Abstract
Since the formal description of fungi in the genus Escovopsis in 1990, only a few studies have focused on the systematics of this group. For more than two decades, only two Escovopsis species were described; however, in 2013, three additional Escovopsis species were formally described along with the genus Escovopsioides, both found exclusively in attine ant gardens. During a survey for Escovopsis species in gardens of the lower attine ant Mycetophylax morschi in Brazil, we found four strains belonging to the pink-colored Escovopsis clade. Careful examination of these strains revealed significant morphological differences when compared to previously described species of Escovopsis and Escovopsioides. Based on the type of conidiogenesis (sympodial), as well as morphology of conidiogenous cells (percurrent), non-vesiculated conidiophores, and DNA sequences, we describe the four new strains as a new species, Escovopsis kreiselii sp. nov. Phylogenetic analyses using three nuclear markers (Large subunit RNA; translation elongation factor 1-alpha; and internal transcribed spacer) from the new strains as well as available sequences in public databases confirmed that all known fungi infecting attine ant gardens comprise a monophyletic group within the Hypocreaceae family, with very diverse morphological characteristics. Specifically, Escovopsis kreiselii is likely associated with gardens of lower-attine ants and its pathogenicity remains uncertain.
Introduction
The diversity of microbial symbionts found within nests of attine ants, as well as the complex relationships among the various participants, makes the attine ant-microbial association among the most complex multipartite interactions known in nature. The tribe Attini comprises 257 ant species within 16 extant genera, including the recently described Cyatta abscondita, and one ichnogenus (Attaichnus Laza) [1–3]. As has been known for over a century, attine ants cultivate mutualistic basidiomycetous fungi as their primary food source [4]. During the 50 million-year evolutionary history of this tribe, five distinct types of agriculture have arisen: (i) lower attine agriculture (Agaricaceae); (ii) coral fungus agriculture (Pterulaceae); (iii) yeast agriculture (Agaricaceae); and (iv) higher attine ant agriculture, which includes (v) leaf-cutter ant agriculture (Agaricaceae) [1]. These groups differ in the type of substrates used for fungal cultivation, including insect frass, seeds, plant sap, arthropod exoskeletons, fresh leaves, and/or flower parts [5].
The last three decades have revealed that the fungal cultivars are not the only microbial symbionts that have evolved intimate relationships with attine ants. In 1999, Currie et al. [6] demonstrated the existence of a specific parasite that attacks the mutualistic cultivar. Fungi in the genus Escovopsis were described as the third member of the attine ant-fungal association. Subsequent studies have focused on evolutionary aspects of Escovopsis parasitism [7–11] and it was also demonstrated that the parasite attacks and consumes the cultivar mycelia [12]. Much of this work has focused on leaf-cutting ants, the most derived group within higher attine agriculture [7, 13–16].
Despite becoming a model system for the study of co-evolution and host-parasite dynamics, until recently little attention has been paid to the taxonomy of these fungi. In the 1990s, when the genus Escovopsis was proposed, only two species were known: Escovopsis weberi [17] and E. aspergilloides [18]. In 2013, three additional Escovopsis species—E. microspora, E. moelleri and E. lentecrescens—were described, and a new genus, Escovopsioides, was proposed [19].
The five ex-type strains of Escovopsis species and Escovopsioides nivea (the only described species in the genus), were isolated from gardens of ants that engage in higher attine (including leafcutter) agriculture (i.e. groups (iv) and (v), respectively), which includes the ant genera Sericomyrmex, Trachymyrmex, Acromyrmex and Atta [1]. Numerous studies have confirmed that leaf-cutting ants (Acromyrmex and Atta) cultivate closely related, or even identical, strains of the mutualistic fungus [20–23], which are derived from the fungi cultivated by the higher attine genera Trachymyrmex and Sericomyrmex [24–26]. Nevertheless, no taxonomic study has been carried out on the parasites that infect gardens of attine ants that perform lower attine agriculture (i.e. group (i)); these fungi were separated into groups depending on their macroscopic characteristics, especially their colony (spore) color (brown, yellow, white and pink) [8–9], but no distinct species has been described in these groups.
We sampled fungus gardens of the lower attine ant Mycetophylax morschi and found four strains of a fungus species that are morphologically and phylogenetically distinct from previously described species of Escovopsis and Escovopsioides. The strains are described as a new species, Escovopsis kreiselii, that exhibits percurrent conidiogenous cells with sympodial conidiogenesis; such morphological characteristics may be considered less derived when compared to morphological aspects exhibited by other Escovopsis species described to date (including the presence of phialides in vesiculated conidiophores). Phylogenetic analyses of three nuclear loci confirmed the close relationship between E. kreiselii and the previously described species in a clade of Hypocreaceae fungi associated with attine ants.
Materials and Methods
Ethics statement
Collections made in the first expedition were conducted under collecting permit number 14789–1 issued by the “Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis” (IBAMA) to SES. Collections made in the second expedition were carried out under collecting permit number 31534 issued by the “Instituto Chico Mendes de Conservação da Biodiversidade” (ICMBio) to AR. LAM and QVM joined the second expedition and their names were included in permit 31534. No endangered or protected species were involved in this study.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants, and hence the new names contained in the electronic publication of a PLOS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix http://www.mycobank.org/MB/. The online version of this work is archived and available from the following digital repositories: [PubMed Central, LOCKSS].
Sampling site and fungal isolation
Sampling was conducted during two different expeditions to Florianópolis, Santa Catarina, Brazil. In March of 2009, we isolated E. kreiselii (strain LESF53) from a nest of M. morschi found at Praia da Joaquina (nest AR090306–01, GPS: 27°37′50.01″S 48°27′3.64″W, elev. 1 m). In February 2014, we isolated three additional strains (LESF303, LESF304 and LESF305) of E. kreiselii, all associated with M. morschi. Strain LESF305 was isolated from a nest located at Praia da Joaquina (nest AR140226–01, GPS: 27°37′49.62″S 48°27′3.6″W, elev. 1.7 m) and the strains LESF303 and LESF304 were isolated from a single nest in Praia de Moçambique, on the northeast shore of the island of Florianópolis (nest AR140227–05, GPS: 27°31′24.96″S 48°25′3.78″W, elev. 1m). All nests were located in a coastal sand dune environment, a typical habitat of M. morschi [2, 27]. The whole fungus garden was sampled along with tending workers using a sterile spatula. Fungus gardens were kept in UV-sterilized plastic containers until they reached the laboratory at UNESP in Rio Claro, São Paulo, Brazil. Worker ants were subsequently identified by Dr. Rodrigo Feitosa and deposited in the entomological collection “Padre Jesus Santiago Moure” at Universidade Federal do Paraná, Curitiba, Brazil.
Small (0.5–1 mm in diameter) fragments of the fungus garden were plated on potato dextrose agar (PDA) supplemented with chloramphenicol [150 mg L-1, Sigma] and incubated at 25°C in the dark for seven days. Plates were monitored daily for fungal growth. Once filamentous fungi emerged from garden fragments, mycelia were subcultured on new PDA plates. Monosporic cultures were obtained for each strain and cultures were stored in 10% glycerol at -80°C at UNESP–Microbial Resource Center.
Morphological characterization
Four different culture media were used for measuring the radial growth and to determine colony morphology: potato-dextrose agar (PDA), malt extract agar 2% (MA2%), cornmeal agar (CMD) and synthetic nutrient agar (SNA). Strains were cultivated in triplicates at four different temperatures for each medium (10, 20, 25, 30 and 35°C). Microscopic morphological characters were examined on slide culture preparations. For this, we placed a 5 mm2 block of PDA on a microscopic slide and inoculated the fungus; after that, the material was covered with a cover slip and incubated at 25°C for seven days. Then, slides were examined under a light microscope. Conidia, conidiophores and conidiogenous cells were measured using the software LAS EZ (Leica Application Suite) using measurements of 30 replicates for each structure.
To visualize these same structures using scanning electron microscopy, fungus samples were fixed in osmium tetroxide vapor for 72 h, dehydrated sequentially through a series of acetone concentrations (50, 75, 90, 95 and 100%) and then dried until the critical point using liquid CO2 (Balzers CPD030). The dried material was mounted in stubs, sputtered with gold (Balzers SCD050) and examined with a scanning electron microscope (TM3000, Hitachi).
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted from mycelia grown on PDA for 7 days following a modified version of the CTAB method [8, 28]. Three different nuclear DNA markers were amplified for phylogenetic analysis: (i) large subunit RNA—LSU; (ii) translation elongation factor 1-alpha—tef1; and (iii) internal transcribed spacer—ITS. The primers and conditions used for amplification are described in Table 1. PCR products were cleaned up with Kit Wizard SV Gel and PCR Clean-up System (Promega) following the manufacturer’s protocol. Cycle sequencing reactions were carried out with 20 ng of template using BigDye Terminator v. 3.1 Cycle Sequencing Kit (Life Technologies) following the manufacturer instructions. Sequences were generated using ABI3500 (Life Technologies). Contigs were assembled in Bioedit v. 7.1.3 [29] and queried using the NCBI—GenBank to find the closest known relatives. Sequences of strain LESF53 were deposited in GenBank under accessions KJ808765—KJ808767. We also sequenced the partial tef1 gene for Escovopsis microspora CBS 135751T, which was deposited in GenBank under accession KJ935030.
Table 1. Molecular markers, primers and PCR conditions used in this study.
| Marker | Primers | Conditions |
|---|---|---|
| LSU | CLA-F (5’- GCATATCAATAAGCGGAGGA); CLA-R (5’- GACTCCTTGGTCCGTGTTTCA) [7] | 2 min of denaturation at 95°C, 40 cycles consisting of 30 s at 95°C, 60 s at 62°C, 90 s at 72°C and 5 min of extension at 72°C [19] |
| tef1 | EF6–20F (5’-AAGAACATGATCACTGGTACCT-3’); EF6–1000R (5’-CGCATGTCRCGGACGGC-3’) [10] | 96°C for 3 min, 35 cycles at 96°C for 30 s, 61°C for 45 s and a final extension step at 72°C for 1 min [53] |
| ITS | ITS4 (5’-TCCTCCGCTTATTGATATGC-3’); ITS5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) [54] | 96°C for 3 min, 35 cycles at 94°C for 1 min, 55°C for 1 min and a final extension step at 72°C for 2 min [54] |
Phylogenetic analyses
To determine the phylogenetic position of the new strains, three different phylogenetic reconstructions were performed: (i) a phylogeny within the Hypocreaceae family using LSU partial sequences; (ii) a phylogeny within the Escovopsis clade using tef1 partial sequences (previously examined strains of these fungi have been sequenced for the gene tef1 and their sequences are available in GenBank); and finally (iii), a phylogeny including only the formally described species of Escovopsis and Escovopsioides using concatenated sequences of ITS and tef1.
Alignments were obtained independently for each molecular marker using MAFFT v.7 [30] and nucleotide substitution models were selected using the Akaike information criterion (AIC) with a confidence interval of 95% in jModelTest 2 [31]. Independent runs in jModelTest 2 were performed for each molecular marker of the concatenated analysis (analysis number iii). Phylogenies were reconstructed using maximum likelihood (ML) in RAxML v.8 [32] and Bayesian Inference (BI) in MrBayes v.3.2.2 [33].
For positioning our strain within the Hypocreaceae, we first reconstructed the phylogeny of several species and genera belonging to this family using the LSU partial sequences. We selected 23 sequences used by Augustin et al. [19] and rooted using two members of Clavicipitaceae. We reconstructed 1000 independent ML trees under the GTR+I+G model in RAxML, and kept the tree with the best score. We evaluated the reliability of the tree topology by performing 2000 bootstrap replicates (bootstrap values converged at 1150 replicates [34]). Bayesian inference also used the GTR+I+G model of nucleotide substitution in two independent runs in MrBayes, each with three heated chains and one cold chain; each run consisted of Markov Chain Monte Carlo (MCMC) sampling for 2 million generations. Convergence occurred when the standard deviation (SD) of split frequencies fell below 0.01; the first 25% of MCMC generations were discarded as burn-in.
To further refine the position of the new strains within the Escovopsis clade, we used phylogenetic inference for tef1 partial sequences using the same conditions described above for both ML and BI analyses. In this case, bootstrap values converged after 850 replicates. Two million MCMC generations were again sufficient for achieving SD of split frequencies of less than 0.01. This tree was rooted using three sequences of other Hypocreaceae (Hypomyces polyporinus, Hypocrea lutea and Trichoderma sp.) used in previous studies [7, 9].
A third analysis used concatenated alignments of ITS and tef1 sequences generated using Winclada v.1.00.08 [35]. A partitioned ML analysis was conducted in RAxML using GTR+G for each partition. The alpha shape parameter of the gamma model of rate heterogeneity, empirical base frequencies, and evolutionary rates in the GTR matrix were estimated independently for each partition [32]. As before, 1000 ML trees were reconstructed and 2000 bootstraps replicates were performed (bootstraps values converged at 1950 replicates). For BI, a partitioned analysis was performed in MrBayes under GTR+G for each partition. The number of independent runs, number of chains, and the burn-in were the same as described above, but each run consisted of 1 million MCMC generations, which was sufficient to reach a SD of split frequencies of less than 0.01. We selected three other Hypocreaceae species (Trichoderma hamatum, T. pubens and Hypocrea rufa) that had ITS and tef1 sequences available in GenBank (NCBI) as the outgroup. The final trees were edited in FigTree v1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/) and Adobe Illustrator CS6 (Adobe Systems).
Results
Morphology
Morphological differences were observed between the E. kreiselii strains (LESF53, LESF303, LESF304 and LESF305) and all previously described species of Escovopsis and Escovopsioides associated with attine ants [19]. The most distinguishing feature of the new species compared with others is the type of conidiogenesis: E. kreiselii has holoblastic sympodial conidiogenesis with percurrent conidiogenous cells (Figs. 1 and 2). The colony color also differs from previously described species: E. kreiselii spores are pink while other described species of Escovopsis are brown, and Escovopsioides is white (Fig. 1). Such color differences appear to be important to distinguish fungi within the Escovopsis clade [6, 9, 19]. Escovopsis kreiselii grows better at 25°C (but also grows well at 20°C, see S1 Fig.) which is consistent with thermal preferences described for some attine ants [36]. Moreover, no growth was observed at 30°C, indicating that E. kreiselii is sensitive to high temperatures. E. kreiselii also has chlamydospores (Fig. 1M and 2D) similar to Escovopsioides nivea.
Figure 1. General morphological characteristics of Escovopsis kreiselii CBS 139320 (= LESF53).

A-D: cultures grown in PDA, MA2%, CMD and SNA after 14 days at 25°C, respectively; E: Conidiophores pattern formation on the aerial mycelia; F-H: Conidiophore branching pattern; I-L: Percurrent conidiogenous cells; M: Chlamydospores developed in chains and N: Globose to subglobose conidia.
Figure 2. SEM of Escovopsis kreiselii CBS 139320 (= LESF53) showing morphological aspects.
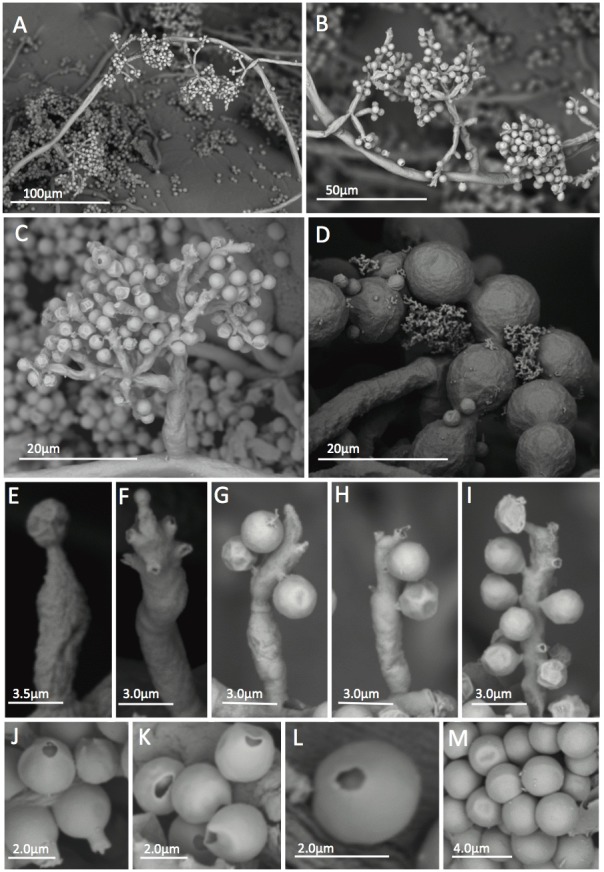
Slide cultures grown in PDA and SNA for five days at 25°C. A-B: Conidiophore growing patterns on the aerial mycelia; C: Conidiophore branching pattern; D: Chlamydospores; E-I: Percurrent conidiogenous cells showing conidia attached to the cells by denticles; J-M: Conidia. J: Conidia with attached denticle; K-L: Conidia without denticles and M: smooth walled conidia.
Phylogenetic analyses
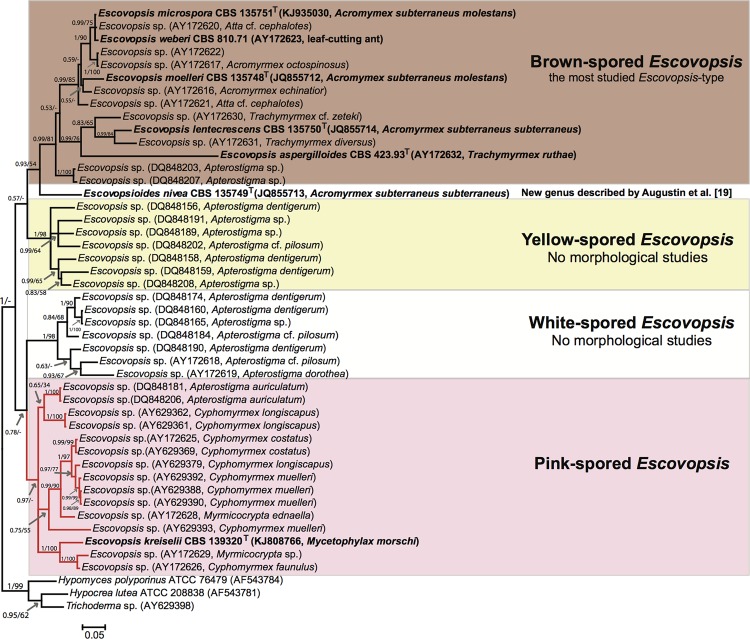
The tree topologies reconstructed using both ML and BI in three different phylogenetic analyses were very similar; the final Bayesian trees are displayed in Figs. 3, 4, and S2 Fig. with posterior probabilities and ML bootstrap values to indicate support for individual nodes. The LSU analysis grouped E. kreiselii as closely related to Escovopsis and Escovopsioides species within the phylogeny including other Hypocreaceae members. These fungi are grouped within an “attine-ants associated clade” (S2 Fig.). To compare the new strain with all Escovopsis diversity previously explored in other studies, we also performed a phylogenetic analysis using tef1 (Fig. 3). In this phylogeny, E. kreiselii grouped within the clade of pink-colored Escovopsis described in Gerardo et al. [9]. Unfortunately, it is not possible to include more genes within this phylogenetic analysis because most studies on the parasite phylogeny used only tef1 sequences and the strains are not available in public culture collections. Therefore, aiming to increase the reliability of our analysis, we also reconstructed a tree using two markers (ITS and tef1) of all described species of Escovopsis and Escovopsioides, similar to the analysis conducted by Augustin et al. [19]. Overall, our results suggest that E. kreiselii is a sister group to the clade that contains all previously described species of Escovopsis (Fig. 4), but the phylogenetic position of Escovopsioides nivea remains uncertain, varying depending on the molecular marker used (see Figs. 3, 4 and S2 Fig.). These results along with the morphological data support E. kreiselii as a new species within the hypocrealean fungi associated with attine ants.
Figure 3. Phylogenetic position of Escovopsis kreiselii within the Escovopsis clade based on tef1 sequences reconstructed using Bayesian Inference.
All Escovopsis species described so far are denoted in bold. In addition to the newly described species, forty-three tef1 sequences representing all Escovopsis morphotypes used in previous studies were retrieved from GenBank. Sequences of tef1 from other Hypocreaceae were used as outgroup. The voucher accession numbers in culture collections follow the taxon names. GenBank accessions and the ant species from which the fungi were isolated are given in parentheses. Different colors indicate the fungal morphotypes found in the Escovopsis clade. Bootstrap values from ML analyses are also indicated from a similar topology. Only PP and bootstrap values ≥ 0.5 or 50 are shown. Phylogeny based on Gerardo et al. [9]. T: ex-type strains. Bar: 0.05 substitutions per nucleotide position.
Figure 4. Phylogeny of described species within the Escovopsis clade.

This tree was generated from Bayesian analyses of two different markers: ITS and tef1. Sequences of other Hypocreaceae species were used as outgroup. The voucher accession numbers in culture collections follow the taxon names, and GenBank accessions and the ant species from which the fungi were isolated are given in parentheses. The new species, Escovopsis kreiselii is denoted in bold. ML analyses resulted in an identical topology and ML bootstraps values are shown above nodes. T: ex-type strains. Bar: 0.05 substitutions per nucleotide position.
Taxonomy
Emendation of the genus Escovopsis Muchovej & Della Lucia. Augustin et al. [19] added information regarding the conidia morphology to the genus originally described by Muchovej and Della Lucia as Escovopsis [17]. However, until now, the genus is described as “vesiculate; vesicles cylindrical to globose, evanescent; phialides hyaline, swollen at base, extending to a narrow neck; conidia in short basipetal chains, aseptate, hyaline at first becoming pigmented with an ornamented or mucilaginous brown outer coat or sheath, phoretic” [19]. To accommodate morphological variations observed in E. kreiselii, we propose an emendation on the description of the genus Escovopsis as follows: non-vesiculated conidiophores are present in less derived strains, with non-phialidic conidigenous cells producing solitary conidia (as indicated in Figs. 1 and 2).
Escovopsis kreiselii L.A. Meirelles, Q.V. Montoya, S.E. Solomon & A. Rodrigues sp. nov.
Mycobank: MB809176
Colonies on PDA, MA2%, CMD and SNA growing slowly, up to 5.3–6.0 cm, 6.6–8.1 cm, 3.9–4.5 cm and 4.3–5.2 cm in diameter after 14 days at 25°C. Mycelium reaching the edge of a 9 cm Petri dish in 24 and 22 days on PDA and MA2%, respectively, and after 30 days on CMD and SNA. Inconspicuous growth observed at 10°C; no growth at 30°C. Concentric growth observed in colonies cultured on PDA and MA2% (S1 Fig.). Hyaline hypha present in the aerial mycelia, stolon-like structures not observed. Colonies at first white, later attaining pink to brownish colors depending on the media (Fig. 1A-D). No soluble pigments observed in all media. Colony reverse is uncolored. Intercalary concatenated chlamydospores found in the submerged and in the aerial mycelia, 10.8–21.4 μm in length × 8.3–16.6 μm in width. Conidiophores arising from fertile aerial hypha in opposite and intercalary patterns (Fig. 1E and Fig. 2A-B), at first white and quickly becoming light to dark pink. A septum is often observed at the base of each conidiophore. Irregular conidiophore branching pattern, some have verticils (of 3 to 4 branches), some have 2 opposite branches and some are solitary; smooth walled, hyaline, 29.0–229.00 μm in length and 2.5–8.6 μm in diameter. Conidiogenous cells are holoblastic, formed at the apex of each branch, intercalary or solitary on the fertile hypha. Each branch of the conidiophore has up to 4–6 conidiogenous cells measuring 5.3–13.4 μm in length and 2.5–7.7 cm in width. Conidiogenous cells are percurrent, hyaline, arising in verticils often with numerous denticle-like structures (Figs 1K, 2E-I). Conidia are globose to subglobose, brown, 2.4–3.2 μm in diameter, thick walled with smooth or rough surface (Figs. 1N, 2C, and 2E-M). Conidia often bear denticle-like structures (Fig. 2J).
Specimen examined: BRAZIL. Santa Catarina, Florianópolis, Praia da Joaquina, GPS: 27°37′50.01″S; 48°27′3.64″W, elev. 1 m, Fungus garden, 03, 2009. A. Rodrigues. Ex-type strain LESF53 (= CBS 139320, = CBMAI 1691). Holotype: CBS H-22062 (dried culture on PDA).
Additional specimens examined: BRAZIL. Santa Catarina, Florianópolis, Praia de Moçambique, GPS: 27°31′24.96″S; 48°25′3.78″W, elev. 1 m, Fungus garden, 02, 2014. A. Rodrigues. LESF303 (= CBS 139321, = CBMAI 1692) and LESF304. BRAZIL. Santa Catarina, Florianópolis, Praia da Joaquina, GPS: 27°37′49.62″S; 48°27′3.6″W, elev. 1.7 m, Fungus garden, 02, 2014. A. Rodrigues. LESF305 (= CBS 139322, = CBMAI 1693).
Sequences: LSU: KJ808765, tef1: KJ808766, ITS: KJ808767
Etymology: Named in honor of Dr. Hanns Kreisel, who first described the fungal parasite Phialocladus currently known as Escovopsis. Kreisel did not designate a type strain for Phialocladus [37], thus this name was considered a nomen invalidum [17].
Habitat: isolated from fungus gardens of the attine ant Mycetophylax morschi.
Discussion
The intricacy of symbioses between animals and microbes is emerging as a major theme in 21st century biology (e.g. the human microbiome project). Despite a new appreciation of the ubiquity and importance of such symbioses, the attine ants and their fungal symbionts have long been recognized as an example of the complex interactions between animals and microbes. Although the fungal cultivar used as a food source for ants was first recognized in 1874 [38], it was not until the 1990s, and especially the 2000s, that the high fungal diversity associated with attine ants began to be recognized [6, 13, 17–19, 39–46].
The genus Escovopsis was likewise first observed in the 19th century [47]. During the 20th century, some early studies documented the presence of these fungi in attine ant nests [37, 48], but the genus and the first two species were not formally described until the 1990s [17, 18]. Recently, Augustin et al. [19] described three additional species within Escovopsis and the new genus Escovopsioides (originally studied by Möller in 1893 [47]; however he interpreted this genus to be a “weak form” of the fungus cultivated by leaf-cutting ants). Moreover, no taxonomic study was performed for Escovopsis associated with lower attine ants and the morphological aspects of these fungi are still poorly known.
Gerardo et al. [9] examined different Escovopsis morphotypes, documenting the existence of at least four different colored groups (pink, white, yellow and brown) associated with Apterostigma and Cyphomyrmex ants. However, that study aimed to explore the phylogenetic diversity of the fungus, and no detailed morphological examination was carried out. In our phylogenetic analyses, E. kreiselii falls within the pink-colored Escovopsis clade (Fig. 3), suggesting that species of the pink clade may exhibit significant morphological differences when compared to other described Escovopsis species; nevertheless, detailed morphological analyses on these strains are necessary to confirm this hypothesis.
Currie et al. [6, 7] provided evidence that Escovopsis is a specific parasite of the mutualistic fungus cultivated by attine ants and that Escovopsis has co-evolved with the ants’ fungal cultivar, as evidenced by co-cladogenesis between species of both fungi. The morphological analyses conducted by Currie et al. [7] were not sufficient to identify detailed differences in conidiophore structure or conidiogenesis type for the distinct Escovopsis clades. Our study extends the findings of Currie et al. [7] and corroborates that Escovopsis is a genus that exhibits diverse morphological features. This concept is consistent with the known diversity of this group of ants [1, 3] and that of their mutualistic fungi [24, 26, 49]. Morphological characteristics of E. kreiselii support its distinction from other Escovopsis species due to the conidiogenesis type, which is sympodial in E. kreiselii and phialidic for the other Escovopsis described so far. Moreover, no vesicles were observed in the conidiophores of E. kreiselii, which is contrary to the vesiculated conidiophores found in other Escovopsis species. In addition, all the described Escovopsis species are brown, whereas E. kreiselii is pink. The conidiogenous cells of the new species are percurrent, contrary to the phialidic conidiogenous cells observed in Escovopsis from higher attines. Furthermore, E. kreiselii produces solitary conidia. Due to the inclusion of E. kreiselii in the genus Escovopsis, it is clear that this group exhibits very diverse morphological aspects and an extension of the genus is necessary to accommodate the new morphological diversity. Future taxonomic work will enhance our understanding of this diversity. Although previous phylogenetic approaches have hinted at the vast genetic diversity of Escovopsis [7, 9–11], detailed examination of the morphology of the yellow, white and other strains of the pink-spored Escovopsis is essential to better understand the systematics of this group. Such examination will also aid in studies regarding evolutionary and ecology of host-pathogen dynamics in this system.
An important event in the study of this group was the description of Escovopsioides by Augustin et al. [19]. However, the phylogenetic position of this taxon remains uncertain. In our LSU phylogeny, Escovopsioides was an outgroup to the clade consisting of all formally described Escovopsis species (S2 Fig.); nevertheless, in the phylogeny reconstructed with tef1, Escovopsioides is sister to the brown-spored Escovopsis, which together with the yellow-spored Escovopsis form a clade that is sister to the white-spored and pink-spored Escovopsis (Fig. 3). A similar topology was suggested by the concatenated (ITS and tef1) phylogeny, but with low statistical support for Escovopsioides position (Fig. 4). Under a morphological approach, Escovopsioides seems to be more similar to species described within the brown-spored Escovopsis group (both have phialides with conidia produced in chains). The main difference is that the phialides for the Escovopsioides are lageniform and produced on terminal and intercalary globose vesicles [19]. Escovopsioides nivea has no pigmentation while all Escovopsis species exhibiting phialidic conidiogenese are brown in color [19]. Moreover, the percurrent conidiogenous cells of E. kreiselii may be considered a less derived character when compared to the phialides of brown Escovopsis and Escovopsioides. Nevertheless, only one species of Escovopsioides is known, represented by a single DNA sequence. Thus, sampling more strains of Escovopsioides may resolve these incongruences in the phylogenetic reconstructions using different genes and clarify its position. If this fungus is in fact more related to the brown Escovopsis clade than to the other color clades (i.e. if it groups within the Escovopsis clade), future studies might reclassify E. nivea as an Escovopsis species, maintaining the monophyly of the hypocrealean fungi specialized in infecting gardens of attine ants. It seems that, over evolutionary time, this group of fungi developed different conidiogenesis types and such morphological aspects might indicate adaptations related to their interactions with the ants and/or the mutualistic cultivar.
Finally, as a member of the Escovopsis group, E. kreiselii is presumed to be a fungal parasite of attine fungal gardens; however, the pathogenicity of this species remains unknown. Currie [50] demonstrated that Escovopsis is a specific parasite of the mutualistic fungus that decreases garden biomass accumulation. Reynolds and Currie [12] suggested that Escovopsis attacks the mutualistic fungus using chemical compounds and Gerardo et al. [8] demonstrated specificity in host-parasite interactions among the pink-spored Escovopsis. Moreover, several studies have tested the in vitro pathogenicity of brown-spored Escovopsis of leaf-cutting ants [51, 52]. However, no study to date has considered this approach for the other Escovopsis clades. It is possible that E. kreiselii attacks the mutualistic fungus of M. morschi, but this hypothesis remains to be supported empirically. It is noteworthy that all the three M. morschi nests we sampled were apparently healthy, suggesting that E. kreiselii was either in a dormant state within the fungus gardens at the time of sampling or that E. kreiselii is not in fact a virulent pathogen of lower attine fungal gardens.
Supporting Information
PDA: potato-dextrose agar; MA2%: malt extract agar 2%; CMD: cornmeal agar; SNA: synthetic nutrient agar.
(TIFF)
The phylogeny of 22 Hypocreaceae species and two Clavicipitaceae species as outgroup was reconstructed using Bayesian Inference. The voucher accession numbers in culture collections follow the taxon names and the GenBank accession numbers are given in parentheses. The clade highlighted in gray represents fungi strictly associated with attine ants. Posterior probabilities of nodes are given along with ML bootstraps values for a similar topology; only PP and bootstrap values ≥ 0.5 or 50 are shown on branches, respectively. Phylogeny based on Augustin et al. [19]. Bar: 0.05 substitutions per nucleotide position. T: ex-type strains.
(TIFF)
Acknowledgments
We are grateful to MSc. Sergio Kakazu for sequencing operations and Dr. Pablo Henrique Nunes for SEM assistance. Also, we would like to thank the editor Dr. Nicole Gerardo and the two reviewers for valuable comments on this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for providing financial support (grant # 2011/16765-0) and a scholarship to LAM and NSF IRFP (United States National Science Foundation International Research Fellowship Program no. 07012333) for supporting SES. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schultz TR, Brady SG (2008) Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA 105: 5435–5440. 10.1073/pnas.0711024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klingenberg C, Brandão CRF (2009) Revision of the fungus-growing ant genera Mycetophylax Emery and Paramycetophylax Kusnezov rev. stat., and description of Kalathomyrmex n. gen. (Formicidae: Myrmicinae: Attini). Zootaxa 2052: 1–31. [Google Scholar]
- 3. Sosa-Calvo J, Schultz TR, Brandão CR, Klingenberg C, Feitosa RM, et al. (2013) Cyatta abscondita: taxonomy, evolution, and natural history of a new fungus-farming ant genus from Brazil. PloS One 8: e80498 10.1371/journal.pone.0080498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber NA (1972) Gardening Ants: The Attines. American Philosophical Society, Philadelphia.
- 5. Mehdiabadi NJ, Schultz TR (2010) Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecol News 13: 37–55. [Google Scholar]
- 6. Currie CR, Mueller UG, Malloch D (1999) The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci USA 96: 7998–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, et al. (2003) Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299: 386–388. [DOI] [PubMed] [Google Scholar]
- 8. Gerardo NM, Mueller UG, Price SL, Currie CR (2004) Exploiting a mutualism: parasite specialization on cultivars within the fungus-growing ant symbiosis. Proc Biol Sci 271: 1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerardo NM, Mueller UG, Currie CR (2006) Complex host-pathogen coevolution in the Apterostigma fungus-growing ant-microbe symbiosis. BMC Evol Biol 6: 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taerum SJ, Cafaro MJ, Little AE, Schultz TR, Currie CR (2007) Low host-pathogen specificity in the leaf-cutting ant-microbe symbiosis. Proc Biol Sci 274: 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taerum SJ, Cafaro MJ, Currie CR (2010) Presence of multiparasite infections within individual colonies of leaf-cutter ants. Environ Entomol 39: 105–113. 10.1603/EN09137 [DOI] [PubMed] [Google Scholar]
- 12. Reynolds HT, Currie CR (2004) Pathogenicity of Escovopsis weberi: The parasite of the attine ant-microbe symbiosis directly consumes the ant-cultivated fungus. Mycologia 96: 955–959. [PubMed] [Google Scholar]
- 13. Rodrigues A, Bacci M Jr, Mueller UG, Ortiz A, Pagnocca FC (2008) Microfungal “weeds” in the leafcutter ant symbiosis. Microb Ecol 56: 604–614. 10.1007/s00248-008-9380-0 [DOI] [PubMed] [Google Scholar]
- 14. Little AE, Currie CR (2008) Black yeast symbionts compromise the efficiency of antibiotic defenses in fungus-growing ants. Ecology 89: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 15. Mendes TD, Rodrigues A, Dayo-Owoyemi I, Marson FAL, Pagnocca FC (2012) Generation of nutrients and detoxification: possible roles of yeasts in leaf-cutting ant nests. Insects 3: 228–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Bael SA, Seid MA, Wcislo WT (2012) Endophytic fungi increase the processing rate of leaves by leaf-cutting ants (Atta). Ecol Entomol 37: 318–321. [Google Scholar]
- 17. Muchovej JJ, Della Lucia TMC (1990) Escovopsis, a new genus from leaf-cutting ant nests to replace Phialocladus nomen invalidum. Mycotaxon 37: 191–195. [Google Scholar]
- 18. Seifert KA, Samson RA, Chapela IH (1995) Escovopsis aspergilloides, a rediscovered hyphomycete from leaf-cutting ant nests. Mycologia 87: 407–413. [Google Scholar]
- 19. Augustin JO, Groenewald JZ, Nascimento RJ, Mizubuti ESG, Barreto RW, et al. (2013) Yet more “weeds” in the garden: fungal novelties from nests of leaf-cutting ants. PLoS One 8: e82265 10.1371/journal.pone.0082265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silva-Pinhati AC, Bacci M Jr, Hinkle G, Sogin ML, Pagnocca FC, et al. (2004) Low variation in ribosomal DNA and internal transcribed spacers of the symbiotic fungi of leaf-cutting ants (Attini: Formicidae). Braz J Med Biol Res 37: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 21. Mikheyev AS, Mueller UG, Abbot P (2006) Cryptic sex and many-to-one coevolution in the fungus-growing ant symbiosis. Proc Natl Acad Sci USA 103: 10702–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mikheyev AS, Mueller UG, Boomsma JJ (2007) Population genetic signatures of diffuse co-evolution between leaf-cutting ants and their cultivar fungi. Mol Ecol 16: 209–216. [DOI] [PubMed] [Google Scholar]
- 23. Mueller UG, Scott JJ, Ishak HD, Cooper M, Rodrigues A (2010) Monoculture of leafcutter ant gardens. PloS One 5: e12668 10.1371/journal.pone.0012668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapela IH, Rehner SA, Schultz TR, Mueller UG (1994) Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science 266: 1691–1694. [DOI] [PubMed] [Google Scholar]
- 25. Mikheyev AS, Vo T, Mueller UG (2008) Phylogeography of post-Pleistocene population expansion in a fungus-gardening ant and its microbial mutualists. Mol Ecol 20: 4480–4488. 10.1111/j.1365-294X.2008.03940.x [DOI] [PubMed] [Google Scholar]
- 26. Mikheyev AS, Mueller UG, Abbot P (2010) Comparative dating of attine ant and lepiotaceous cultivar phylogenies reveals coevolutionary synchrony and discord. Am Nat 175: E126–133. 10.1086/652472 [DOI] [PubMed] [Google Scholar]
- 27. Klingenberg C, Brandão CRF, Engels W (2007) Primitive nest architecture and small monogyneous colonies in basal Attini inhabiting sandy beaches of Southern Brazil. Stud Neotrop Fauna E 42, 121–126. [Google Scholar]
- 28. Möller EM, Bahnweg G, Sandermann H, Geiger HH (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res 20: 6115–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hall TA (1999) BioEdit 5.0.9: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 30. Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat methods 9: 772 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, et al. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pattengale ND, Alipour M, Bininda-Emonds OR, Moret BM, Stamatakis A (2010) How many bootstrap replicates are necessary? J Comput Biol 17: 337–354. 10.1089/cmb.2009.0179 [DOI] [PubMed] [Google Scholar]
- 35.Nixon KC (2002) WinClada ver. 1.0000 Published by the author, Ithaca, New York, USA.
- 36. Bollazzi M, Roces F (2002) Thermal preference for fungus culturing and brood location by workers of the thatching grass-cutting ant Acromyrmex heyeri . Insectes Soc 49: 153–157. [Google Scholar]
- 37. Kreisel H (1972) Pilze aus Pilzgarten von Atta insularis in Kuba. Z Allg Mikrobiol 12: 643–654. [PubMed] [Google Scholar]
- 38.Belt T (1874) The Naturalist in Nicaragua. The University of Chicago Press, Chicago.
- 39. Middelhoven WJ, Fonseca A, Carreiro SC, Pagnocca FC, Bueno OC (2003) Cryptococcus haglerorum, sp. nov., an anamorphic basidiomycetous yeast isolated from nests of the leaf- cutting ant Atta sexdens . Antonie Van Leeuwenhoek 83: 167–174. [DOI] [PubMed] [Google Scholar]
- 40. Carreiro SC, Pagnocca FC, Bacci M Jr, Lachance MA, Bueno OC, et al. (2004) Sympodiomyces attinorum sp. nov., a yeast species associated with nests of the leaf-cutting ant Atta sexdens . Int J Syst Evol Microbiol 54: 1891–1894. [DOI] [PubMed] [Google Scholar]
- 41. Rodrigues A, Mueller UG, Ishak HD, Bacci M Jr, Pagnocca FC (2011) Ecology of microfungal communities in gardens of fungus-growing ants (Hymenoptera: Formicidae): a year-long survey of three species of attine ants in Central Texas. FEMS Microbiol Ecol 78: 244–255. 10.1111/j.1574-6941.2011.01152.x [DOI] [PubMed] [Google Scholar]
- 42. Rodrigues A, Passarini MR, Ferro M, Nagamoto NS, Forti LC, et al. (2014) Fungal communities in the garden chamber soils of leaf-cutting ants. J Basic Microbiol 54: 1186–1196. 10.1002/jobm.201200458 [DOI] [PubMed] [Google Scholar]
- 43. Van Bael SA, Fernández-Marín H, Valencia MC, Rojas EI, Wcislo WT, et al. (2009) Two fungal symbioses collide: endophytic fungi are not welcome in leaf-cutting ant gardens. Proc Biol Sci 276: 2419–2426. 10.1098/rspb.2009.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pagnocca FC, Legaspe MFC, Rodrigues A, Ruivo CCC, Nagamoto NS, et al. (2010) Yeasts isolated from a fungus-growing ant nest, including the description of Trichosporon chiarellii sp. nov., an anamorphic basidiomycetous yeast. Int J Syst Evol Microbiol 60: 1454–1459. 10.1099/ijs.0.015727-0 [DOI] [PubMed] [Google Scholar]
- 45. Attili-Angelis D, Duarte APM, Pagnocca FC, Nagamoto NS, Vries M, et al. (2014) Novel Phialophora species from leaf-cutting ants (tribe Attini). Fungal Divers 65: 65–75. [Google Scholar]
- 46. Melo WG, Arcuri SL, Rodrigues A, Morais PB, Meirelles LA, et al. (2014) Starmerella aceti f.a., sp. nov., an ascomycetous yeast species isolated from fungus garden of the leafcutter ant Acromyrmex balzani . Int J Syst Evol Microbiol 64: 1428–1433. 10.1099/ijs.0.058818-0 [DOI] [PubMed] [Google Scholar]
- 47.. Möller A (1893) Die Pilzgärten einiger Südamerikanischer Ameisen. In: Schimper AFW, editor. Botanische Mittheilungen aus den Tropen. Jena: Gustav Fischer
- 48. Weber NA (1966) Fungus-growing ants. Science 153: 587–604. [DOI] [PubMed] [Google Scholar]
- 49. Mueller UG, Rehner SA, Schultz TR (1998) The evolution of agriculture in ants. Science 281: 2034–2038. [DOI] [PubMed] [Google Scholar]
- 50. Currie C (2001) Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 128: 99106. [DOI] [PubMed] [Google Scholar]
- 51. Folgarait P, Gorosito N, Poulsen M, Currie CR (2011) Preliminary in vitro insights into the use of natural fungal pathogens of leaf-cutting ants as biocontrol agents. Curr microbiol 63: 250–258 10.1007/s00284-011-9944-y [DOI] [PubMed] [Google Scholar]
- 52. Folgarait P, Marfetán JA, Cafaro MJ (2011) Growth and conidiation response of Escovopsis weberi (Ascomycota: Hypocreales) against the fungal cultivar of Acromyrmex lundii (Hymenoptera: Formicidae). Environ Entomol 40: 342–349 [Google Scholar]
- 53. Meirelles LA, Mendes TD, Solomon SE, Bueno OC, Pagnocca FC, et al. (2014) Broad Escovopsis-inhibition activity of Pseudonocardia associated with Trachymyrmex ants. Environ Microbiol Rep 6: 339–345 10.1111/1758-2229.12132 [DOI] [PubMed] [Google Scholar]
- 54. White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: PCR Protocols: a guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, New York, pp 315–322 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDA: potato-dextrose agar; MA2%: malt extract agar 2%; CMD: cornmeal agar; SNA: synthetic nutrient agar.
(TIFF)
The phylogeny of 22 Hypocreaceae species and two Clavicipitaceae species as outgroup was reconstructed using Bayesian Inference. The voucher accession numbers in culture collections follow the taxon names and the GenBank accession numbers are given in parentheses. The clade highlighted in gray represents fungi strictly associated with attine ants. Posterior probabilities of nodes are given along with ML bootstraps values for a similar topology; only PP and bootstrap values ≥ 0.5 or 50 are shown on branches, respectively. Phylogeny based on Augustin et al. [19]. Bar: 0.05 substitutions per nucleotide position. T: ex-type strains.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.