Abstract
The basal ganglia (BG) have long been considered to play an important role in the control of movement and the pathophysiology of movement disorders, such as Parkinson’s disease (PD). Studies over the past decades have considerably broadened this view, indicating that the BG participate in multiple, parallel, largely segregated, cortico-subcortical reentrant pathways involving motor, associative and limbic functions. Research has shown that dysfunction within individual circuits is associated not only with movement disorders, but also with neuropsychiatric disorders. Accordingly, a number of movement disorders and neuropsychiatric disorders such as obsessive compulsive disorder and Tourette’s syndrome are viewed as “circuit disorders.” We here discuss the changes in our current understanding of the anatomic and functional organization of BG circuits and related circuit disorders.
Keywords: Basal Ganglia, Circuit Disorder, Dopamine, Pallidum, Parkinson’s Disease, Pathophysiology, Striatum, Subthalamic Nucleus
INTRODUCTION
Advances in neuroscience research have led to a detailed understanding of the anatomical and functional organization of the BG and the relations of these subcortical nuclei with the cerebral cortex, brainstem and thalamus. These studies have resulted in the construction of circuit models in which the BG are seen as components of cortico-subcortical circuits that also include the thalamus and cerebral cortex. Anatomic, physiologic and neuroimaging studies in animals and human subjects have led to the concept that disease states may affect the function of one or more of these specific circuits. Diseases for which the “circuit disorder” concept may apply include movement disorders, such as PD or dystonia, as well as neuropsychiatric disorders, such as Tourette’s Syndrome, obsessive compulsive disorder (OCD) and depression. This perspective of these diseases has provided a physiologic basis and strong rationale for the surgical treatment of these conditions with ablative procedures or deep brain stimulation (DBS). This article provides a brief discussion of these issues. For a more extended discussion of these and related issues see.1–3
GENERAL ANATOMY OF THE BG
The BG are a collection of interconnected subcortical nuclei, including the striatum, globus pallidus, substantia nigra, and subthalamic nucleus (STN) which closely interact with the cerebral cortex and thalamus. The striatum, the major input structure of the BG, receives projections from the cerebral cortex, brainstem, and thalamus. The globus pallidus consists of two anatomically and functionally separate nuclei, the external and internal pallidal segments (GPe and GPi, respectively). The substantia nigra is also comprised of two separate nuclei, the GABAergic pars reticulata (SNr) and the pars compacta (SNc), which contains pigmented dopamine-containing neurons. The dopaminergic neurons of the SNc project primarily to the striatum, but also to the other nuclei of the BG and to the thalamus. Functionally, GPi and SNr are viewed as a single output structure of the BG, which is divided anatomically by the internal capsule. The glutamatergic STN is a small nucleus which is intercalated between GPe and GPi. Interestingly, the STN also functions as a BG input structure, as it receives input from the cerebral cortex.
Cortical inputs enter the BG via the striatum and STN, and are then sent to GPi and SNr. Two major pathways between striatum and GPi/SNr have been identified: (1) a direct, monosynaptic connection to both output nuclei (GPi and SNr) and (2) an indirect, polysynaptic pathway that first targets GPe, and involves additional projections from GPe to the output nuclei, both directly, and via a loop through the STN (see below).
The output nuclei project to specific thalamic and brain stem nuclei. Thalamic projections are directed to widespread areas of the frontal lobe and give collaterals back to the striatum. Descending projections from the BG to the brainstem, specifically the pedunculopontine nucleus (PPN), also exist. These may directly influence brainstem and spinal motor mechanisms, especially those related to gait and balance. The PPN is also part of several feedback circuits with projections to the BG and the thalamus. Other projections from the SNr reach the superior colliculus, which is involved in head and eye movements, while GPi projections are also directed to the lateral habenula, and may play a role in the modulation of reward and limbic mechanisms.
FUNCTIONAL ORGANIZATION OF THE BG CIRCUITS
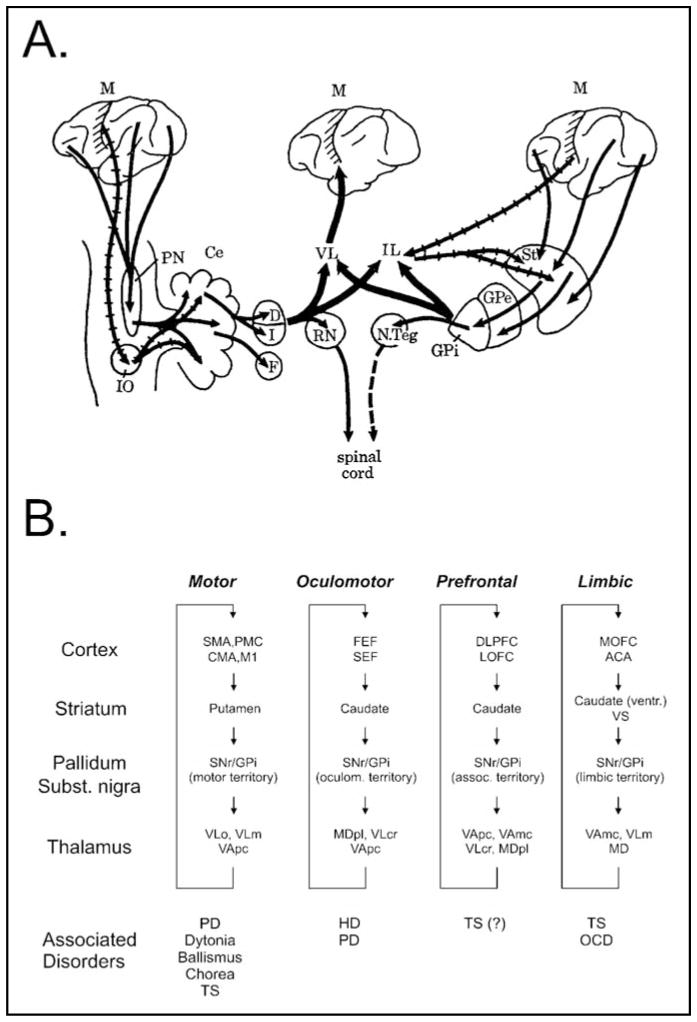
Earlier concepts of the functional organization of the BG emphasized that the subcortical BG and cerebellar circuits converged in the thalamus and sent integrated output to the motor cortex. The BG were believed to “funnel” input from wide areas of the cerebral cortex to the motor cortex, thus, providing a route whereby the planning and execution of movements could be carried out or influenced by input from disparate cortical areas [Figure 1A and ref.4]. This scheme has been replaced with the concept that BG and cortex interact through parallel, segregated cortical-subcortical re-entrant circuits, whereby different areas of the cerebral cortex project topographically upon the striatum with little convergence at the striatal or subsequent levels of processing in the BG, thalamus or cerebral cortex [Figure 1B and ref.5,6].
Figure 1.
Changing views of the organization of the BG and their integration in the control of motor and non-motor functions. A. View of the BG as developed by Kemp and Powell in the 1970s.4 Multiple motor and non-motor cortical inputs converge in the BG. BG and cerebellum interact to regulate motor cortical functions. B. Current view of the circuit anatomy of the cortex-basal ganglia-thalamocortical circuits. Motor and non-motor circuits are segregated throughout their subcortical course, involving specific territories in the BG and associated areas of thalamus and cortex. Abnormal activity in specific circuits gives rise to “circuit disorders.” Abbreviations: ACA, anterior cingulate area; CMA, cingulate motor area; DLPFC, dorsolateral prefrontal cortex; FEF, frontal eye fields; LOFC, lateral orbitofrontal cortex; M1, primary motor cortex; MD, mediodorsal nucleus of the thalamus; MDpl, mediodorsal nucleus of thalamus, pars lateralis; MOFC, medial orbitofrontal cortex; PMC, premotor cortex; SMA, supplementary motor area; SEF, supplementary eye field; VApc, ventral anterior nucleus of thalamus, pars parvocellularis; VAmc, ventral anterior nucleus of thalamus, pars magnocellularis; VLm, ventrolateral nucleus of thalamus, pars medialis; VLo, ventrolateral nucleus of thalamus, pars oralis; VLcr, ventrolateral nucleus of thalamus, pars caudalis, rostral division. See text for other abbreviations. Figure 1A reproduced, with permission, from [4]. Figure 1B reproduced, with permission, from [1].
The topographic termination zones of cortical inputs in the striatum establish functional domains that mirror those in the cortex. Domain-specific information is then carried forward throughout the basal ganglia-thalamocortical circuitry by virtue of further highly topographic projections, eventually reaching the same cortical areas from which it originated, thus closing a family of cortico-subcortical re-entrant loops. Specific regions of the BG, therefore, appear to participate in the functions of individual cortical areas, an arrangement that may allow different aspects of behavior to be processed in parallel.6 Importantly, the intrinsic organization and basic physiologic function(s) of the BG structures and thalamic and other targets of BG output appears to be the same, independent of the functional domain. The BG are, thus, comprised of separate parallel “modules” that carry out the same principal processing operation(s) on different types of cortical inputs.
The larger functional circuits are named after the presumed functions of the regions of frontal cortex from which they originate, leading to the designation of a motor circuit, an oculomotor circuit, two prefrontal (associative) circuits, and a limbic circuit. The cortical source regions for these circuits are shown in Figure 1B. Each of these large circuits are, of course, comprised of subcircuits, centered on cortical subregions of the larger domains with more specific functions.7
Although evidence for interactions between circuits and open components of the described circuits was lacking initially, evidence for interactions between the different loops and exceptions to the closed loop hypothesis outlined above have been pointed out by several investigators.8,9 Anatomic and physiologic studies of the motor circuit have indicated a high degree of somatotopic organization within the BG structures at each level, as well as a high degree of neuronal specificity, but with indications of partial overlap and interactions between adjacent subcircuits.10 Interactions between the limbic/associative and motor circuits have been proposed to occur at the level of the SNc which may participate in spiral-like interactions with the striatum, so that SNc afferents from limbic areas of the striatum are projected back to striatal associative and motor regions.11,12 While there is some anatomical support for this hypothesis, there is no clear physiologic evidence for it. Furthermore, in a study carried out by Kelly and Strick,7 a region of the (non-motor) caudo-ventral putamen, a region that is known to receive input from the amygdala, was retrogradely labeled following injections of virus into the motor cortex. It is not known what functional relevance this might have, but the influences of mood and motivational disturbances on movement disorders is well known and may involve such (limited) interdomain interactions. Of course, involvement of non-motor areas within and outside of the BG has also been documented to occur in movement disorders, and likely accounts for most of the non-motor disturbances.
The view that BG and cerebellar systems are non-overlapping is also undergoing a limited revision. Recent studies by Strick and colleagues13 have demonstrated that cerebellar output directed to motor and associative cortical areas via the thalamus may also reach the corresponding areas of the striatum. Conversely, the BG may also influence the cerebellum via a bisynaptic pathway from the STN that involves the pontine nuclei.14 There is also evidence that cerebellar and BG outputs converge on the same cortical areas [e.g., ref.15–17]. The finding that BG and cerebellar circuits interact may provide an explanation for the increasing evidence from functional imaging and other studies for involvement of the cerebellum in dystonia and PD.18–21
INTRINSIC BG CIRCUITRY
Detailed anatomical models of the intrinsic circuitry of the BG, including the aforementioned concept of direct and indirect striatal output pathways were first developed in the 1980s [Figure 2 and ref.6,22–24]. Direct and indirect pathways originate from separate populations of striatal projection neurons, whose activity is strongly dependent on their cortical inputs. The activity at corticostriatal synapses (and thus, the activity of direct and indirect pathways) is regulated by dopamine (DA) release from terminals of the SNc. Although anatomic studies have shown that the separation of direct and indirect pathways is less than initially thought, due to extensive collaterals [e.g.,25], the general notion of separate direct and indirect pathways has received strong support from work in transgenic mice.26–29
Figure 2.
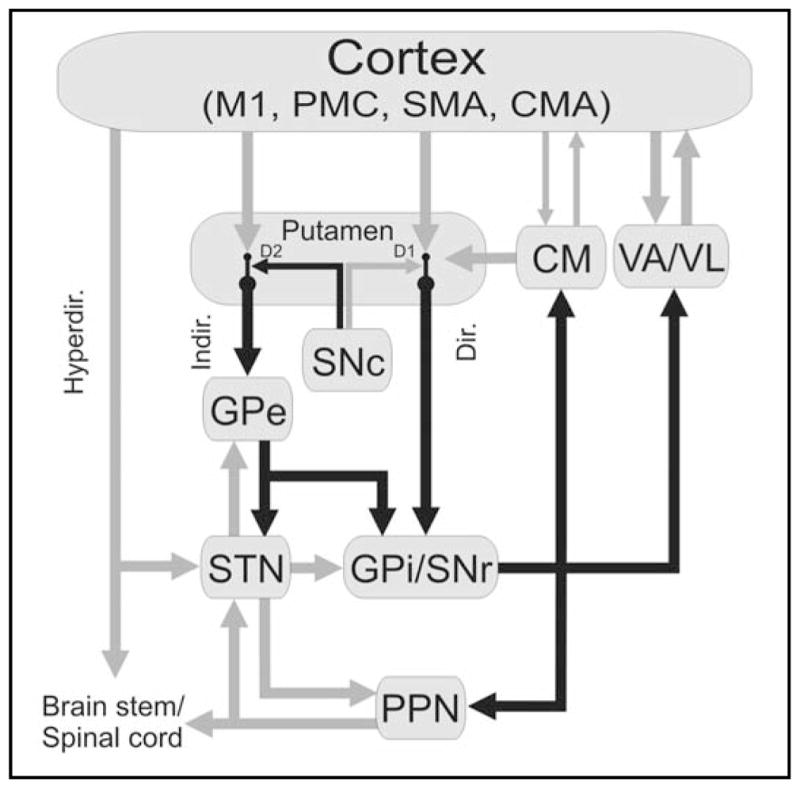
Motor circuit of the BG. Black arrows indicate inhibitory connections; gray arrows indicate excitatory connections. The thickness of the arrows corresponds to their presumed activity. Abbreviations: CM, centromedian nucleus of thalamus; CMA, cingulate motor area; Dir., direct pathway; Hyperdir., hyperdirect pathway; D1, D2, dopamine receptor subtypes; Indir., indirect pathway; M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area; VA, ventral anterior nucleus of thalamus; VL, ventrolateral nucleus of thalamus. See text for other abbreviations. Reproduced, with permission, from [74].
An important component of the direct/indirect pathway model is that the activity of the two pathways may be differentially regulated by DA. Striatal neurons that give rise to the direct pathway neurons express excitatory D1-like receptors. Activation of these receptors by DA would result in increased activity along the direct pathway, mostly through facilitation of corticostriatal transmission onto these neurons. In contrast, the striatal neurons that give rise to the indirect pathway (projecting to GPe), express D2-like receptors. The activity of these neurons is decreased in the presence of DA, mostly by gating of corticostriatal transmission onto these neurons.
According to this model, activation of the direct and indirect pathways upon the output nuclei would lead to different effects on BG output. Thus, activation of direct pathway neurons would lead to inhibition, while activation of neurons of the indirect pathway would lead to disinhibition of GPi/SNr output neurons. Accordingly, activation of the direct pathway would increase (by disinhibition) thalamocortical neuronal activity, while activation of the indirect pathway would lead to greater inhibition of thalamocortical neuronal activity. Others have emphasized a broader and important role of the GPe30 in the intrinsic processing of afferent input rather than as merely passing information forward as part of the indirect pathway. The STN has been emphasized as a key player in functions of the so-called “hyper-direct” pathway that links the cortex with GPi via the STN as discussed below.31–33
ANATOMY AND FUNCTION OF THE MOTOR CIRCUIT
We describe the anatomy and function of the motor circuits in some detail because of its proposed involvement in some of the major disorders of BG origin (see below). As shown in Figure 2, the motor circuit originates in the pre-central motor and post-central sensory fields which project to the same areas of the putamen in a somatotopic fashion. Topographically specific projections from the putamen are then directed to the motor regions of GPe, and GPi and SNr. The motor portions of the GPi and SNr project, in turn, to specific motor-related areas of three thalamic nuclei: the ventral lateral nucleus (VL), the ventral anterior nucleus (VA), and the centromedian nucleus (CM). The motor circuit is at least partially closed by projections from VA and VL to the motor cortex, supplementary motor area, and other premotor fields. CM sends a massive projection to the putamen and a smaller projection to the STN, forming additional feedback loops.
Attempts have been made to explain two movement-related functions, “scaling” and “focusing,” on the basis of the direct/indirect pathway organization of the BG. It was hypothesized that the BG play a role in controlling the speed and amplitude of movement, by allowing movements to occur via activation of the direct pathway, and by terminating them through subsequent activation of the indirect pathway. Evidence for a role of the BG in such movement scaling was demonstrated in studies of the activity of pallidal neurons in monkeys trained to perform movements of different amplitudes.34
An alternative model, proposed early on by Denny-Brown, is that the BG act as a “clearing house,” by selecting the most appropriate action from the numerous competing options for a given situation. This general view was incorporated by Kemp and Powell4 into their model, as discussed earlier. Albin, Young and Penney22 later suggested, based on the direct/indirect pathways schema, that the BG act to select which movements should be carried out in response to competing programs and to suppress unwanted movements. This model was further elaborated on by Mink,35 suggesting that the selection of specific movements involved activation of the direct pathway, while the inhibition of competing movements would involve activation of the indirect pathway, with the latter supplying a blanket inhibition in GPi out of which the direct pathway carved the intended movement.
The problems with these hypotheses are several and significant. The first is that the onset of discharge of corticostriatal neurons from the motor cortex occurs later than that of nearby pyramidal cells and must travel through the entire motor loop before influencing the motor areas of the cortex and brainstem.36,37 This is further compounded by the relatively slow conduction along the indirect pathway, as compared to that in the direct pathway, which would result in premature activation of the “focus” compared to the postulated surround inhibition. This problem was addressed by subsequent authors,32 invoking a non-striatal route for cortical inputs to reach the BG to produce the hypothesized inhibition, the so-called “hyperdirect” cortico-subthalamo-pallidal pathway. Rapid activation of the STN-GPi route via the hyperdirect pathway could thereby generate an early increase in inhibitory output from GPi upon which a “focus” of disinhibition could be placed via the direct pathway to facilitate a specific movement. A major argument against this, however, is that the phasic changes in neuronal activity in the STN and GPi, in relation to the onset of limb movement, are too late to have a significant role in focusing.34,38–41 Moreover, the proposed widespread surround activation of GPi neurons that would serve a braking or suppressing function during a motor act has not been found. Furthermore, while both forms of the focusing hypothesis are based on the idea that the projections from the STN to the GPi are diffuse, in order to provide the postulated surround inhibition,42 studies in primates have shown that the projections within the indirect pathway are, in fact, highly topographic.43 However, the level of specificity of the cortico-subthalamic projections has not been determined, and could, conceivably, be lower than that of the intrinsic BG projections.
One of the obvious difficulties with the scaling or focusing hypotheses is that they require an active role of the BG in the selection process, while the close and highly specific relationship between the individual BG modules and their cortical inputs, suggests that the selection process has already taken place at the cortical level. It would, therefore, seem most likely that focusing, scaling, and action selection, are primarily cortical rather than BG processes, and that the BG do not play a primary role in the initiation or selection of movement.
A more recent attempt to assign a specific role to the direct/indirect pathway architecture is that the BG may serve a role in response inhibition.44 For example, for eye movements, it has been suggested that the STN may play a role in inhibiting automatic eye movements when switching to voluntary eye movements.45 While physiologic studies of eye movements are consistent with a direct role of the BG in the initiation and control of eye movements, such is lacking for body movements. Studies by Houk and colleagues have, however, provided evidence for a role of GPi output in corrective movements, arguing for a role of the BG in both action selection and corrective movements.46
In terms of functional interpretations, the current models do not take into consideration many of the more recent electrophysiologic and anatomical findings such as the fact that BG neurons tend to (autonomously) generate oscillatory firing patterns, the influence of thalamostriatal projections,47 or the extrastriatal actions of DA [e.g.,48]. A particularly important “neglected” aspect of the BG circuitry is that BG output is directed not only at the thalamus, but also at the brain stem.49 The organization, topography, and functional importance of these connections remain unclear.
A direct role of the BG in motor control is often taken for granted, because of the strong association between BG pathology and the emergence of disturbances of movement, the results of neuroimaging studies during movement in healthy individuals and in movement disorder patients, and the results of neuronal recordings in primates performing movement tasks. Furthermore, a wide variety of focal disturbances within the BG, such as lesions or inactivation of the STN,50 injection of GABA-A receptor antagonists into GPe or striatum,51–53 and electrical stimulation of the primate putamen54 produce involuntary movements, suggesting that abnormal activity in the BG is capable of generating involuntary movements. However, the simple hypothesis that the BG are important for the on-line control of movement is increasingly unclear, since lesions of the BG output nuclei have little or no effect on posture, movement initiation, or movement execution in normal animals or humans.55 Indeed, patients undergoing pallidotomy, an obviously major interruption of the motor circuit, have no perceived complaints or observable disturbances of voluntary movement.
Given the available evidence it seems now more likely that the BG are more prominently involved in other functions, specifically including higher-level aspects of behavior, such as motor and procedural learning, than in the online-control of movement [e.g., ref.56–58]. Indeed, recent neuroimaging and physiologic studies suggest that components of the associative circuit and the caudate nucleus may play an important role in motor learning, while the putamen and the motor circuit is activated after learning has occurred and is more prominently involved in aspects of the automatic execution of the movements.59 The higher level motor and non-motor functions of the BG are among the most actively investigated areas of BG physiology at this time.
CIRCUIT DISORDERS
Considerable evidence has accumulated to indicate that dysfunction within the motor circuit is associated with movement disorders, such as PD, dystonia and hyperkinetic disorders, that dysfunction within the associative circuits is involved with disturbances of cognition and executive function, and that dysfunction within the limbic circuit is associated with psychiatric disorders such as OCD and depression (Figure 1B). Tourette’s syndrome, which is characterized by the occurrence of motor and vocal tics, as well as comorbid depression and OCD, may involve combined disturbances in both the motor and limbic circuits. It thus seems profitable to view these neurologic and psychiatric disorders as “circuit disorders,” resulting from specific forms of neural dysfunction within specific circuits, and involving BG dysfunction as well as the function of the associated thalamic and cortical areas.
The most obvious application of the circuit disorder concept is with regard to movement disorders. In fact, when the direct/indirect circuit models were initially formulated,22,24 it was clear that movement disorders do not only reflect BG activity, but rather the activities of all components of the basal ganglia-thalamocortical circuitry. For instance, the model of intrinsic connections of the BG in which DA modulates the balance between direct and indirect pathways, suggests that striatal neurons that give rise to the indirect pathway become hyperactive because of the loss of dopaminergic inhibition, resulting in reduced activity in GPe. This, in turn, would disinhibit the STN-GPi axis, and lead to increased (inhibitory) GPi output. The change in BG output would be further aggravated by the loss of the facilitating actions of DA on direct pathway neurons, which would lead to disinhibition of GPi. Increased GPi output was believed to excessively inhibit thalamocortical projection neurons, resulting in an inhibition of frontal cortical areas. The simple model that parkinsonism was due to gross activity changes in the BG was supported by neurophysiologic single cell recordings in primate models of PD,60–63 by studies of brain metabolism [see review in 64], and by studies demonstrating the antiparkinsonian effects of lesions of the hyperactive GPi and STN in animals with experimental parkinsonism [e.g.,65,66], and in humans with PD [e.g.,67,68]. As further support for the model that the level of BG activity regulates the overall amount of movement, it was shown that STN inactivation resulted in the predicted decrease in GPi neuronal activity,50 and excessive movement (hemichorea).
This model, however, could not explain several key findings, for example, the fact that lesions of the motor thalamus69 do not result in bradykinesia or akinesia (as would be expected if excessive inhibition of the thalamus was a fundamental problem in parkinsonism), and that GPi lesions in parkinsonian patients and primates do not result in dyskinesias. Accordingly, subsequent models of the pathophysiology of PD have focused not on overall change in firing rates, but on abnormal firing patterns, such as bursts and oscillatory patterns and abnormal synchronization70 that may involve all components of the motor circuit. The currently favored model ascribes parkinsonism to a disruption of normal cortical activities resulting from excessive beta-band oscillations, and a reduction in gamma-band oscillations throughout the BG motor circuit.70 Although this model has experimental support from animal and human recordings, it remains controversial. Thus, while recordings utilizing implanted DBS electrodes and local field potential recording in parkinsonian patients have demonstrated beta-band oscillations, and weak gamma oscillatory activity, this has not been consistently demonstrated.71 Furthermore, animal studies have shown that oscillatory firing patterns emerge at a late stage of DA depletion,72 after the development of parkinsonism, and that systemic DA receptor antagonists induce parkinsonism without inducing substantial oscillations in the BG and cortex.73
Much less is known about other diseases that involve the BG, but it has been convincingly demonstrated that abnormal activity within the cortico-basal ganglia- thalamocortical circuits may give rise to a large number of movement and neuropsychiatric conditions (see above). The basic insight that diseases within the BG circuitry may have functional consequences that go beyond the confines of these nuclei has helped to revitalize the attempts at treating patients who suffer from these conditions with focal BG interventions, such as lesions or DBS. DBS of pallidum or STN has become the preferred treatment for patients with advanced stages of movement disorders (such as PD or primary generalized dystonia), and, more recently, has also shown promise for the treatment of non-motor disorders, such as OCD, treatment-resistant depression and Tourette’s syndrome, targeting the limbic circuit.
CONCLUSION
The anatomical/physiologic and pathophysiologic circuit models have had a major impact on our thinking about the physiological role of the BG, and the pathophysiologic changes that occur in BG disorders. They have also stimulated research and the development of new therapeutic approaches.
Some aspects of the earlier pathophysiologic models of BG diseases have fallen by the wayside, most likely because the anatomical models were too rigidly transformed into static functional models. The focus has shifted from changes in discharge frequency and excitation/inhibition to the role of abnormal circuit-wide oscillations, synchrony and burst discharges [see also ref.74]. However, many of the features and hypotheses related to the early models have stood the test of time. The modular, closed-loop arrangement of the basal ganglia-thalamocortical circuitry and the basic circuit model of the intrinsic connections between the BG remain widely accepted, although evidence of some interactions and open loop influences are apparent and may lead to revisions of these models in the future. The recent evidence for closer anatomical connections between the BG and cerebellar circuitry may help to explain the findings from neuroimaging and physiologic studies in both animal models and patients for a role of the cerebellum in what have been viewed as traditional BG disorders. These findings suggest new opportunities for understanding of the pathophysiology and treatment of these disorders.
From a functional point of view, the time is ripe to seriously question the traditional view that the BG play a major role in movement initiation and execution. It seems far more likely that the BG have a role in learning, habit formation, and other higher-order behavioral and cognitive functions, than in the on-line control of movement.
Footnotes
DISCLOSURE AND CONFLICT OF INTEREST
M. DeLong and T. Wichmann have no conflicts of interest in relation to this article.
References
- 1.Wichmann T, DeLong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Wichmann T, DeLong MR. Anatomy and physiology of the basal ganglia: relevance to Parkinson’s disease and related disorders. In: Koller W, Melamed E, editors. Handbook Clinical Neurology. Vol. 83. New York, NY: Elsevier; 2007. pp. 1–18. [DOI] [PubMed] [Google Scholar]
- 3.DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord. 2009;15 (suppl 3):S237–240. doi: 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemp JM, Powell TPS. The connexions of the striatum and globus pallidus: synthesis and speculation. Philosophical Transactions of the Royal Society of London - Series B: Biol Sciences. 1971;262:441–457. doi: 10.1098/rstb.1971.0106. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 6.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 7.Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- 8.Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neurosci. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 9.Joel D, Weiner I. The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Res Rev. 1997;23:62–78. doi: 10.1016/s0165-0173(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 10.Romanelli P, Esposito V, Schaal DW, Heit G. Somatotopy in the basal ganglia: experimental and clinical evidence for segregated sensorimotor channels. Brain Res Brain Res Rev. 2005;48:112–128. doi: 10.1016/j.brainresrev.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 14.Bostan AC, Dum RP, Strick PL. The basal ganglia communicates with the cerebellum. Soc Neurosci Annual Meeting Abstracts. 2009;39 661.666/CC650. [Google Scholar]
- 15.Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to “AIP”. Cerebr Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- 17.Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- 19.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–154. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 23.Crossman AR. Neural mechanisms in disorders of movement. Comp Biochem Physiol. 1989;93A:141–149. doi: 10.1016/0300-9629(89)90201-6. [DOI] [PubMed] [Google Scholar]
- 24.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 25.Parent A, Sato F, Wu Y, Gauthier J, Levesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci. 2000;23:S20–27. doi: 10.1016/s1471-1931(00)00022-7. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord. 2008;23 (suppl 3):S548–559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- 31.Nambu A, Mori S, Stuart DG, Wiesendanger M. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- 32.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 33.Nambu A. A new approach to understand the pathophysiology of Parkinson’s disease. J Neurol. 2005;252(suppl 4):iv1–iv4. doi: 10.1007/s00415-005-4002-y. [DOI] [PubMed] [Google Scholar]
- 34.Georgopoulos AP, DeLong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 36.Turner RS, DeLong MR. Corticostriatal activity in primary motor cortex of the macaque. J Neurosci. 2000;20:7096–7108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauswein E, Fromm C, Preuss A. Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res. 1989;493:198–203. doi: 10.1016/0006-8993(89)91018-4. [DOI] [PubMed] [Google Scholar]
- 38.Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. I. Functional properties in intact animals. J Neurophysiol. 1994;72:494–506. doi: 10.1152/jn.1994.72.2.494. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell SJ, Richardson RT, Baker FH, DeLong MR. The primate globus pallidus: neuronal activity related to direction of movement. Exp Brain Res. 1987;68:491–505. doi: 10.1007/BF00249793. [DOI] [PubMed] [Google Scholar]
- 40.Anderson ME, Turner RS. A quantitative analysis of pallidal discharge during targeted reaching movement in the monkey. Exp Brain Res. 1991;86:623–632. doi: 10.1007/BF00230536. [DOI] [PubMed] [Google Scholar]
- 41.Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- 42.Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 43.Shink E, Bevan MD, Bolam JP, Smith Y. The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neurosci. 1996;73:335–357. doi: 10.1016/0306-4522(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 44.Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hikosaka O, Isoda M. Brain mechanisms for switching from automatic to controlled eye movements. Prog Brain Res. 2008;171:375–382. doi: 10.1016/S0079-6123(08)00655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tunik E, Houk JC, Grafton ST. Basal ganglia contribution to the initiation of corrective submovements. Neuroimage. 2009;47:1757–1766. doi: 10.1016/j.neuroimage.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Kliem MA, Maidment NT, Ackerson LC, Chen S, Smith Y, Wichmann T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol. 2007;89:1489–1500. doi: 10.1152/jn.00171.2007. [DOI] [PubMed] [Google Scholar]
- 49.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in reduced pallidal neuronal activity during active holding. J Neurophysiol. 1992;68:1859–1866. doi: 10.1152/jn.1992.68.5.1859. [DOI] [PubMed] [Google Scholar]
- 51.Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004 Sep;127:2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- 52.McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- 53.Darbin O, Wichmann T. Effects of striatal GABAA-receptor blockade on striatal and cortical activity in monkeys. J Neurophysiol. 2008;99:1294–1305. doi: 10.1152/jn.01191.2007. [DOI] [PubMed] [Google Scholar]
- 54.Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. I. Physiological properties of striatal microexcitable zones. J Neurophysiol. 1985;53:1401–1416. doi: 10.1152/jn.1985.53.6.1401. [DOI] [PubMed] [Google Scholar]
- 55.Obeso JA, Jahanshahi M, Alvarez L, Macias R, Pedroso I, Wilkinson L, et al. What can man do without basal ganglia motor output? The effect of combined unilateral subthalamotomy and pallidotomy in a patient with Parkinson’s disease. Exp Neurol. 2009;220:283–292. doi: 10.1016/j.expneurol.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 56.Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 58.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opinion Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Restivo L, Frankland PW. Shifting to automatic. Front Integr Neurosci. 2010;4:1. doi: 10.3389/neuro.07.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: Carpenter MB, Jayaraman A, editors. The Basal Ganglia II. New York: Plenum Press; 1987. pp. 415–427. [Google Scholar]
- 61.Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- 62.Filion M. Effects of interruption of the nigrostriatal pathway and of dopaminergic agents on the spontaneous activity of globus pallidus neurons in the awake monkey. Brain Res. 1979;178:425–441. doi: 10.1016/0006-8993(79)90704-2. [DOI] [PubMed] [Google Scholar]
- 63.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 64.Crossman AR. Functional anatomy of movement disorders. J Anat. 2000;196:519–525. doi: 10.1046/j.1469-7580.2000.19640519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 66.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez L, Macias R, Lopez G, Alvarez E, Pavon N, Rodriguez-Oroz MC, et al. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain. 2005;128:570–583. doi: 10.1093/brain/awh397. [DOI] [PubMed] [Google Scholar]
- 68.Fine J, Duff J, Chen R, Chir B, Hutchison W, Lozano AM, Lang AE. Long-term follow-up of unilateral pallidotomy in advanced Parkinson’s disease. N Engl J Med. 2000;342:1708–1714. doi: 10.1056/NEJM200006083422304. [DOI] [PubMed] [Google Scholar]
- 69.Canavan AG, Nixon PD, Passingham RE. Motor learning in monkeys (Macaca fascicularis) with lesions in motor thalamus. Exp Brain Res. 1989;77:113–126. doi: 10.1007/BF00250573. [DOI] [PubMed] [Google Scholar]
- 70.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Rossi L, Marceglia S, Foffani G, Cogiamanian F, Tamma F, Rampini P, et al. Subthalamic local field potential oscillations during ongoing deep brain stimulation in Parkinson’s disease. Brain Res Bull. 2008;76:512–521. doi: 10.1016/j.brainresbull.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 72.Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- 73.Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]