Abstract
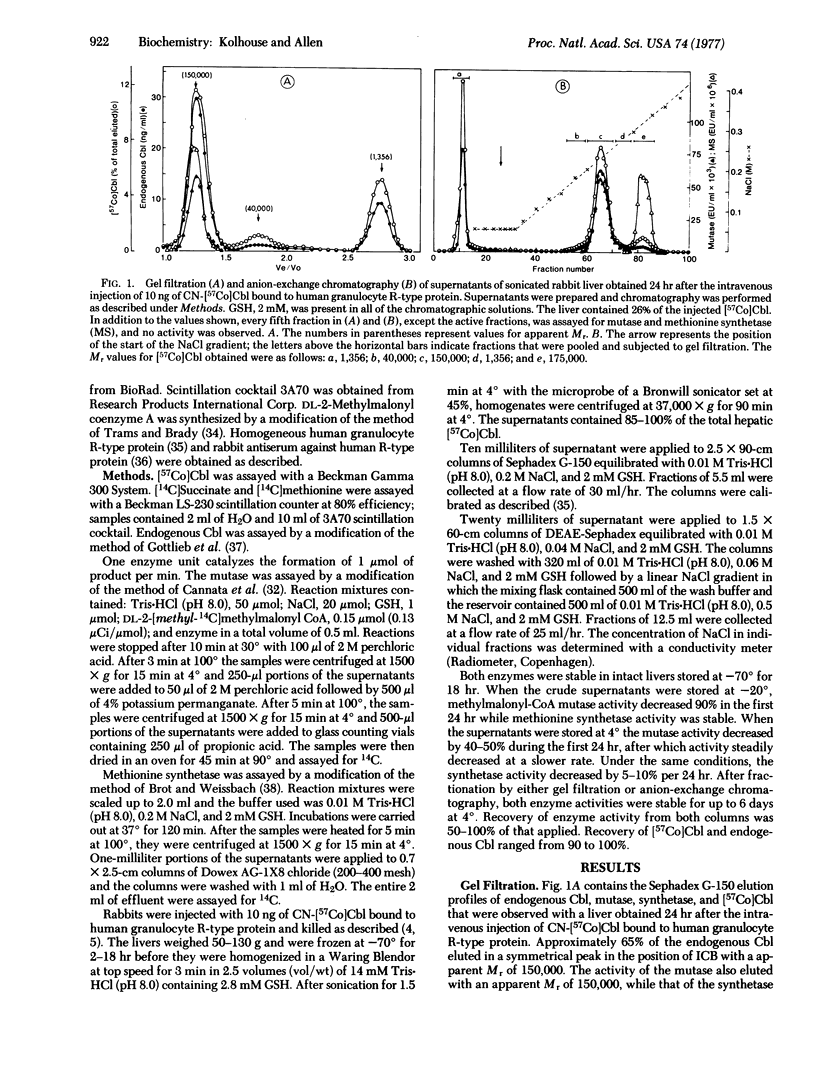
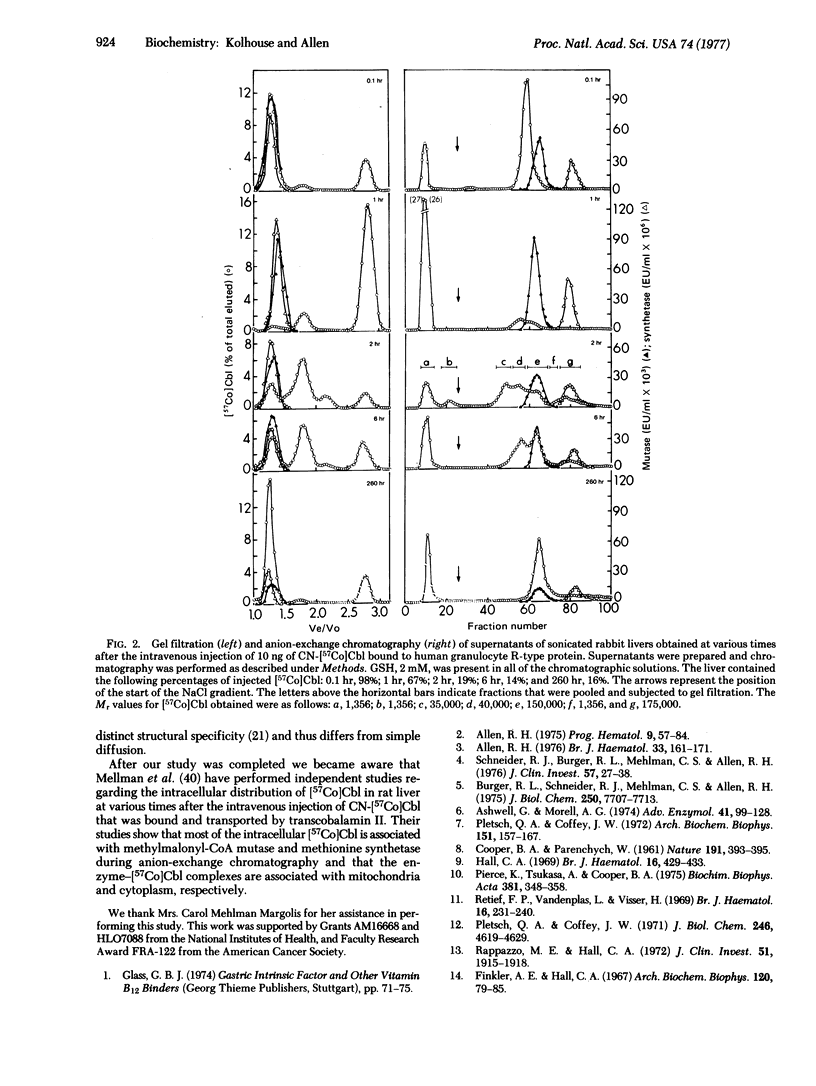
The granulocyte R-type cobalamin binding protein delivers cobalamin (Cbl) exclusively to hepatocytes, and transcobalamin II delivers Cbl to various mammalian cells. Both protein-Cbl complexes enter cells by pinocytosis, and the protein moieties are rapidly degraded in lysosomes. The liberated Cbl is subsequently bound to a high-molecular-weight intracellular cobalamin binding protein (ICB). The nature of ICB-Cbl is unknown but appears important because ICB-[57Co]Cbl is missing from cultured fibroblasts of a group of patients whose cells take up CN-[57Co]Cbl normally but do not convert it to either of its coenzyme forms. We have examined supernatants of sonicated rabbit livers and have found that 65% of the total endogenous Cbl elutes from Sephadex G-150 as ICB-Cbl and that this fraction also contains the two mammalian Cbl-dependent enzymes, methylmalonyl-CoA mutase (methylmalonyl-CoA CoA-carbonylmutase;EC 5.4.99.2) and methionine synthetase (tetrahydropteroylglutamate methyltransferase; 5-methyltetrahydropteroyl-L-glutamate:L-homocysteine-S-methyltransferase; EC 2.1.1.13). Gradient elution from DEAE-Sephadex reveals that 90--95% of the ICB--Cbl elutes with methylmalonyl-CoA mutase and 5--10% elutes with methionine synthetase. ICB--[57Co]Cbl first appears 2 hr after the intravenous injection of CN[57Co]Cbl bound to granulocyte R-type protein. This ICB-[57Co]Cbl is associated with either methylmalonyl-CoA mutase or methionine synthetase although the latter appears to be formed at a relatively faster rate. Our studies indicate that mammalian cells contain two ICBs, that these proteins are methylmalonyl-CoA mutase and methionine synthetase, and that the primary abnormality in the group of patients mentioned above lies at a step that is common to the formation of both Cbl coenzymes and that precedes the stable binding of Cbl to both methylmalonyl-CoA mutase and methionine synthetase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H. Human vitamin B12 transport proteins. Prog Hematol. 1975;9:57–84. [PubMed] [Google Scholar]
- Allen R. H., Majerus P. W. Isolation of vitamin B12-binding proteins using affinity chromatography. II. Purification and properties of a human granulocyte vitamine B12-binding protein. J Biol Chem. 1972 Dec 10;247(23):7702–7708. [PubMed] [Google Scholar]
- Allen R. H. The plasma transport of vitamin B12. Br J Haematol. 1976 Jun;33(2):161–171. doi: 10.1111/j.1365-2141.1976.tb03527.x. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Barker H. A. Corrinoid-dependent enzymic reactions. Annu Rev Biochem. 1972;41:55–90. doi: 10.1146/annurev.bi.41.070172.000415. [DOI] [PubMed] [Google Scholar]
- Brot N., Weissbach H. The role of cobamides in methionine synthesis. Enzymatic formation of holoenzyme. J Biol Chem. 1966 May 10;241(9):2024–2028. [PubMed] [Google Scholar]
- Burger R. L., Allen R. H. Characterization of vitamin B12-binding proteins isolated from human milk and saliva by affinity chromatography. J Biol Chem. 1974 Nov 25;249(22):7220–7227. [PubMed] [Google Scholar]
- Burger R. L., Schneider R. J., Mehlman C. S., Allen R. H. Human plasma R-type vitamin B12-binding proteins. II. The role of transcobalamin I, transcobalamin III, and the normal granulocyte vitamin B12-binding protein in the plasma transport of vitamin B12. J Biol Chem. 1975 Oct 10;250(19):7707–7713. [PubMed] [Google Scholar]
- CANNATA J. J., FOCESI A., Jr, MAZUMDER R., WARNER R. C., OCHOA S. METABOLISM OF PROPIONIC ACID IN ANIMAL TISSUES. XII. PROPERTIES OF MAMMALIAN METHYLMALONYL COENZYME A MUTASE. J Biol Chem. 1965 Aug;240:3249–3257. [PubMed] [Google Scholar]
- COOPER B. A., PARANCHYCH W. Selective uptake of specifically bound cobalt-58 vitamin B12 by human and mouse tumour cells. Nature. 1961 Jul 22;191:393–395. doi: 10.1038/191393a0. [DOI] [PubMed] [Google Scholar]
- DICKERMAN H., REDFIELD B. G., BIERI J. G., WEISSBACH H. THE ROLE OF VITAMIN B12 IN METHIONINE BIOSYNTHESIS IN AVIAN LIVER. J Biol Chem. 1964 Aug;239:2545–2552. [PubMed] [Google Scholar]
- Finkler A. E., Hall C. A. Nature of the relationship between vitamin B12 binding and cell uptake. Arch Biochem Biophys. 1967 Apr;120(1):79–85. doi: 10.1016/0003-9861(67)90600-5. [DOI] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- Goodman S. I., Moe P. G., Hammond K. B., Mudd S. H., Uhlendorf B. W. Homocystinuria with methylmalonic aciduria: two cases in a sibship. Biochem Med. 1970 Dec;4(5):500–515. doi: 10.1016/0006-2944(70)90080-3. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Mahoney M. J., Ruddle F. H., Rosenberg L. E. Genetic complementation in heterokaryons of human fibroblasts defective in cobalamin metabolism. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3181–3185. doi: 10.1073/pnas.72.8.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. A. Transport of vitamin B 12 in man. Br J Haematol. 1969 May;16(5):429–433. doi: 10.1111/j.1365-2141.1969.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Linnell J. C., Hoffbrand A. V., Hussein H. A., Wise I. J., Matthews D. M. Tissue distribution of coenzyme and other forms of vitamin B12 in control subjects and patients with pernicious anaemia. Clin Sci Mol Med. 1974 Feb;46(2):163–172. doi: 10.1042/cs0460163. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Hart A. C., Steen V. D., Rosenberg L. E. Methylmalonicacidemia: biochemical heterogeneity in defects of 5'-deoxyadenosylcobalamin synthesis. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2799–2803. doi: 10.1073/pnas.72.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M. J., Rosenberg L. E., Mudd S. H., Uhlendorf B. W. Defective metabolism of vitamin B 12 in fibroblasts from children with methylmalonicaciduria. Biochem Biophys Res Commun. 1971 Jul 16;44(2):375–381. doi: 10.1016/0006-291x(71)90610-3. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Youngdahl-Turner P., Willard H. F., Rosenberg L. E. Intracellular binding of radioactive hydroxocobalamin to cobalamin-dependent apoenzymes in rat liver. Proc Natl Acad Sci U S A. 1977 Mar;74(3):916–920. doi: 10.1073/pnas.74.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G., 3rd, Barness L. A., Cardinale G. J., Abeles R. H., Flaks J. G. Congenital methylmalonic acidemia: enzymatic evidence for two forms of the disease. Proc Natl Acad Sci U S A. 1969 May;63(1):191–197. doi: 10.1073/pnas.63.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Levy H. L., Abeles R. H., Jennedy J. P., Jr A derangement in B 12 metabolism leading to homocystinemia, cystathioninemia and methylmalonic aciduria. Biochem Biophys Res Commun. 1969 Apr 10;35(1):121–126. doi: 10.1016/0006-291x(69)90491-4. [DOI] [PubMed] [Google Scholar]
- Newmark P. A. The mechanism of vitamin B 12 by the kidney of the rat in vivo. Biochim Biophys Acta. 1972 Jan 28;261(1):85–93. doi: 10.1016/0304-4165(72)90317-0. [DOI] [PubMed] [Google Scholar]
- Newmark P., Newman G. E., O'Brien J. R. Vitamin B12 in the rat kidney. Evidence for an association with lysosomes. Arch Biochem Biophys. 1970 Nov;141(1):121–130. doi: 10.1016/0003-9861(70)90114-1. [DOI] [PubMed] [Google Scholar]
- Peirce K., Abe T., Cooper B. A. Incorporation and metabolic conversion of cyanocobalamin by Ehrlich ascites carcinoma cells in vitro and in vivo. Biochim Biophys Acta. 1975 Feb 13;381(2):348–358. doi: 10.1016/0304-4165(75)90240-8. [DOI] [PubMed] [Google Scholar]
- Pletsch Q. A., Coffey J. W. Properties of the proteins that bind vitamin B 12 in subcellular fractions of rat liver. Arch Biochem Biophys. 1972 Jul;151(1):157–167. doi: 10.1016/0003-9861(72)90484-5. [DOI] [PubMed] [Google Scholar]
- Rappazzo M. E., Hall C. A. Transport function of transcobalamin II. J Clin Invest. 1972 Jul;51(7):1915–1918. doi: 10.1172/JCI106995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retief F. P., Vandenplas L., Visser H. Vitamin B12 binding proteins in liver disease. Br J Haematol. 1969 Mar;16(3):231–240. doi: 10.1111/j.1365-2141.1969.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A. C., Hsia Y. E., Rosenbloom F. M. Vitamin B12 dependent methylmalonicaciduria: defective B12 metabolism in cultured fibroblasts. Biochem Biophys Res Commun. 1969 Nov 6;37(4):607–614. doi: 10.1016/0006-291x(69)90853-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A., Hsia Y. E. Methylmalonic aciduria: metabolic block localization and vitamin B 12 dependency. Science. 1968 Nov 15;162(3855):805–807. doi: 10.1126/science.162.3855.805. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Patel L., Lilljeqvist A. C. Absence of an intracellular cobalamin-binding protein in cultured fibroblasts from patients with defective synthesis of 5'-deoxyadenosylcobalamin and methylcobalamin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4617–4621. doi: 10.1073/pnas.72.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryel E. M., Meyer L. M., Gams R. A. Uptake and subcellular distribution of vitamin B12 in mouse L1210 leukemic lymphoblasts. Blood. 1974 Sep;44(3):427–433. [PubMed] [Google Scholar]
- Schneider R. J., Burger R. L., Mehlman C. S., Allen R. H. The role and fate of rabbit and human transcobalamin II in the plasma transport of vitamin B12 in the rabbit. J Clin Invest. 1976 Jan;57(1):27–38. doi: 10.1172/JCI108265. [DOI] [PMC free article] [PubMed] [Google Scholar]



