Abstract
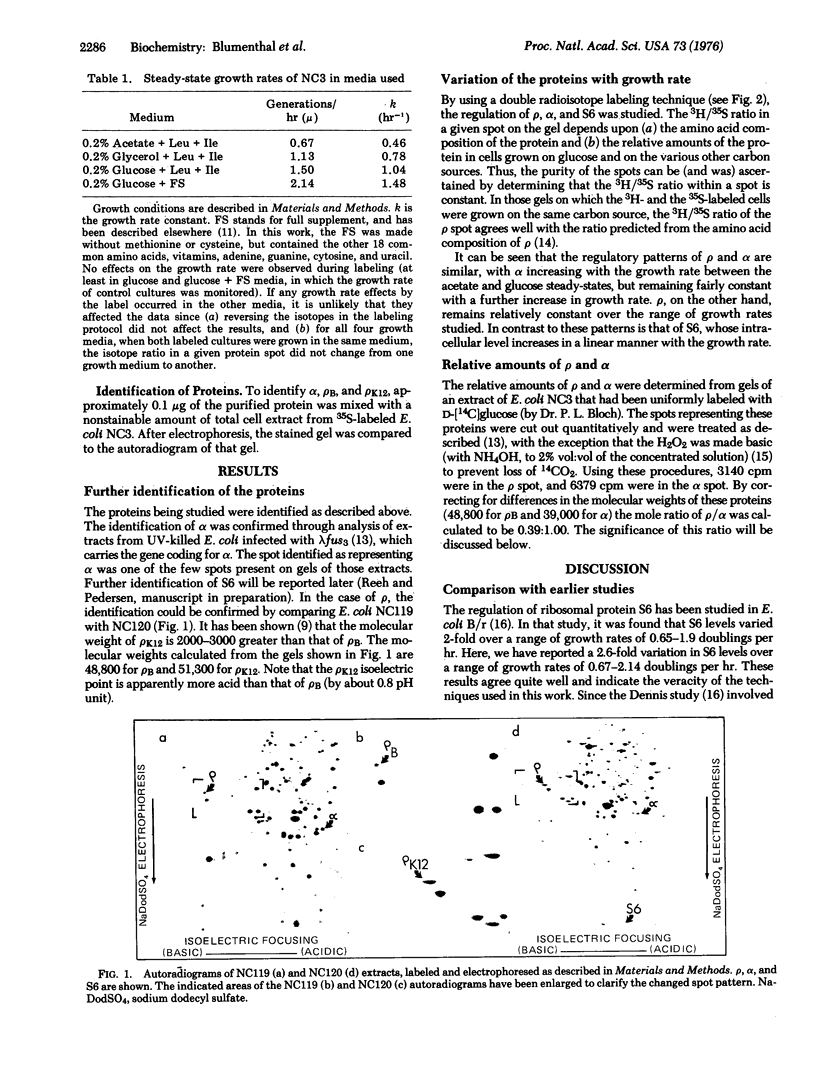
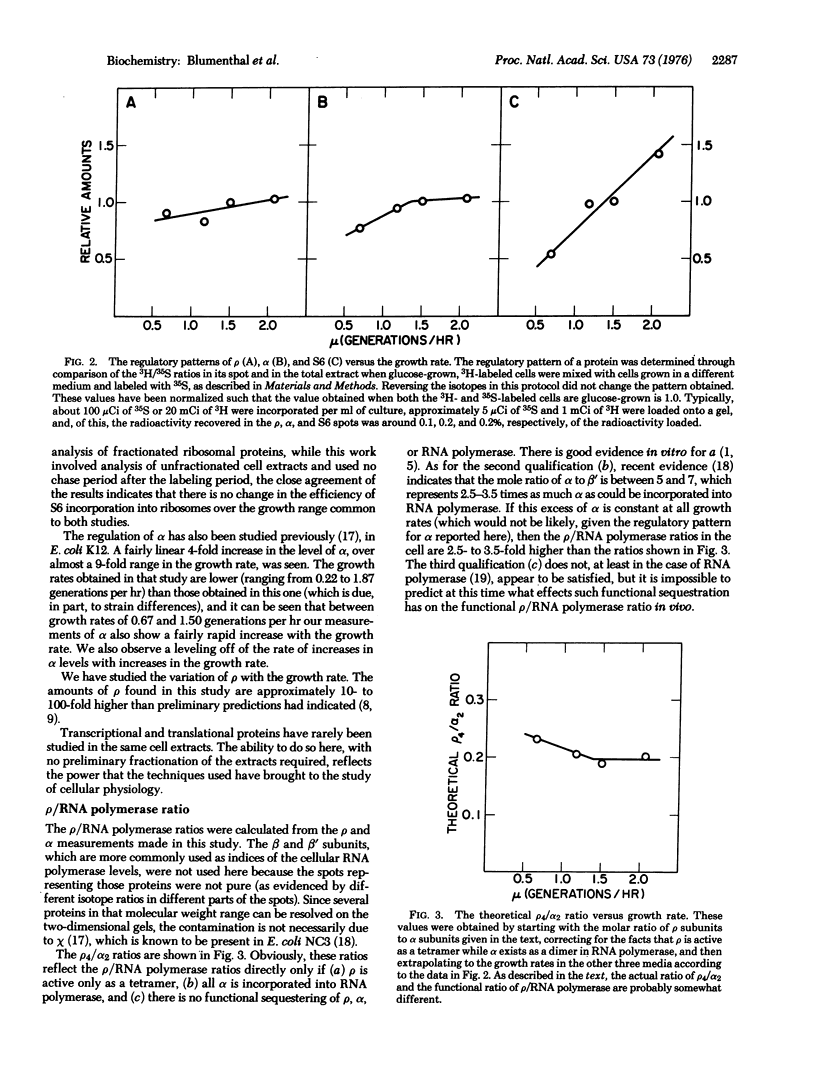
Transcriptional termination factor rho, the alpha subunit of RNA polymerase (RNA nucleotidyltransferase nucleosidetriphosphate: RNA nucleotidyltransferase, EC 2.7.7.6), and ribosomal protein S6 were resolved from whole-cell extracts of E. coli B/r by a high-resolution, two-dimensional polyacrylamide gel electrophoretic technique, and were identified through coelectrophoresis with the purified proteins. The regulation of rho, alpha, and S6 was studied, in steady-state cultures of E. coli B/r growing at rates ranging from 0.6 to 2.1 generations per hr, through the use of this gel technique and a double radioisotope labeling procedure. The regulatory patterns of rho and alpha are distinct from, but similar to, one another. Neither rho nor alpha shows the sharply increasing levels with increasing growth rate shown by the ribosomal proteins was exemplified by S6. The difference between the levels of rho and alpha, on the one hand, and S6, on the other, is most pronounced during rapid growth. The regulatory pattern of alpha is interesting, given the recent suggestion that the gene coding for alpha is contranscribed with genes coding for ribosomal proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter T., Newton A. New polarity suppressors in Escherichia coli: suppression and messenger RNA stability. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2962–2966. doi: 10.1073/pnas.68.12.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L. Stimultaneous purification of Escherichia coli termination factor rho, RNAase III and RNAase H. Eur J Biochem. 1975 Feb 21;51(2):369–376. doi: 10.1111/j.1432-1033.1975.tb03937.x. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. In vivo stability, maturation and relative differential synthesis rates of individual ribosomal proteins in Escherichia coli B/r. J Mol Biol. 1974 Sep 5;88(1):25–41. doi: 10.1016/0022-2836(74)90293-9. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Hurwitz J. Studies on termination of in vitro ribonucleic acid synthesis by rho factor. J Biol Chem. 1972 Sep 10;247(17):5637–5645. [PubMed] [Google Scholar]
- Goodman D., Matzura H. An improved method of counting radioactive acrylamide gels. Anal Biochem. 1971 Aug;42(2):481–486. doi: 10.1016/0003-2697(71)90062-5. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Burgess R. R., Nomura M. Identification of a gene for the alpha-subunit of RNA polymerase at the str-spc region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5036–5040. doi: 10.1073/pnas.72.12.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Guertin M. Amber suA mutations which relieve polarity. J Mol Biol. 1972 Feb 14;63(3):605–608. doi: 10.1016/0022-2836(72)90453-6. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Wickner W., Kornberg A. A novel form of RNA polymerase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4425–4428. doi: 10.1073/pnas.71.11.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Yarbrough L. R., Hillel Z., Wu F. Y. Sigma cycle during in vitro transcription: demonstration by nanosecond fluorescence depolarization spectroscopy. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3019–3023. doi: 10.1073/pnas.72.8.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]



