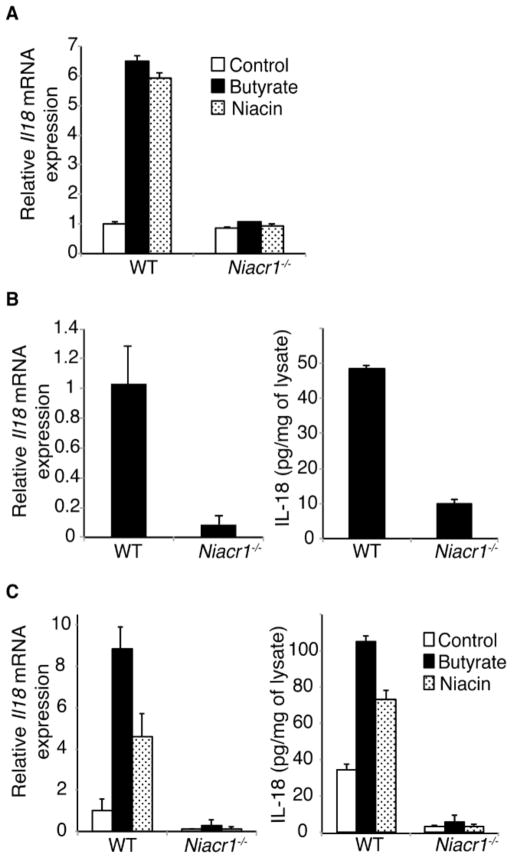
Butyrate or niacin induces IL-18 expression in colonic epithelium in a Gpr109a-dependent manner
Colons of germ-free (GF) mice expressed lower amount of Il18 mRNA than those of conventionally housed mice (Figure S3A). Toll-like receptor (TLR) ligands do not influence Il18 expression in the colon (Larsson et al., 2012), but butyrate does (Kalina et al., 2002). To test the role of Gpr109a in butyrate-mediated induction of Il18 mRNA, we cultured neonatal colons from WT mice in the presence of butyrate and niacin for one day. Both butyrate and niacin up-regulated Il18 mRNA in neonatal colon organ cultures (Figure S3B) but lipopolysaccharide did not (Figure S3B). Butyrate- and niacin-mediated induction of Il18 mRNA in neonatal colon was dependent on Gpr109a (Figure 3A). Expression of IL-18 in colonic epithelium protects colon against inflammation and carcinogenesis in animal models (Dupaul-Chicoine et al., 2010; Elinav et al., 2011; Salcedo et al., 2010). Therefore, we analyzed the expression of IL-18 in colonic epithelium of WT and Niacr1−/− mice. Colonic epithelium from Niacr1−/− mice contained significantly reduced amount of Il18 mRNA and protein compared to their WT counterparts (Figure 3B). Next we tested the role of Gpr109a in butyrate- and niacin-mediated induction of Il18 mRNA in vivo. One day after administration of butyrate or niacin, colonic epithelium from WT and Niacr1−/− mice were tested for expression of Il18. Both butyrate and niacin induced expression of Il18 in colonic epithelium of WT mice, but failed to do so in those of Niacr1−/− mice (Figure 3C).
Figure 3. Gpr109a is required for butyrate- and niacin-mediated IL-18 induction.
(A) Induction of IL-18 in neonatal colon. Colons of 6-day-old WT or Niacr1−/− mice were cultured in medium with or without butyrate or niacin for 24 h, and Il18 mRNA was quantified using qPCR (n=3).
(B) Expression of Il18 mRNA and protein in colonic epithelial cells isolated from WT or Niacr1−/− mice were quantified using qPCR and ELISA, respectively (n=3).
(C) One day after oral administration of butyrate or niacin in vivo, expression of Il18 in colonic epithelium of WT or Niacr1−/− mice was quantified (n=3).
Error bars represent SD of mean. A representative of 3 independent experiments is shown.
Gpr109a deficiency enhances susceptibility to lethal colitis and colonic inflammation
Because Gpr109a regulated the expression of IL-18, IL-10, Aldh1a1 and the presence of Treg cells in colon, we examined the role of Gpr109a in colonic inflammation. WT and Niacr1−/− mice were subjected to 3% DSS in drinking water for 6 days and their survival was monitored. Niacr1−/− mice were highly susceptible to this treatment and started dying on 5th day of DSS administration and all of them succumbed to death by day 10 (Figure S4A). In contrast, all the WT mice were alive all through the completion of the study.
To monitor both colonic inflammation and carcinogenesis in a single model, we used a well-characterized mouse model of inflammation-associated colon cancer in which DSS-mediated injury induces inflammation that contributes to colon carcinogenesis caused by azoxymethane (AOM). Mice were subjected to intraperitoneal injection of AOM, followed by cyclic DSS (2%) treatment (Figure 4A). We first evaluated the development of colonic inflammation after the first cycle of DSS treatment. Compared to WT mice, Niacr1−/− mice showed severe weight loss, diarrhea, rectal bleeding and by day 15 they had lost ~10% of body weight (Figures 4B-D). Following AOM+DSS treatment, colons of Niacr1−/− mice shrank and showed increased weight per unit length compared to colons of WT mice (Figure S4B). Myeloperoxidase activity, a hallmark of colonic inflammation, was up-regulated in colons of Niacr1−/− mice after AOM+DSS treatment (Figure S4C). Colons from untreated WT and Niacr1−/− mice showed no morphological sign of damage or inflammation. Colonic sections from (AOM+DSS) treatment group revealed extensive damage to mucosa with epithelial erosion and frequent ulcerations, loss of crypt structures and infiltration by immune cells in colons of Niacr1−/− mice compared to colons of similarly treated WT mice; this resulted in a higher histological score (inflammation + epithelial damage) in the former group (Figures 4E and S4D). Colons of Niacr1−/− mice showed profoundly decreased staining for a tight junction protein claudin-3, following treatment with AOM+DSS (Figure 4F), indicating epithelial barrier breakdown. This was confirmed by increased translocation of bacteria into liver and spleen, increase in FITC-dextran in serum following oral gavage, and enhanced systemic inflammation as shown by elevated levels of serum amyloid A, IL-6, IL-17, CCL2, IL-1β and CXCL1 in the serum (Figures S4E, F and data not shown).
Figure 4. Increased susceptibility of Niacr1−/− mice to colonic inflammation.
(A) Experimental paradigm for induction of colonic inflammation and inflammation-associated colon cancer in mice; azoxymethane (AOM) by intraperitoneal injection; DSS in drinking water. At days 20 and 70, colons of mice were analyzed for inflammation and cancer, respectively.
(B–D), Change in body weight, diarrhea and rectal bleeding of WT and Niacr1−/− mice subjected to AOM+DSS (n≥4). On 20th day of AOM+DSS treatment, colons were harvested and analyzed for histopathology.
(E) Representative images of H&E-stained colonic sections from untreated or (AOM+DSS)-treated WT and Niacr1−/− mice (original magnification, 200×).
(F) Claudin-3 staining of colonic sections from untreated (UT) or (AOM+DSS)-treated (20th day) WT and Niacr1−/− mice (original magnification, 200×).
(G) IL-10, IL-17 and IL-18 levels in colons of WT and Niacr1−/− mice before and after AOM+DSS treatment (n=5). Error bars represent standard deviation of mean.
Values are mean ± SD or representative of at least 2 independent experiments.
Colons of unmanipulated Niacr1−/− mice expressed reduced amount of IL-10 compared to those of WT mice. After (AOM+DSS) treatment, colons of WT mice showed a modest reduction in IL-10 amounts, whereas colons of Niacr1−/− mice exhibited a severe impairment of IL-10 production (Figure 4G). Expression of several other genes that inhibit colitis and colon carcinogenesis such as Tgfb1, Tgfb2, Tgfbr1 and Tgfbr2 was also drastically reduced in colons of Niacr1−/− mice compared to WT mice following (AOM+DSS) treatment (Figure S4G). In line with defective IL-18 production by colonic epithelium, colons of untreated Niacr1−/− animals expressed significantly decreased amount of IL-18 compared to those of WT counterparts. Compared to untreated animals, IL-18 expression was higher in colons of WT mice following (AOM+DSS) treatment. In contrast, IL-18 amounts in colons of Niacr1−/− mice were drastically decreased compared to colons of WT mice following (AOM+DSS) treatment (Figure 4G). Reduction in IL-18 production was not due to defective inflammasome activation because colons and sera of (AOM+DSS)-treated Niacr1−/− mice contained higher amounts of IL-1β (Figure S4F and S4H). Increased amounts of IL-17 are associated with colonic inflammation. Consistent with hyper-production of IL-17 by colonic CD4+ T cells, colons of untreated and (AOM+DSS)-treated Niacr1−/− mice expressed higher amounts IL-17 compared to their WT counterparts (Figure 4G). Amounts of other cytokines that promote colonic inflammation and carcinogenesis such as Il1a, IL-6, CXCL1, CCL2, and IL−1β were also markedly increased in colons of Niacr1−/− mice following (AOM+DSS) treatment compared to their WT counterparts (Figure S4G and S4H). Taken together, these data demonstrate that an imbalance in the production of anti-inflammatory molecules versus pro-inflammatory molecules in favor of the later increases the susceptibility of Niacr1−/− mice to colonic inflammation.
GPR109a deficiency promotes inflammation-induced as well as ApcMin/+ -driven colon carcinogenesis
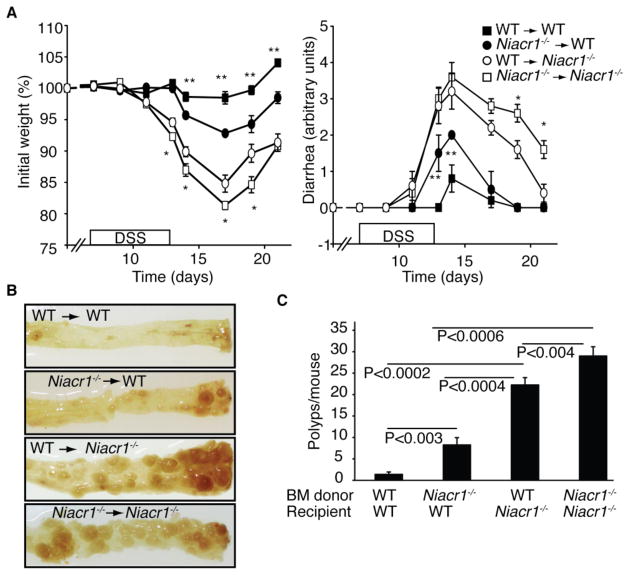
We asked whether Niacr1−/− mice are more susceptible to development of colon cancer. In colons of untreated WT and Niacr1−/− mice, 80% and 20% of the crypts contained proliferating cells in lower 1/3 and 2/3 compartments, respectively. Following (AOM+DSS) treatment, in colon of WT mice, 53% and 47% of crypts showed proliferating cells in their lower 1/3 and 2/3 compartments, respectively, and no crypt showed proliferation along its full length. In contrast, 33% of crypts in colons of (AOM+DSS)-treated Niacr1−/− mice showed proliferating cells all along its entire length (Figures S5A,B). Expression of cyclin-D1, cyclin-B1, and cyclin-dependent kinase 1, which promote development of colon cancer, was highly increased in colons of Niacr1−/− mice following AOM+DSS regime. In contrast, expression of tumor suppressors Slc5a8, Msh2 and Msh3 were decreased in colons of (AOM+DSS)-treated Niacr1−/− mice compared to WT mice (Figure S5C). At the end of the (AOM+DSS) treatment regime, colons of Niacr1−/− mice showed increased number of large polyps (22.8 ± 4.0 polyps/mouse) compared to WT mice (2.3 ± 1.8 polyps/mouse) (Figures 5A-C). Furthermore, at completion of experiment, Niacr1−/− animals also exhibited anemia (Figure S5D). Mutations in adenomatous polyposis coli (APC) gene cause an inherited form of colon cancer in humans. Because IL-10, and Treg cell suppress, whereas IL-17 enhances colon carcinogenesis in ApcMin/+ mice (mice expressing a point mutation in one copy of Apc gene) (Erdman et al., 2005; Grivennikov et al., 2012), we also analyzed ApcMin/+-driven intestinal and colon carcinogenesis in Niacr1−/− mice. At 3-months of age, Niacr1−/−ApcMin/+ mice had strikingly more polyps in the colon and small intestine than ApcMin/+ mice (Figures 5D,E). These data from two different animal models demonstrating accelerated progression of colon carcinogenesis upon deletion of Niacr1 provide strong evidence for the tumor-suppressive role of this receptor in colon.
Figure 5. Increased susceptibility of Niacr1−/− mice to colon cancer.
(A) Representative photographs of dissected colons from WT and Niacr1−/− mice on day 70 after animals were treated with AOM+DSS as described in Figure 4A.
(B and C) Number and size distribution of colonic polyps induced by AOM+DSS treatment in WT and Niacr1−/− mice (n=6).
(C) Representative photographs of dissected colons of 3-month-old ApcMin/+ and Niacr1−/−ApcMin/+ mice.
(D) Number of polyps in colon and small intestine in ApcMin/+ and Niacr1−/−ApcMin/+ mice (n=4).
Values are mean ± SD or representative of 2 independent experiments.
Gpr109a expressed in immune cells as well as in colonic tissue is necessary for protection against colitis and colon carcinogenesis
To address the role of hematopoietic versus non-hematopoietic cells expressed-Gpr109a, reciprocal bone marrow (BM) chimeras between WT and Niacr1−/− mice were generated and subjected to (AOM+DSS) treatment. Gpr109a expressed in hematopoietic cells played a critical role in AOM+DSS-induced colonic inflammation and carcinogenesis because WT or Niacr1−/− host that received Niacr1−/− BM exhibited significantly more AOM/DSS-induced weight loss, diarrhea and colonic polyps than corresponding recipients of WT BM cells (Figures 6A-C). Similarly, Gpr109a expressed in non-hematopoietic cells was also important in AOM+DSS-induced colonic inflammation and carcinogenesis because Niacr1−/− mice receiving WT or Niacr1−/− BM cells developed more severe weight loss, diarrhea and colon carcinogenesis than corresponding WT host receiving same BM cells (Figure 6A-C). The contribution by non-hematopoietic Gpr109a was quantitatively greater than that by immune-cell Gpr109a because WT→ Niacr1−/− group developed more colonic polyps and showed more weight loss and diarrhea than Niacr1−/− →WT group (p<0.004). These data demonstrate that Gpr109a expressed by both hematopoietic as well as non-hematopoietic cells is necessary for effective protection against colonic inflammation and colon cancers.
Figure 6. Role of Gpr109a expressed in hematopoietic and non-hematopoietic cells in regulation of colitis and colitis-associated colon cancer.
Reciprocal bone marrow chimeras of WT and Niacr1−/− mice were subjected to (AOM+DSS)-induced model of colitis-associated colon cancer.
(A) Weight loss and diarrhea after the first cycle of DSS (n≥4). *; significant difference between WT → Niacr1−/− and Niacr1−/− → Niacr1−/− group for weight loss (p<0.05) and diarrhea (p<0.02). **; Significant difference between WT → WT and Niacr1−/− → WT group for weight loss and diarrhea (p<0.02).
(B) Representative photographs of dissected colons from bone marrow chimeric mice.
(C) Tumor burden in bone marrow chimeras subjected to AOM+DSS induced colon cancer (n=4).
Error bars represent SD of mean. A representative of 2 independent experiments is shown.
Activation of Gpr109a suppresses colonic inflammation and carcinogenesis in absence of gut microbiota or dietary fiber
We then examined the relevance of niacin, a pharmacologic agonist for GPR109A, to colonic inflammation. For this, we first depleted gut microbiota with antibiotics, which reduces the production of butyrate, the endogenous agonist for GPR109A. Antibiotic treatment resulted in >300 fold reduction in aerobic and anaerobic bacterial counts in the stool (data not shown). Antibiotic treatment increased DSS-induced weight loss, diarrhea and bleeding in WT mice (Figures 7B and S6A). Consistent with increased inflammation, we found that antibiotic treatment increased the number of polyps (8.2 ± 2.2 polyps/mouse with antibiotics; 1.6 ± 1.5 polyps/mouse without antibiotics) in WT mice (Figures 7C,D). We then tested whether administration of niacin protects antibiotic-treated mice against colonic inflammation and carcinogenesis. Niacin was added to drinking water along with antibiotic cocktail. Niacin ameliorated (AOM+DSS)-induced weight loss, diarrhea and bleeding, and reduced colon cancer development in antibiotic-treated WT mice (Figures 7B–D and S6A). Consistent with a role of niacin in IL-18 induction, the protective effect of niacin in DSS-induced weight loss and diarrhea in antibiotic-treated Il18−/− mice was significantly blunted (Figure S6B). Niacin did not alter the development of weight loss, diarrhea, rectal bleeding, and colon cancer in antibiotic-treated Niacr1−/− mice, suggesting an essential role of Gpr109a in niacin-mediated promotion of colonic health (Figures 7B–D and S6A). Antibiotic treatment reduced colonic inflammation and number of polyps in Niacr1−/− mice. This may be likely due to presence of altered colitogenic gut microbiota in Niacr1−/− animals. Increased representation of Prevotellaceae and TM7 groups of bacteria are associated with enhanced risk of colitis in IL-18-deficient mice (Casp1−/−, Asc−/−, Nlrp6−/− or Il18−/−) (Elinav et al., 2011). We found that feces of Niacr1−/− mice exhibited increased representation of Prevotellaceae and TM7 groups of commensal bacteria compared to WT mice (Figure S6C). Bacteroides and Bacillus groups of bacteria were present in comparable numbers in feces of both WT and Niacr1−/− mice. To test whether Gpr109a deficiency promotes preferential accumulation of Prevotellaceae and TM7 bacterial groups in their intestines independent of parental contribution, we performed similar studies with fecal samples from littermates of Niacr1+/− and Niacr1−/− genotypes. We observed that feces from Niacr1−/− mice contained significantly more Prevotellaceae and TM7 bacteria that feces from Niacr1+/− littermates (Figure S6D). This provides evidence for significant alterations in relative abundance of indicated microbial species in the colons of Niacr1−/− mice and explains the reduction of colonic inflammation and colon carcinogenesis by antibiotic treatment in Niacr1−/− animals (Figure 7B–D, S6A). Both exogenously administered rIL-10 and rIL-18 were able to reduce AOM+DSS-induced weight loss, diarrhea and colon carcinogenesis in Niacr1−/− mice (Figure 7E,F).
Figure 7. Niacin suppresses colonic inflammation and carcinogenesis in absence of gut microbiota and dietary fibers via Gpr109a.
(A) Experimental paradigm for antibiotic treatment (gentamicin sulfate, ciprofloxacin, streptomycin and bacitracin in drinking water) and niacin administration to mice in the (AOM+DSS)-induced colon cancer model.
(B) Weight loss in WT and Niacr1−/− mice treated with antibiotics in the presence or absence of antibiotics and subjected to AOM+DSS as in A.
(C) Tumor burden in WT and Niacr1−/− mice under various treatment conditions. Error bars represent standard deviation of mean (n≥4).
(D) Representative photographs of dissected colons of WT and Niacr1−/− mice subjected to various treatments as described in A.
(E) Niacr1−/− mice were treated as in Figure 4A. Some mice also received rIL-10 or rIL-18 intraperitoneally (50 ng/mouse/injection) starting day 2 and every alternate day till day 60. Shown are the weight loss and diarrhea during first cycle of DSS.
(F) Tumor burden on day 70 in Niacr1−/− mice treated as described in (E) (n=4).
(G) Two-month-old, ApcMin/+ and Niacr1−/−ApcMin/+ mice were fed with dietary fiber containing normal chow (FC) or fiber-free (FF) chow. Some mice in FF diet group also received niacin in drinking water ad libitum. Five weeks later, mice were sacrificed and colonic polyps were counted (n=2–5). * p<0.007
(H) A representative photographs of dissected colon from mice subjected to experimental protocol described in G.
Values represent mean ± SD or representative of at least 2 independent experiments.
Antibiotic treatment reduces the number of colonic polyps in mice with Apc mutation, implying a cancer-promoting role of gut microbiota in this model (Grivennikov et al., 2012; Li et al., 2012). Our data indicate that gut microbial metabolite butyrate and hence gut microbiota reduce colon carcinogenesis in Apcmin/+ mice. We hypothesized that gut microbiota has both cancer promoting and suppressing effects in Apcmin/+ mice, and microbiota-depleted animals show the balance between cancer promoting and suppressing properties of gut microbiota. To define the protective role of Gpr109a in promoting colonic health through butyrate and gut microbiota, we fed Apcmin/+ mice and Niacr1−/−Apcmin/+ mice with fiber-free (FF) diet, which eliminates butyrate production in colon. Figure 7G and H show that Apcmin/+ mice fed FF-diet had significantly increased number of colonic polyps, which was effectively suppressed by the Gpr109a agonist niacin. FF-diet in the presence or absence of niacin did not affect development of colonic polyps in Niacr1−/−Apcmin/+ mice. Collectively, these data clearly indicate that Gpr109a agonists suppress development of colon cancer. Butyrate, the endogenous Gpr109a agonist in colon, is produced following fermentation of dietary fiber by commensal bacteria. Therefore, the data presented here suggest a role of Gpr109a in suppression of colonic inflammation and carcinogenesis by butyrate-producing commensals and dietary fibers.
DISCUSSIONS
The current study defines an essential role of Gpr109a in the suppression of colonic inflammation and carcinogenesis. Commensals induce Treg cells and IL-10-producing T (Tr1) cells in colon (Atarashi et al., 2011; Geuking et al., 2011; Mazmanian et al., 2008; Round and Mazmanian, 2010). They also induce IL-10 expression in colonic DCs and macrophages, which promote differentiation of Tr1 cells (Jeon et al., 2012; Ueda et al., 2010). Our studies provide a molecular mechanism by which the commensals elicit these effects. The bacterial metabolite butyrate functions as a messenger between the commensals and the host. This SCFA induces expression of antiinflammatory molecules in macrophages and DCs and enables them to support differentiation of Treg and IL-10-producing T cells. The present study also implicates a tumor-suppressive role of Gpr109a-butyrate signaling in colon and suggests that commensals in the gut provide protection to the host not only against colonic inflammation but also against colon cancer. Our conclusion that butyrate is responsible, at least partly, for the actions of gut microbiota on the host colon with regard to suppression of inflammation and carcinogenesis is congruent with previous findings that the frequency of butyrate-producing bacteria and rate of butyrate production are greatly diminished in the colon during ulcerative colitis and colon cancer (Frank et al., 2007; Wang et al., 2012). Butyrate enemas decrease colonic inflammation in ulcerative colitis (Hamer et al., 2008). The present studies also highlight the biological significance of dietary fiber and its relevance to butyrate production. Dietary fiber suppresses colonic inflammation and colorectal cancer (Davis and Milner, 2009; Hamer et al., 2008). The most important aspect of the present studies is the identification of Gpr109a as one of the mediators of the biological effects of butyrate.
Niacin is a vitamin, which when taken in pharmacological doses, modifies lipid profile in circulation by acting as a GPR109A agonist in adipocytes. At these high doses, niacin is likely to reach the colon at concentrations high enough to exert GPR109A-dependent effects. Therefore, the present studies suggest that pharmacological doses of niacin may have anti-inflammatory and tumor-suppressive effects in the colon.
Although it has been known for decades that the commensal metabolite butyrate suppresses inflammation and carcinogenesis in colon, the exact identity of molecular target(s) of butyrate in this process remained elusive. The present studies identify Gpr109a as an important mediator of butyrate effects in colon and also as a critical molecular link between colonic bacteria and dietary fiber and the host. These findings have important implications for prevention as well as treatment of inflammatory bowel disease and colon cancer and suggest that under conditions of reduced dietary fiber intake and/or decreased butyrate production in colon, pharmacological doses of niacin might be effective to maintain GPR109A signaling and consequently protect colon against inflammation and carcinogenesis.
EXPERIMENTAL PROCEDURES
Mice
Age-matched conventional and germ-free mice (Swiss Webster strain) were obtained from Taconic Farms, Inc., (Petersburg, NY) and used for experiments on day of arrival. Il18−/− and ApcMin/+ mice on C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME). Fiber-free diet (TD00278) was from Harlan Laboratories (Indianapolis, Indiana). Niacr1+/−ApcMin/+ mice were derived from intercross of Niacr1−/− mice and ApcMin/+ mice. Niacr1+/−ApcMin/+ mice were bred with Niacr1−/− mice to obtain Niacr1−/−ApcMin/+ mice. All animal procedures were approved by the Institutional Animal Care and Use Committee.
Antibodies
Antibodies against mouse CD4 (clone GK1.5), CD45 (clone 104), Foxp3 (clone FLK-16s), IL-10 (clone JES5-16E3), IL-17 (clone eBio17B7) CD11b (clone M1/70), CD11c (clone N418), I-Ab(clone 25-9-17), CD90 (clone 53-2.1), CD19 (clone SJ25C1), Gr1 (clone RB6-8C5), against human CD3 (clone UCHT1), CD14 (61D3), CD123 (6H6) and CD56 (CMSSB) were from eBioscience. Anti-human GPR109A (clone 245106), anti-Ki67 (clone TEC-3) and anti-claudin3 (34-1700) were from R&D Systems, Dako and Life technologies respectively.
Cell isolation and analysis
B cells (CD19+), T cells (Thy1.1+), macrophages (CD11b+CD11c−), and dendritic cells (CD11c+CD11b−) from spleens were sorted using MoFlo (Dakocytomation) cell sorter or CD11b or CD11c antibodies coupled to maganetic beads (Miltenyi Biotec). For isolation of colonic epithelium, washed and everted colons were incubated in Hanks balanced salt solution (HBSS) containing 2 mM EDTA at 37 °C with gentle shaking. Supernatants were centrifuged, and pellets were resuspended in 40% Percoll and centrifuged. The cells on top layer were collected and used as colonic epithelium. For lamina propria lymphocytes, colons and small intestines were opened and shaken with HBSS containing 2 mM EDTA at 37 °C to remove epithelial cells. After that, pieces of colon or small intestine were incubated with collagenase D (1 mg/ml) and DNAse (0.1 mg/ml) for 20 min. The cell suspension was collected, washed and stained with antibodies specific for mouse CD4 and Foxp3 and analyzed by FACS. In some experiments, mononuclear cells from colonic LP or spleen of WT and Niacr1−/− mice were cultured with phorbol myristate acetate plus ionomycin in the presence of GolgiStop and Golgiplug for 5 h. Cells were fixed and stained for CD4, IL-10 and IL-17. CD45+I-Ab+CD11c+ (DCs) or CD45+I-Ab+CD11b+ (macrophages) cells from colonic LP were sorted by FACS. Splenic CD11c+ or CD11b+ cells were sorted using.
Cell cultures
Sorted colonic LP CD45+I-Ab+CD11c+ or CD45+I-Ab+CD11b+ cells were cultured with naïve OT-II (CD4+CD25-) T cells in the presence of egg albumin peptide (ISQVHAAHAEINEA) (0.5 μg/ml), TGFβ1 (1 ng/ml), and IL2 (5 ng/ml). Four days later, cells were harvested and stained for CD4 and FoxP3. Alternatively, cells were rested overnight and re-stimulated with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml). Twenty-four hours later, supernatants was collected and analyzed for IL-10 and IL-17a by ELISA. Splenic CD11c+ (DCs) and CD11b+ (macrophages) were cultured (105 cells/well) in the presence or absence of butyrate (0.5 mM) or niacin (0.5 mM). Two days later, cells were harvested and analyzed for IL-10 and Aldh1a expression or tested for their ability to induce differentiation of naïve OT-II CD4+ T cells as above. In some experiments, retinoic acid receptor (RAR) inhibitor LE135 was used (1 μM).
Bone marrow chimeras
WT (CD45.1) or Niacr1−/− (CD45.2) mice were irradiated (900 rads) and injected intravenously with donor bone marrow cells (2 × 106 cells/mouse). Reconstitution was confirmed by staining for donor specific CD45 allele (CD45.1 versus CD45.2) in blood. Two months after reconstitution, mice were used for colonic inflammation-associated colon cancer experiments.
Antibiotics and niacin treatment
Mice were given a cocktail of antibiotics (0.2 μg/ml of gentamicin, 0.15 μg/ml of ciprofloxacin, 2 mg/ml streptomycin and 1 mg/ml bacitracin) in drinking water at the indicated period of time. Where indicated, antibiotic cocktail was supplemented with 25 mM of niacin. For IL-18 induction assays, mice were treated with butyrate or niacin (25 mM) as described (Kalina et al., 2002)
Induction of colonic inflammation and inflammation-associated colon cancer
Inflammation-associated colon cancer was induced by intraperitoneal injection of azoxymethane (10 mg/kg body weight). Seven days later, DSS (36–50 kDa) was added in a cyclic manner to drinking water at indicated doses. Some mice were also given rIL-10 or rIL-18 intraperitoneally (50 ng/mouse) at indicated points. Mice were monitored for weight changes, diarrhea, and rectal bleeding. Diarrhea was scored as (0) normal stool, (1) soft but formed pellet, (2) very soft pellet, (3) diarrhea (no pellet), (4) dysenteric diarrhea. Rectal bleeding was recorded as (0) no bleeding, (2) presence of occult blood in stool, (4) gross macroscopic bleeding.
Histopathology and immunohistochemistry
5-μm sections from formalin-fixed and paraffin-embedded colons or polyps were placed onto glass slides. H & E stained sections were blindly scored for severity of colonic inflammation. The degree of inflammation was scored as follows: (0) physiologic inflammation, (1) mild inflammation or prominent lymphoid aggregates, (2) moderate inflammation, (3) moderate inflammation associated with crypt loss, and (4) severe inflammation with crypt loss and ulceration. Crypt destruction was graded as follows: (0) no destruction, (1) 1–33% of crypts destroyed, (2) 34–66% of crypts destroyed, and (3) 67–100% of crypts destroyed. The individual scores from inflammation and crypt damage were summed to derive histological score for colonic inflammation (maximum score 7). For immunohistochemistry, sections were de-paraffinized with xylene, and antigen retrieval was performed using target antigen retrieval solution (Dako). The staining was visualized using Vectstain ABC kit and diaminobenzidine.
Quantitative real-time-PCR (qPCR)
cDNA was synthesized from 2 μg of total RNA using Superscript III reverse transcription system (Invitrogen). qPCR was performed using SYBR green PCR mix and StepOnePlus machine (Applied Biosystems). PCR primers are listed in Table S1. Gapdh was used as internal control..
ELISA
Colonic tissue or colonic epithelium extracts were prepared in PBS containing 0.1% NP-40 and protease inhibitors (Thermo Fisher Scientific). ELISA was performed using antibody pairs for IL-6, IL-1β, and IL-17. ELISA kits for IL-18 and Ccl2 were from R&D systems and eBioscience respectively. Cxcl1 was quantified using a kit from Peprotech. Serum amyloid A was detected using Kit from Immunology Consultants Laboratory Inc.
Measurement of intestinal permeability
Mice were given fluorescein isothiocyanate (FITC)-dextran by oral gavage at a dose of 0.5 mg/g of body weight. Four hours later, mice were bled and FITC-dextran was quantified in the serum using a fluorescence spectrophotometer.
Myeloperoxidase (MPO) activity
Pieces of colon (100 μg weight) were homogenized in phosphate buffer (20 mM, pH 7.4) and centrifuged. Pellet was resuspended in phosphate buffer (50 mM, pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma). The sample was freeze-thawed, sonicated followed by warming to 60 °C for 2 h and centrifuged. Redox reaction of 3,3′,5,5′-tetramethylbenzidine (Sigma) by supernatant was used to determine MPO activity. Reaction was terminated with 2N HCl, and absorbance was read at 450 nm.
Organ culture
Colons from 7-day-old pups were opened, cut into ~0.5-cm pieces and cultured with butyrate or niacin (2 mM) for 24 h. The tissues were then used for analysis of Il18 mRNA using qPCR. For DSS-treated animals, colon segments (100 mg weight) were chopped into smaller pieces (1–2 mm) and cultured in medium containing penicillin and streptomycin. Twenty-four hours later, supernatants were collected, and cytokines were measured by ELISA.
Supplementary Material
HIGHLIGHTS.
Commensal metabolite butyrate and niacin induce IL-18 in colon via Gpr109a
Butyrate and niacin induce IL-10 and Aldh1a in APCs in a Gpr109a-dependent manner
Niacr1−/− mice exhibit increased risk for colitis and colon cancer
Gpr109a signaling protects colon health during deficiency of gut bacteria and dietary fiber
Acknowledgments
This research was supported by National Institutes of Health grants AI085440 (N.S.) and CA152396 (V.G.). We thank Dr. L. Ignatowicz for helpful discussions.
References
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–461. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. The Journal of nutritional biochemistry. 2009;20:743–752. doi: 10.1016/j.jnutbio.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer research. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nature genetics. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol. 2013 doi: 10.1016/j.coph.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Punit S, Gallini CA, Michaud M, Zhang D, Sigrist KS, Lord GM, Glickman JN, Glimcher LH. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 2009;16:208–219. doi: 10.1016/j.ccr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annual review of pharmacology and toxicology. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. International journal of dermatology. 2004;43:1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annual review of immunology. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O’Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annual review of immunology. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS pathogens. 2012;8:e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina U, Koyama N, Hosoda T, Nuernberger H, Sato K, Hoelzer D, Herweck F, Manigold T, Singer MV, Rossol S, Bocker U. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur J Immunol. 2002;32:2635–2643. doi: 10.1002/1521-4141(200209)32:9<2635::AID-IMMU2635>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Larsson E, Tremaroli V, Lee YS, Koren O, Nookaew I, Fricker A, Nielsen J, Ley RE, Backhed F. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61:1124–1131. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Karre K, Pettersson S, Greicius G. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231–1238. doi: 10.1093/carcin/bgs137. [DOI] [PubMed] [Google Scholar]
- Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121:1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clinical and experimental immunology. 2002;130:245–255. doi: 10.1046/j.0009-9104.2002.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’HUigin C, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- Takagawa T, Tamura K, Takeda N, Tomita T, Ohda Y, Fukunaga K, Hida N, Ohnishi K, Hori K, Kosaka T, et al. Association between IL-18 gene promoter polymorphisms and inflammatory bowel disease in a Japanese population. Inflamm Bowel Dis. 2005;11:1038–1043. doi: 10.1097/01.mib.0000182868.67025.b9. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kayama H, Jeon SG, Kusu T, Isaka Y, Rakugi H, Yamamoto M, Takeda K. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. International immunology. 2010;22:953–962. doi: 10.1093/intimm/dxq449. [DOI] [PubMed] [Google Scholar]
- Urbanska AM, Bhathena J, Martoni C, Prakash S. Estimation of the potential antitumor activity of microencapsulated Lactobacillus acidophilus yogurt formulation in the attenuation of tumorigenesis in Apc(Min/+) mice. Dig Dis Sci. 2009;54:264–273. doi: 10.1007/s10620-008-0363-2. [DOI] [PubMed] [Google Scholar]
- Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Morinobu A, Horiuchi M, Liu J, Kumagai S. Butyrate inhibits functional differentiation of human monocyte-derived dendritic cells. Cellular immunology. 2008;253:54–58. doi: 10.1016/j.cellimm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.