Abstract
The underlying etiology of parkinsonian anterocollis has been the subject of recent debate. The purpose of this study is to test the hypothesis that anterocollis in parkinsonian syndromes is associated with dystonia of the deep cervical flexors (longus colli and capitis). Eight patients with anterocollis, six in the setting of parkinsonism and two primary cervical dystonia control subjects with anterocollis underwent prospective structured clinical evaluations (interview, examination and rating scales), systematic electromyography of the cervical extensor musculature and 18F-FDG PET/CT studies of cervical muscles to examine evidence of hypermetabolism or overactivity of deep cervical flexors. Subjects with parkinsonian anterocollis were found to have hypermetabolism of the extensor and sub-occipital muscles but not in the cervical flexors (superficial or deep). EMG abnormalities were observed in all evaluated patients, but only one patient was definitely myopathic. Meanwhile, both dystonia controls exhibited hypermetabolism of cervical flexors (including the longus colli). In conclusion, we were able to demonstrate hypermetabolism of superficial and deep cervical flexors with muscle 18F-FDG PET/CT in dystonic anterocollis patients, but not in parkinsonian anterocollis patients. The hypermetabolic changes seen in parkinsonian anterocollis patients in posterior muscles may be compensatory. Alternative explanations for anterocollis include myopathy of the cervical extensors, or unbalanced rigidity of the cervical flexors, but this remains to be proven.
Keywords: Anterocollis, FDG-PET, Parkinson’s disease, EMG, Dystonia
1. Introduction
Anterocollis in parkinsonian syndromes is defined as marked neck flexion (>45°), disproportionate to concomitant truncal flexion [1,2]. The etiology of this condition is a matter of recent debate, which has been reviewed extensively [2–4]. Multiple hypotheses have been proposed including cervical extensor myopathy, cervical flexor dystonia, and disproportionate rigidity of the flexors [5–7]. Evidence of myopathic changes has been presented in multiple studies [8–14], however, critics argue that the observed changes may be secondary to muscle stretching and tearing [15], that electromyography (EMG) interpretation can be subjective and non-specific, and that biopsy and EMG findings are difficult to interpret in the absence of appropriate controls [11]. The dystonia and rigidity hypotheses are criticized based on the fact that effective therapies for dystonia (botulinum toxin) and rigidity (dopaminergic medication) are not effective in reversing parkinsonian anterocollis [11,15]. Dystonia of deep flexors could explain why the SCMs are often inactive or atrophied [5,15], and why targeting this muscle group is not effective.
In this study, we sought to find relative hyperactivity of the deep cervical flexors to support the hypothesis that focal dystonia of these muscles is the primary underlying etiology of anterocollis in parkinsonian syndromes. 18F-2-fluoro-2-deoxy-D-glucose (FDG) uptake has been shown to correlate with muscle activity in normal subjects, and is capable of spatially differentiating the involved muscles [16]. Furthermore, FDG positron emission tomography combined with computed tomography (PET/CT) is capable of localizing dystonic muscles in patients with cervical dystonia (CD) [17–20]. In this study we used 18F-FDG PET/CT as a measure of muscle metabolism of glucose, to determine the activity of cervical muscles in parkinsonian anterocollis.
2. Methods
To be included in the study subjects had a clinical diagnosis of idiopathic PD (according to UK Brain Bank criteria) or multiple system atrophy of the parkinsonian sub-type (MSA-P, according to consensus statement [21]) accompanied by anterocollis of at least 45° of neck flexion. The degree of neck flexion was determined by using a protractor and wall chart [22]. Nine patients with a diagnosis of idiopathic PD (n = 7) or MSA (n = 2) and concomitant anterocollis were recruited from the Emory University Movement Disorder clinic. None of these patients had prior botulinum toxin injections. One of these patients was excluded from analysis for not meeting criteria for anterocollis (≥45° neck flexion) and two did not complete the FDG-PET, therefore six patients completed all portions of the study. This group of patients will be referred to as the target group. In addition, two control subjects with idiopathic cervical dystonia and predominant anterocollis were included. This group of patients will be referred to as the dystonia control group. A total of 5 CD controls were evaluated, two were excluded since they had prior botulinum toxin injections within three months of the study, and another was excluded due to treatment with deep brain stimulation that led to resolution of symptoms.
Records documenting diagnosis, disease progression, treatment and investigations on all subjects were reviewed. Subjects in the target group underwent prospective structured interviews aimed at gathering details regarding the nature of their anterocollis. The full Unified Parkinson’s Disease Rating Scale (UPDRS) and the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) were administered to the target group. The TWSTRS was administered to the dystonic control group. The Institutional Review Board of Emory University approved all investigational protocols. Informed consent was obtained from all subjects enrolled in the study.
2.1. PET/CT protocol
Target and dystonia control group subjects underwent 18F-FDG PET of the cervical musculature.
PET/CT scans were performed with a Discovery LS PET/CT scanner (GE Healthcare). Prior to FDG administration, blood serum glucose levels (BSG) were obtained from all subjects. During the uptake period patients were sitting without reclining, and advised to assume the natural posture most likely to result in anterocollis. They were instructed not to resist the abnormal posture. Limited field-of-view CT from head to thoracic inlet was performed with a continuous spiral technique on an 8-slice helical CT scanner with the following parameters: 80 mAs (tube current [mA] · scan time [s]), 140 keV, 5-mm section width, and table feed rate of 5 mm per rotation. Next, an emission scan was performed from skull base to thoracic inlet at 4 min per frame at 60 min after the intravenous administration of 370 MBq of 18F-FDG. CT data were used for attenuation correction, and PET images were reconstructed with an ordered-subset expectation maximization algorithm (28 subsets, 2 iterations). CT and PET scan data were co-registered on a GE AW workstation (GE Healthcare). Maximum standardized uptake values (SUVmax) were acquired from attenuation-corrected images, and the amount of 18F-FDG injected, patient body weight, and cross-calibration factors between PET and the dose calibrator were recorded. CT, PET, PET/CT, and maximum-intensity-projection PET images were reviewed by one nuclear medicine physician (J.M.). When increased 18F-FDG uptake was observed in head and neck muscles, the SUVmax and the name of each hypermetabolic muscle were recorded. Uninvolved musculature (without any uptake) was utilized to determine background SUVmax. A muscle was considered hypermetabolic when an increase of twice the background SUVmax was observed. The radiologist was not aware of the diagnosis at the time of assignment of SUVs.
2.2. EMG protocol
Systematic needle EMG was performed of the cervical paraspinal musculature at multiple levels for the purpose of documenting myopathic findings only. In order to document the distribution of observed electromyographic abnormalities, needle EMG was also performed of proximal shoulder-girdle muscles including the deltoid, rhomboid and infraspinatus. Spontaneous activity as well as motor unit morphology and firing pattern were documented at each level. Findings were then classified as normal, myopathic, neurogenic, or a combination of the latter two. Designation of units as myopathic was based on their morphology (short duration, low amplitude and polyphasic) and firing pattern (i.e. increased recruitment). The presence of fibrillation potentials and positive sharp waves was documented as evidence of either ongoing axonal injury (in the context of neurogenic motor unit potentials) or an active myopathic process with muscle membrane irritability (in the context of myopathic motor unit potentials). All EMGs were performed and interpreted by an experienced electromyographer (M.B.). Once all data was collected, it was de-identified and each case was interpreted individually blind to any other clinical information. At this time the electromyographer made a final determination based on the documented findings as to whether they were possibly myopathic, definitely myopathic, or not myopathic at all (normal or neurogenic).
Descriptive statistics including medians, range, and percentages were calculated for variables of interest.
3. Results
3.1. Demographic, clinical and historical data
For the target group median (range) age was 62.5 (30–83) years, and there were 4 males. The median modified Hoehn and Yahr scale was 3 [1–3] for PD and 4 for both MSA patients. The median duration of primary diagnosis from onset of symptoms to the date of the study evaluation was 10 years [5–13] for PD and 6 years [4–8] for MSA. The median duration of anterocollis from onset of symptoms to the study evaluation was 3.5 years [2–5] for MSA and 2 (0.2–13) years for PD. Clinical findings (including history and exam findings) were published separately as part of a larger cohort [23]. Patients with MSA had more severe motor impairment in the UPDRS part 3 (mean: 57; SD: 55–59) and disability in the UPDRS part 2 (mean: 36; SD: 27–49) compared to PD (motor impairment mean: 22; SD: 12–39, and disability mean: 13.5; SD: 8–20).
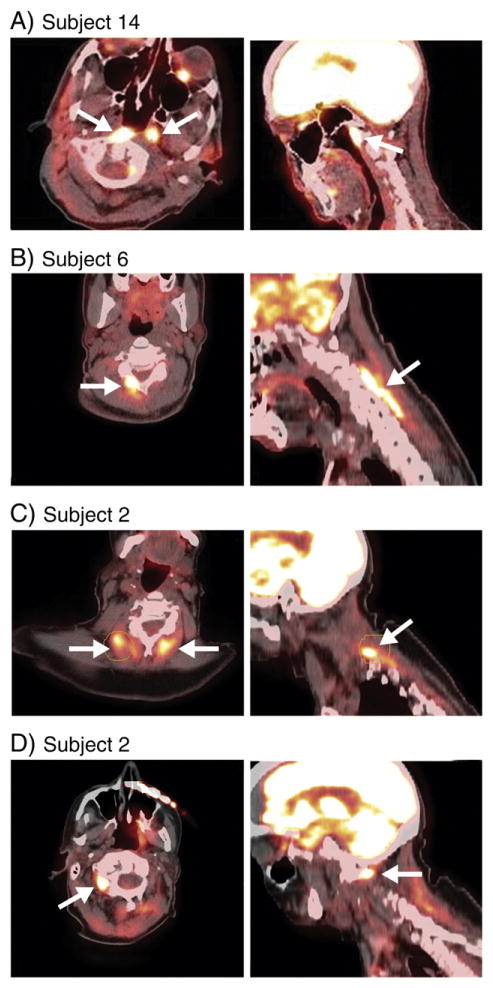
The two subjects in the control group (subjects 13 and 14) were aged 30 and 51 years, respectively. Duration of illness was 15 and 7 years, respectively. Both patients had anterocollis as well as left torticollis. TWSTRS scores were 26 and 19 for section I, respectively. Subject 13 had received botulinum toxin injections for a period of ten years but had diminishing benefit. His last injection was more than fifteen weeks prior to the FDG-PET. Subject 14 had received three prior botulinum toxin injections, none of which were effective. His last botulinum toxin injection was 14 weeks prior to the FDG-PET.
3.2. PET data
Results of the 18F-FDG PET/CT studies are summarized in Table 1. All of the parkinsonian patients showed hypermetabolism of sub-occipital muscles and neck extensors. Of the two dystonic controls that were included in the study, one showed hypermetabolism of the longus colli, and the other of the SCMs. One of these also had extensor hypermetabolism (See Fig. 1).
Table 1.
FDG-PET and EMG findings.
| # | Dx | Longus colli/capitis
|
SCM
|
Suboccipitals
|
Splenius Capitis
|
Semispinallis
|
EMG | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | |||
| 1 | PD | 2.87 | 3.1 | 3.7 | Neurogenic | |||||||
| 2 | PD | 9.12 | 6.76 | 7.94 | Possibly myopathic | |||||||
| 3 | MSA | 2.33 | 3.71 | Neurogenic | ||||||||
| 4 | PD | 4.24 | Neurogenic | |||||||||
| 6 | PD | 3.88 | 8.71 | Possibly myopathic | ||||||||
| 8 | MSA | 4.58 | 7.6 | 5.3 | Myopathic | |||||||
| 13 | CD | 2.19 | 1.78 | 3.46 | N/a | |||||||
| 14 | CD | 3.16 | 6.84 | 1.79 | 1.92 | N/a | ||||||
Fig 1.
Numerical representation of maximum standard uptake values (SUVmax) on FDG-PET.
3.3. Electromyography data
Results of the EMG evaluations are summarized in Table 1. Only one patient (subject 8) had weakness on extension and was found to have myopathy (definite) of the extensors. Myopathic findings were isolated to the extensors in all subjects. Two patients with neurogenic findings had ongoing denervation.
4. Discussion
Our study of anterocollis in patients with parkinsonism was designed to determine which of the competing hypotheses regarding the underlying etiology of this condition was correct. We used a novel non-invasive approach as evidence of muscle activation. Although well established tools to identify and quantify myopathy are available (EMG, creatine kinase levels, and biopsy), there are few, if any, well established, objective measures to identify and quantify dystonia. Since, by definition, dystonia must involve a sustained muscle contraction [24], any tool developed to assess dystonia should be able to capture this accurately and quantitatively. Needle or surface EMG is used for this purpose, however, it can be invasive, particularly in deep musculature, relatively subjective, technically difficult and has not been standardized for this purpose. Validated scales can be useful in determining the severity and distribution of abnormal postures but do not address whether they are the result of underlying muscle contraction. Therefore, the lack of direct evidence of dystonia in parkinsonian anterocollis may be a function of the lack of adequate tools to document this phenomenon, particularly in deep, inaccessible muscles. For this reason we used 18F-FDG PET/CT to study muscle hyperactivity in deep muscles as would be expected if the appropriate muscles were truly dystonic and responsible for this persistent abnormal posture.
18F-FDG PET/CT is well suited for the task of identifying muscle activity and can be used as a quantitative measure. It has been shown that increases in muscle resistance correlate with FDG uptake in normal subjects [16]. Furthermore, in CD, deep cervical flexors have been shown to be hypermetabolic in cases of dystonic anterocollis and 18F-FDG uptake correlated with the severity of dystonia and diminished in proportion with symptom improvement after botulinum toxin injections [18,20].
EMG evaluations revealed a combination of myopathic and neurogenic changes. These findings are consistent with those reported in previous case series [5,6,11,15]. Although myopathic features were present in three of the six patients who completed the study, they were not universal, and findings, when present, were not sufficient to warrant a definitive diagnosis of myopathy in two of the three. Only one of six anterocollis patients in our study met criteria for definite myopathy after reviewing all EMG data in a blinded fashion. This was also the only patient to be weak in extension. Ongoing myopathic and neurogenic changes highlight the fact that this is an evolving process.
Findings with 18F-FDG PET evaluation in parkinsonian patients revealed a homogeneous pattern of muscle hypermetabolism in the cervical extensors and sub-occipital musculature. We propose that these changes may be due to overactivity of these muscle groups to compensate for the abnormal anterocollic posture. A normal control group was not studied since thousands of oncology patients undergo FDG-PET and this pattern of posterior muscle hypermetabolism is not seen. Of note, some of these patients have shown hypermetabolism in the neck and chest attributed to either tension or brown fat, but the pattern of activation is completely different than in our cohort [25,26]. There was no correlation with the presence of myopathy since parkinsonian patients without myopathy (e.g. patients 1 and 3) and dystonia control patients showed a similar pattern of activation of the cervical extensors. In this group there was no activation of superficial (SCM) or deep cervical flexors (longus colli/capitis). Conversely, the two dystonic control patients included in the study did show increased metabolic activity of anterior cervical musculature; one with longus colli involvement and one with SCM involvement.
Limitations of this study include the small size of the cohort, and the fact that anterocollis was fairly chronic in this study population, which may affect the findings of both EMG and PET; however, there is no precedent for dystonia evolving into myopathy. Although FDG-PET of muscle is not a validated tool for quantifying muscle contraction in cervical dystonia, we feel that it can be a useful tool to investigate the contraction pattern responsible for complex dystonic postures, particularly of deep and inaccessible muscles.
5. Conclusions
Our findings do not support the hypothesis that dystonia (as it is currently defined) of the deep cervical flexors is the underlying etiology of parkinsonian anterocollis. These findings do not support or justify invasive procedures to paralyze this muscle group as a therapeutic strategy in this patient population. Myopathy and rigidity remain possible explanations for this phenomenon, in some patients. Muscle 18F-FDG PET/CT is a useful tool for identifying muscle overactivity in dystonic conditions.
Acknowledgments
This study was supported by a grant from the Bachmann Strauss Dystonia Parkinson Foundation, the NIH (Award Number U54 NS065701) and the NIH/NCRR (Award Number UL1RR029882). Statistical support was provided by Amy E. Wahlquist, MS.
Footnotes
Conflict of interest
Dr. Revuelta has received grants from Bachmann-Strauss Dystonia Parkinson Foundation, Barmore Fund, Phytopharm, Biotie, NIH, Chelsea has previously participated in speaker bureaus with TEVA Neurosciences, Lundbeck, UCB and advisory boards with Chelsea and Lundbeck. Dr. Montilla has nothing to disclose. Dr. Benatar has consulted with Cytokinetics and has received grants from FDA, MDA, ALS Association. Dr. Freeman has participated in advisory boards with Allergan, Ipsen, Merz, UCB Pharma, and received grants from EMD Serono and Phytopharm. Dr. Wichmann has current grant support from NIH/NINDS: R01NS071074, P50NS071669, R01NS054976, R01NS042937 (Co-I), R01NS062876, R01NS037948 (co-I), RJG foundation, and has participated in scientific advisory boards with Bachmann-Strauss Foundation, previous member of Dystonia Medical Research Foundation Board. Dr. Jinnah has participated in advisory boards with Dystonia Medical Research foundation and Tyler’s Hope, has received previous grant support from Allergan Inc., the Bachmann-Strauss Dystonia & Parkinson’s Foundation, the Lesch-Nyhan Syndrome Children’s Research Foundation, Merz Pharmaceuticals, the Tomorrow Foundation, and Tyler’s Hope for a Cure. He currently is funded by grants from the Dystonia Medical Research Foundation and the NIH (NS067501, HD53312, NS061349, and NS03592). Dr. DeLong has been a consultant for the RJG Foundation, has served on the advisory board for the Dystonia Foundation, has received honoraria from Merck and grants from the APDA. Dr. Factor has received research grant funding from TEVA Neurosciences, Ceregene, Michael J Fox Foundation, Consolidated Anti-aging Foundation and NIH (1 P01 ES 016731-01), educational grants from Allergan and Lundbeck, has been a consultant for Boehringer Ingelheim (Expert testimony), Allergan, Lundbeck and UCB, has served as section editor for Current Treatment Options in Neurology and guest editor for Neurotherapeutics.
References
- 1.Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord. 2006 Nov;21(11):1856–63. doi: 10.1002/mds.21058. Epub 2006/08/31. eng. [DOI] [PubMed] [Google Scholar]
- 2.Doherty KM, van de Warrenburg BP, Peralta MC, Silveira-Moriyama L, Azulay JP, Gershanik OS, et al. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011 Jun;10(6):538–49. doi: 10.1016/S1474-4422(11)70067-9. Epub 2011/04/26. eng. [DOI] [PubMed] [Google Scholar]
- 3.Revuelta GJ. Anterocollis and camptocormia in parkinsonism: a current assessment. Curr Neurol Neurosci Rep. 2012 Aug;12(4):386–91. doi: 10.1007/s11910-012-0280-9. Epub 2012/05/29. eng. [DOI] [PubMed] [Google Scholar]
- 4.Savica R, Kumar N, Ahlskog JE, Josephs KA, Matsumoto JY, McKeon A. Parkinsonism and dropped head: dystonia, myopathy or both? Parkinsonism Relat Disord. 2012 Jan;18(1):30–4. doi: 10.1016/j.parkreldis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Kashihara K, Ohno M, Tomita S. Dropped head syndrome in Parkinson’s disease. Mov Disord. 2006 Aug;21(8):1213–6. doi: 10.1002/mds.20948. Epub 2006/05/17. eng. [DOI] [PubMed] [Google Scholar]
- 6.Yoshiyama Y, Takama J, Hattori T. The dropped head sign in parkinsonism. J Neurol Sci. 1999 Aug 1;167(1):22–5. doi: 10.1016/s0022-510x(99)00129-x. Epub 1999/09/29. eng. [DOI] [PubMed] [Google Scholar]
- 7.Oyama G, Hayashi A, Mizuno Y, Hattori N. Mechanism and treatment of dropped head syndrome associated with parkinsonism. Parkinsonism Relat Disord. 2009 Mar;15(3):181–6. doi: 10.1016/j.parkreldis.2008.04.040. Epub 2008/06/25. eng. [DOI] [PubMed] [Google Scholar]
- 8.Askmark H, Eeg-Olofsson K, Johansson A, Nilsson P, Olsson Y, Aquilonius S. Parkinsonism and neck extensor myopathy: a new syndrome or coincidental findings? Arch Neurol. 2001 Feb;58(2):232–7. doi: 10.1001/archneur.58.2.232. Epub 2001/02/15. eng. [DOI] [PubMed] [Google Scholar]
- 9.Lava NS, Factor SA. Focal myopathy as a cause of anterocollis in Parkinsonism. Mov Disord. 2001 Jul;16(4):754–6. doi: 10.1002/mds.1152. Epub 2001/08/02. eng. [DOI] [PubMed] [Google Scholar]
- 10.Gdynia HJ, Sperfeld AD, Unrath A, Ludolph AC, Sabolek M, Storch A, et al. Histopathological analysis of skeletal muscle in patients with Parkinson’s disease and ‘dropped head’/’bent spine’ syndrome. Parkinsonism Relat Disord. 2009 Nov;15(9):633–9. doi: 10.1016/j.parkreldis.2009.06.003. Epub 2009/07/04. eng. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic J. Camptocormia, head drop and other bent spine syndromes: heterogeneous etiology and pathogenesis of Parkinsonian deformities. Mov Disord. 2010 Apr 15;25(5):527–8. doi: 10.1002/mds.23139. Epub 2010/04/29. eng. [DOI] [PubMed] [Google Scholar]
- 12.Margraf NG, Wrede A, Rohr A, Schulz-Schaeffer WJ, Raethjen J, Eymess A, et al. Camptocormia in idiopathic Parkinson’s disease: a focal myopathy of the paravertebral muscles. Mov Disord. 2010 Apr 15;25(5):542–51. doi: 10.1002/mds.22780. Epub 2010/01/29. eng. [DOI] [PubMed] [Google Scholar]
- 13.Spuler S, Krug H, Klein C, Medialdea IC, Jakob W, Ebersbach G, et al. Myopathy causing camptocormia in idiopathic Parkinson’s disease: a multidisciplinary approach. Mov Disord. 2010 Apr 15;25(5):552–9. doi: 10.1002/mds.22913. Epub 2009/12/17. eng. [DOI] [PubMed] [Google Scholar]
- 14.Wanschitz JV, Sawires M, Seppi K, Boesch S, Loescher WN, Schocke M, et al. Axial myopathy in parkinsonism. Mov Disord. 2011 Jul;26(8):1569–71. doi: 10.1002/mds.23492. Epub 2011/04/27. eng. [DOI] [PubMed] [Google Scholar]
- 15.van de Warrenburg BP, Cordivari C, Ryan AM, Phadke R, Holton JL, Bhatia KP, et al. The phenomenon of disproportionate antecollis in Parkinson’s disease and multiple system atrophy. Mov Disord. 2007 Dec;22(16):2325–31. doi: 10.1002/mds.21634. Epub 2007/10/27. eng. [DOI] [PubMed] [Google Scholar]
- 16.Pappas GP, Olcott EW, Drace JE. Imaging of skeletal muscle function using (18)FDG PET: force production, activation, and metabolism. J Appl Physiol. 2001 Jan;90(1):329–37. doi: 10.1152/jappl.2001.90.1.329. Epub 2001/01/03. eng. [DOI] [PubMed] [Google Scholar]
- 17.Lee HB, An YS, Lee HY, Hwang JH, Lee HJ, Jeong KY, et al. Usefulness of (18)f-fluorodeoxyglucose positron emission tomography/computed tomography in management of cervical dystonia. Ann rehab med. 2012 Dec;36(6):745–55. doi: 10.5535/arm.2012.36.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IH, Yoon YC, Sung DH, Kwon JW, Jung JY. Initial experience with imaging-guided intramuscular botulinum toxin injection in patients with idiopathic cervical dystonia. AJR Am J Roentgenol. 2009 Apr;192(4):996–1001. doi: 10.2214/AJR.08.1535. Epub 2009/03/24. eng. [DOI] [PubMed] [Google Scholar]
- 19.Revuelta GJ, Factor SA. Clinical subtypes of Disproportionate Anterocollis in Parkinsonian syndromes. Neurology. 2010;47(supplement 2):A88. [Google Scholar]
- 20.Sung DH, Choi JY, Kim DH, Kim ES, Son YI, Cho YS, et al. Localization of dystonic muscles with 18F-FDG PET/CT in idiopathic cervical dystonia. J Nucl Med. 2007 Nov;48(11):1790–5. doi: 10.2967/jnumed.107.044024. Epub 2007/10/19. eng. [DOI] [PubMed] [Google Scholar]
- 21.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008 Aug 26;71(9):670–6. doi: 10.1212/01.wnl.0000324625.00404.15. Epub 2008/08/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien C, Brashear A, Cullis P, Truong D, Molho E, Jenkins S, et al. Cervical dystonia severity scale reliability study. Mov Disord. 2001 Nov;16(6):1086–90. doi: 10.1002/mds.1226. Epub 2001/12/19. eng. [DOI] [PubMed] [Google Scholar]
- 23.Revuelta GJ, Benatar M, Freeman A, Wichmann T, Jinnah HA, Delong MR, et al. Clinical subtypes of anterocollis in parkinsonian syndromes. J Neurol Sci. 2012 Apr 15;315(1–2):100–3. doi: 10.1016/j.jns.2011.11.017. Epub 2011/12/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdo WF, van de Warrenburg BP, Burn DJ, Quinn NP, Bloem BR. The clinical approach to movement disorders. Nat Rev Neurol. 2010 Jan;6(1):29–37. doi: 10.1038/nrneurol.2009.196. Epub 2010/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 25.Sturkenboom MG, Hoekstra OS, Postema EJ, Zijlstra JM, Berkhof J, Franssen EJ. A randomised controlled trial assessing the effect of oral diazepam on 18F-FDG uptake in the neck and upper chest region. Mol Imaging Biol. 2009 Sep-Oct;11(5):364–8. doi: 10.1007/s11307-009-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook GJ, Fogelman I, Maisey MN. Normal physiological and benign pathological variants of 18-fluoro-2-deoxyglucose positron-emission tomography scanning: potential for error in interpretation. Semin Nucl Med. 1996 Oct;26(4):308–14. doi: 10.1016/s0001-2998(96)80006-7. [DOI] [PubMed] [Google Scholar]