Abstract
Background
Mortality predictions following traumatic brain injury (TBI) may be improved by including genetic risk in addition to traditional prognostic variables. One promising target is the gene coding for brain-derived neurotrophic factor (BDNF), a ubiquitous neurotrophin important for neuronal survival and neurogenesis.
Objective
We hypothesized the addition of BDNF genetic variation would improve mortality prediction models and that BDNF Met-carriers (rs6265) and C-carriers (rs7124442) would have the highest mortality rates post-TBI.
Methods
This study examined BDNF functional single nucleotide polymorphisms (SNPs) rs6265r (val66met) and rs7124442 (T>C) in relation to mortality in a prospective, longitudinal cohort with severe TBI. We examined 315 individuals receiving care for a closed head injury within the University of Pittsburgh Medical Center, aged 16–79. Mortality was examined acutely (0–7 days post-injury) and post-acutely (8–365 days post-injury). A gene risk score (GRS) was developed to examine both BDNF loci. Cox proportional hazards models were used to calculate hazard ratios for survivability post-TBI while controlling for covariates.
Results
BDNF GRS was significantly associated with acute mortality, regardless of age. Interestingly, subjects in the hypothesized no-risk allele group had the lowest survival probability. Post-acutely, BDNF-GRS interacted with age such that younger participants in the no-risk group had the highest survival probability, while older participants in the hypothesized no-risk group had the lowest probability of survival.
Conclusions
These data suggest complex relationships between BDNF and TBI mortality that interact with age to influence survival predictions beyond clinical variables alone. Evidence supporting dynamic, temporal balances of pro-survival/pro-apoptotic target receptors may explain injury and age-related gene associations.
Keywords: Brain Injury, BDNF, TrkB, Mortality, Age, Genetic Association Study
Introduction
In the US, ~52,000 deaths are attributed to traumatic brain injury (TBI) yearly.1 As TBI is a highly heterogeneous disease, it is difficult to predict immediate and long-term outcomes. Understanding factors that predict mortality post-TBI may improve treatment. With the recent focus on personalized medicine, this study utilizes a Rehabilomics2 approach, examining genetic factors that capture innate heterogeneity across recovery to improve mortality predictions beyond traditional prognostic factors.
The International Mission on Prognosis and Analysis of randomized Controlled Trials in TBI (IMPACT) has demonstrated high predictability of post-TBI mortality/outcome using a core model of age, injury severity (motor subscale of the Glasgow Coma Scale, GCS3), and pupillary reactivity.4 Further studies support extending this model by adding neurological findings (evidence of midline shift or presence of subarachnoid hemorrhage5). However, these studies have not examined genetic factors, nor have they addressed the possibility of evolving or dynamic predictors of mortality. As influences on mortality likely change across recovery, it is important to examine mortality predictors over time.
Age is a consistent determinant of TBI survival. Older adults comprise a large segment of the population sustaining TBI with comparatively worse outcomes and higher mortality rates despite similar injury parameters.6 This phenomenon may be due to adverse age effects on secondary injury cascades. Older age leads to greater susceptibility to glutamate-mediated oxidative stress and damage.7 In experimental models, older animals show decreased neuronal survival post-injury8.
A ubiquitous neurotrophin in the brain, brain-derived neurotrophic factor (BDNF), may interact with age to influence TBI pathology. BDNF is important for synaptic plasticity, neurogenesis and neuronal survival.9 Yet in areas like the hypothalamus, BDNF can regulate metabolism10,11. Evidence suggests BDNF may also affect brainstem control of cardiovascular function12–14. Thus, BDNF may affect autonomic regulation and reflect the current energy state15, suggesting multiple mechanisms through which BDNF signaling could influence TBI mortality and recovery. While BDNF may affect mortality in other populations16–18, no study has examined BDNF in mortality post-TBI.
BDNF signals through the tyrosine-related kinase-B (TrkB) receptor, full length (TrkB.FL) and truncated (TrkB.T), as well as the p75NTR receptor, activating antagonistic signaling cascades that are dependent on receptor milieu. Studies suggest TrkB.FL/TrkB.T/p75NTR expression ratios may vary across the lifespan19 and following ischemia.20 Similarly, there are dynamic receptor expression changes following experimental TBI.21 Thus, BDNF’s role in TBI recovery may be dependent on the relative balance of these target receptors.
The BDNF gene has a common, functional, single nucleotide polymorphism (SNP), Val66Met (rs6265) that alters activity-dependent secretion of BDNF in vitro22 and shows an age-dependent relationship in cognition.23 Importantly, rs6265 is in linkage disequilibrium (LD) with another BDNF SNP, rs7124442. The rs7124442 variant reportedly affects neuronal BDNF mRNA trafficking24. While rs626525 and rs712444226 have been associated with TBI recovery, no studies have examined how these variants influence other aspects of TBI recovery, specifically mortality. As rs6265 can influence hypothalamus-pituitary-adrenal (HPA) axis reactivity27,28 and autonomic control of heart rate29, there may be important contributions for BDNF variants in TBI recovery outside of cognitive outcomes.
We examined BDNF variation in survivorship post-TBI. We hypothesized that the Met-allele (rs6265), with its decreased activity-dependent BDNF secretion, and the C-allele (rs7124442), with its impaired BDNF mRNA trafficking, would be risk alleles in mortality predictions. We chose to examine a cumulative gene risk score (GRS) incorporating variation at both loci when developing mortality prediction models. In this report, we demonstrate a dynamic, temporal relationship between BDNF GRS and post-TBI survivorship. Our data show a BDNF gene risk relationship with survivorship 0–7d post-injury that is contrary to hypothesized relationships. Yet, when evaluating mortality models 8–365d post-injury, we show a BDNF age*GRS interaction in survivorship. These findings underscore the importance of understanding age and BDNF signaling relationships following TBI.
Methods
Demographics
This prospective cohort study was approved by the University of Pittsburgh Institutional Review Board. Enrollment criteria for this study included age, ≥16 and <75 years, and an admission GCS score ≤8 indicating severe TBI. Exclusion criteria included documented prolonged hypoxia prior to admission or penetrating head injury. Subjects were consecutively recruited and consent obtained by appropriate proxy. To minimize genetic stratification effects,30 associations are reported in Caucasians only (n=284, reported findings). Important for generalizability of findings, reported analyses in Caucasians-only were similar to results in the total population (n=315, data not shown).
The analyzed cohort (284 participants) was aged 16–74 yrs. (mean: 35.96±15.46; median=33) with closed head injury receiving care within the University of Pittsburgh Medical Center (UPMC). GCS scores3 ranged from 3–15 (mean GCS: 6.20±2.54; median=6) when using the best GCS obtained within 24 hours post-injury. Demographic information (age, race, sex, and mechanism of injury) was collected through clinical chart review and subject/caregiver interviews. Age was treated as a continuous and categorical variable, split at the 75% quartile (Q3) of our population (above and below, Q3=45 yrs). By 365d post-injury, 25.8% of subjects had died and distribution of Glasgow Outcome Scale (GOS)31 scores for survivors was as follows: 2, n=13; 3, n=66; 4, n=49; 5, n=22.
The University of Pittsburgh Trauma Registry provided abstracted information from the acute care medical record regarding post-injury complications. These complications were categorized as: pulmonary, infection, cardiovascular, musculoskeletal, hematological, renal, wound, gastrointestinal, and neurological complications (Table 1).
Table 1.
Complications categories.
| Category | Complications Listed in Trauma Registry |
|---|---|
| Pulmonary | Acute Respiratory Distress Syndrome (ARDS) |
| Acute Respiratory Failure | |
| Aspiration/ Pneumonia Atelectasis | |
| Pleural Effusion | |
| Pneumonia | |
| Pulmonary Embolus | |
| Bronchial Mainstem Intubation | |
| Acute Sinusitis | |
| Empyema | |
| Infection | CNS Infection |
| Sepsis/Septicemia | |
| Cardiovascular | Acute Arterial Occlusion |
| Cardiopulmonary Arrest (not cause of death) | |
| Major Arrhythmia | |
| Myocardial Infarction | |
| Musculoskeletal | Extremity Compartment Syndrome |
| Hematological | Coagulopathy |
| Post-Operative Hemorrhage | |
| Renal | Acute Renal Failure |
| Renal Failure | |
| Urinary Tract Infection | |
| Wounds | Wound Infection |
| Decubitis Ulcer | |
| Wound Dehiscence | |
| Gastrointestinal | C Difficile Colitis |
| Esophageal Intubation | |
| GI Bleed | |
| Bowel Obstruction | |
| Pancreatitis | |
| Small Bowel Obstruction | |
| Neurological | CNS Infection |
| Diabetes Insipidus | |
| Neuro-sequelae | |
| Progression of Neurologic Insult | |
| Seizures | |
Neurological injury assessments were abstracted from admission head CT reports and were categorized by the following injury subtypes: contusion, subdural hematoma, subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), intracranial hemorrhage (ICH), epidural hematoma (EDH), and diffuse axonal injury (DAI). A neurological burden score (NBS) was calculated by summing injury types that significantly impacted survival for a given mortality group. NBS was only utilized in the acute mortality analysis and consisted of SDH, EDH, and contusion. For the post-acute mortality group, only the ICH category survived correction for age and GCS in Cox model predictions of survivorship.
Seizure information was abstracted from available medical records and coded as time to first post-traumatic seizure (PTS), up to 365d post-injury. Time to first seizure was divided into two groups, consistent with mortality cohorts and standard PTS nomenclature: acute (0–7d) and post-acute PTS (8–365d).32 Notation in medical records referring to convulsions, seizures, status epilepticus, or seizure disorder was documented as a PTS episode.
Mortality
Time until death was recorded in days post-injury, up to 1 year post-injury, using the Social Security Death Index.33 Mortality was evaluated over two time-epochs, 0–7d post-injury (acute) and 8d-365d post-injury (post-acute). For 0–7d, survivorship was right censored at 7d post-injury. For 8–365d, subjects were only included if they survived greater than 7d, and survivorship was right censored at 365d. For logistic regression and receiver operating curve (ROC) analysis, mortality was examined as a binary outcome at 7d post-injury (acute) and 365d post-injury (post-acute, excluding subjects who died before 7d). Acutely, 11.62% of subjects died (time until death: median=3d, min=0d, max=7d, Q1=2d, Q3=6d). An additional 14.16% died post-acutely (time until death: median=19d, min=8d, max=301d, Q1=11.5d, Q3=31d).
Genotyping and SNP Selection
DNA was isolated from blood using a simple salting out procedure34 or from cerebrospinal fluid using the Qiamp protocol from Qiagen. BDNF rs6269 and rs7124442 were genotyped by TaqMan allele discrimination assay using Assay on Demand reagents (Applied Biosystems). This assay utilized fluorescent labeled probes to detect allele(s) present for DNA sample. All allele frequencies were in Hardy-Weinberg equilibrium.
Selected SNPs (rs6265, rs7124442) have been reported as functional. Both SNPs have a minor allele frequency >20%. Each SNP represents a different haplotype block of BDNF covering variation corresponding to isoform a. A cumulative BDNF GRS was developed using rs6265 Met (Val/Met or Met/Met) and rs7124442 C (T/C or C/C) carrier status as hypothesized risk alleles based on the literature.22,24 Thus, a GRS of 0 was the hypothesized no risk group (Val/Val, T/T); a GRS of 1 included carriers for 1 risk allele (Val/Val, C-carriers or Met-carriers, T/T); and a GRS of 2 included carriers of both risk alleles (Met-carriers, C-carriers).
Statistical Analysis
Analysis was conducted using Statistical Analysis Software (version 9.2; SAS Institute) and the Statistical Package for Social Sciences (version 21.0; SPSS). Descriptive analysis included mean+standard deviation (STD) for continuous variables. Frequencies were calculated for categorical variables. Genetic analysis utilized categorizations based on allele carrier status, and the BDNF GRS was used to examine cumulative genetic risk associations with mortality. Demographic and clinical information was compared with mortality status and genotype using Student’s t-tests and ANOVA (Mann-Whitney or Kruskal-Wallis where appropriate) to compare means, and Chi-Square or Fisher’s Exact test to compare frequencies.
Demographic, clinical, and genotype information was examined for survivorship associations using either a Kaplan-Meier or Cox proportional hazards model.36 The Log-rank test was used to determine significant differences between two survival curves (significant if p<0.05). To control for covariates, we used multivariate Cox proportional hazards regression. Demographic and clinical variables remained in the final Cox models if they survived correction for age and GCS (p≤0.2). The proportionality of hazards assumption was tested and confirmed for relevant variables.
Mortality status was examined using multivariate receiver operating curve (ROC) analysis to quantify model prediction capacity and to relate to published studies.5 Mortality was assessed at 7d and 365d post-injury. Using area under the curve (AUC), ROCs estimated incremental increases in model sensitivity and specificity gained when including GRS and/or GRS interactions, compared to base models of relevant clinical variables. Base models were compared to final models using a chi-square test for significant differences in AUC (p<0.05 considered significant).
Results
Genetic Associations with Demographics
Demographic distributions were examined for both BDNF loci (Table 2). The mean age for T/T homozygotes (rs7124442) was higher versus C-carriers (38.0±16.3 versus 33.4±14.0, p=0.011). DAI was less common among T/T homozygotes versus C-carriers (24.7% vs. 35.7%, p=0.043). However, subjects with DAI were significantly younger compared to subjects without DAI (28.7±11.9 vs. 39.0±15.8, p=0.038). There were no demographic variable differences by rs6265 Met-carrier status.
Table 2.
Demographic variables by genotypes.
| Rs6265 | Rs7124442 | ||||||
|---|---|---|---|---|---|---|---|
| Val/Val (n=170) |
Val/Met Met/Met (n=114) |
p value |
C/C, C/T (n=126) |
T/T (n=158) |
p value |
||
| Age | 35.55±15.48 | 36.57±15.46 | 0.962 | 33.38±14.02 | 38.01±16.25 | 0.011 | |
| Gender (% Male) |
134 (78.8%) | 96 (84.2%) | 0.283 | 102 (80.9%) | 128 (81.0%) | 0.989 | |
| Mechanism of Injury | 0.779 | 0.776 | |||||
| Automobile/ Motorcycle |
128 (75.3%) | 85 (75.2%) | 97 (77.0%) | 116 (73.9%) | |||
| Fall/Jump | 25 (14.7%) | 19 (16.8%) | 19 (15.1%) | 25 (15.9%) | |||
| Other | 17 (10.0%) | 9 (8.0%) | 10 (7.9%) | 16 (10.2%) | |||
| GCS | 6 | 6 | 0.678 | 6 | 6 | 0.368 | |
| Length of Hospital Stay (days) |
19.72 ± 11.38 | 21.02 ± 12.32 | 0.517 | 19.32 ± 11.69 | 20.99 ± 11.80 | 0.306 | |
| ISS | 35.17 ± 10.01 | 34.69 ± 8.91 | 0.625 | 35.04 ± 9.26 | 34.92 ± 9.83 | 0.925 | |
| Seizures | 25 (14.8%) | 15 (13.8%) | 0.697 | 14 (11.2%) | 26 (16.5%) | 0.208 | |
| Complication Type (% Present) | |||||||
| Pulmonary | 108 (63.5%) | 63 (55.3%) | 0.175 | 75 (59.5%) | 96 (60.8%) | 0.833 | |
| Infection | 32 (15.3%) | 29 (25.4%) | 0.471 | 31 (24.6%) | 34 (21.5%) | 0.538 | |
| Cardiovascular | 6 (3.5%) | 7 (6.1%) | 0.387 | 4 (3.2%) | 9 (5.7%) | 0.312 | |
| MSK | 3 (1.80%) | 0 (0%) | 0.227 | 2 (1.6%) | 1 (0.6%) | 0.434 | |
| Heme | 27 (15.9%) | 12 (10.5%) | 0.222 | 13 (10.3%) | 26 (16.5%) | 0.135 | |
| Renal | 23 (13.5%) | 16 (14.0%) | 0.999 | 19 (15.1%) | 20 (12.7%) | 0.556 | |
| Wounds | 8 (4.7%) | 11 (9.6%) | 0.145 | 10 (7.9%) | 9 (5.7%) | 0.453 | |
| GI | 18 (10.6%) | 9 (7.9%) | 0.538 | 14 (11.1%) | 13 (8.2%) | 0.411 | |
| Neuro | 29 (17.9%) | 22 (19.3%) | 0.639 | 22 (17.5%) | 29 (18.4%) | 0.845 | |
| Neurological Injury Type (% Present) | |||||||
| SDH | 107 (62.9%) | 73 (64.0%) | 0.900 | 82 (65.1%) | 98 (62.0%) | 0.596 | |
| DAI | 51 (30.0%) | 33 (28.9%) | 0.895 | 45 (35.7%) | 39 (24.7%) | 0.043 | |
| EDH | 22 (12.9%) | 23 (20.2%) | 0.135 | 21 (16.7%) | 24 (15.2%) | 0.735 | |
| Contusion | 76 (44.7%) | 60 (52.6%) | 0.226 | 62 (49.2%) | 74 (46.8%) | 0.691 | |
| IVH | 44 (25.9%) | 36 (31.6%) | 0.346 | 35 (27.8%) | 45 (28.5%) | 0.896 | |
| ICH | 67 (39.4%) | 37 (32.5%) | 0.259 | 44 (34.9%) | 60 (38.0%) | 0.596 | |
| SAH | 116 (68.2%) | 74 (64.9%) | 0.608 | 87 (69.0%) | 103 (65.2%) | 0.493 | |
Mortality Associations with Demographics
Demographic associations with acute/post-acute mortality and survivorship were examined (Table 3). Survivors had lower mean age (all group, p<0.001) and higher GCS scores (all group, p=0.002) compared to non-survivors. In Cox proportional hazards regression, age and GCS predicted survival probability for both 0–7d and 8–365d models (p<0.03, all comparisons). Pulmonary (adjusted for age, GCS: p<0.001, HR=0.156) and cardiac (adjusted for age, GCS: p=0.196, HR=2.065) complications survived correction for age and GCS (p<0.2) and were added to the overall models for acute survival. Pulmonary complications were less frequent in subjects who died acutely (Table 3), but subjects with pulmonary complications were significantly younger than those without pulmonary complications (34.1±15.1 versus 38.8±15.6, p=0.009). Cardiac complications were more frequent in subjects who died acutely compared to survivors (12.1% vs. 2.8%, all group, p=0.026). For the 8–365d model, a wound complication (adjusted for age, GCS: p=0.020, HR=2.857) was the only complication to significantly predict survival probability.
Table 3.
Demographic information across mortality groups (acute, post-acute, and survivor).(Kruskal-Wallis, Fischer’s Exact, significance at p<0.05)
| Variable | Acute Mortality (0–7d, n=33) |
Post-Acute Mortality (8–365d, n=40) |
Survivors (at 365d n=211) |
All Groups (pvalue) |
|
|---|---|---|---|---|---|
| Age, years (mean±STD) | 42.69±17.30 | 47.5±16.39 | 32.72±13.54 | <0.001 | |
| Gender (n, % Male) | 23 (69.7%) | 31 (77.5%) | 176 (83.4%) | 0.141 | |
| Mechanism of Injury (n, %) | 0.004 | ||||
| Automobile/Motorcycle | 17 (51.5%) | 27 (67.5%) | 169 (80.5%) | ||
| Fall/Jump | 13 (39.4%) | 9 (22.5%) | 22 (10.5%) | ||
| Other | 3 (9.1%) | 4 (10.0%) | 19 (9.0%) | ||
| GCS, median | 5 | 6 | 6 | 0.002 | |
| ISS, mean ± STD | 35.61±10.86 | 36.58 ± 9.89 | 34.57±9.29 | 0.589 | |
| Length of Hospital Stay, days, mean±STD | 3.82±2.42 | 18.62±8.63 | 23.24±10.92 | <0.001 | |
| Seizures | 1 (3.0%) | 4 (10.0%) | 35 (16.5%) | 0.081 | |
| Complication Type (% Present) | |||||
| Pulmonary | 8 (24.2%) | 28 (70.0%) | 135 (64.0%) | <0.001 | |
| Infection | 4 (12.1%) | 10 (25.0%) | 51 (24.2%) | 0.293 | |
| Cardiovascular | 4 (12.1%) | 3 (7.5%) | 6 (2.8%) | 0.026 | |
| Heme | 4 (12.1%) | 4 (10.0%) | 31 (14.7%) | 0.844 | |
| Renal | 3 (9.1%) | 6 (15.0%) | 30 (14.2%) | 0.746 | |
| Wounds | 0 (0%) | 6 (15.0%) | 13 (6.2%) | 0.037 | |
| GI | 0 (0%) | 3 (7.5%) | 24 (11.4%) | 0.093 | |
| Neuro | 6 (18.2%) | 10 (25.0%) | 35 (16.6%) | 0.440 | |
| Neurological Injury Type (% Present) | |||||
| SDH | 23 (69.7%) | 28 (70.0%) | 129 (61.1%) | 0.254 | |
| DAI | 5 (15.2%) | 7 (17.5%) | 72 (34.1%) | 0.074 | |
| EDH | 2 (6.1%) | 6 (15.0%) | 37 (17.5%) | 0.291 | |
| Contusion | 21 (63.6%) | 24 (60.0%) | 91 (43.1%) | 0.004 | |
| IVH | 7 (21.2%) | 14 (35.0%) | 59 (28.0%) | 0.338 | |
| ICH | 12 (36.4%) | 14 (35.0%) | 78 (37.5%) | 0.947 | |
| SAH | 29 (87.9%) | 30 (75.0%) | 131 (62.1%) | 0.005 | |
While there were no differences in frequency of radiological findings between acute and post-acute mortality groups, there were significant associations with survivorship. For the 0–7d model, the NBS (including SAH, epidural hematoma, and contusion; range of 0–3), tended to predict survivorship (corrected for age, GCS: p=0.046, HR=1.379). SAH and contusions were more frequent in subjects who died (0–7d or 8–365d) versus survivors (Table 3). For the 8–365d model, those with ICH tended to have higher survival frequencies (age and GCS corrected, p=0.079, HR=0.541).
Genetic Associations with Mortality
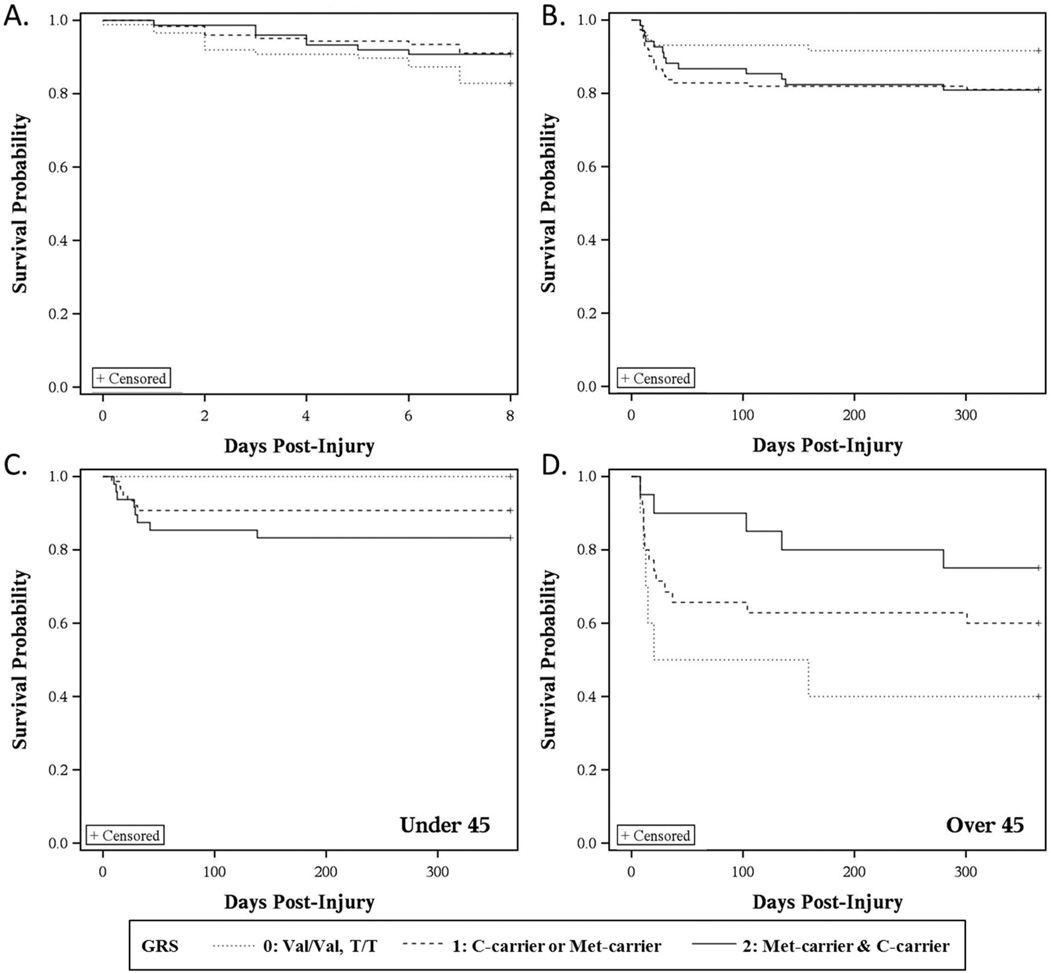
Kaplan-Meier curves reflecting mortality 0–7d post-injury tended to have different survivorship probabilities based on BDNF GRS (p=0.144, Figure 1A). Those with a GRS=0 hypothesized risk alleles had the lowest probability of survival. Multivariate Cox regression predicting survivorship 0–7d post-injury showed BDNF GRS became significant after adjusting for age, GCS, NBS, pulmonary complications, and cardiac complications (Table 4A). Those with a GRS of 1 or 2 had a higher probability of survival compared to those with a GRS of 0 (GRS=1, p=0.0107, HR=0.351, 95% CI: 0.157–0.784; GRS=2, p=0.0286, HR=0.349, 95% CI: 0.136–0.895). For 8–365d post-injury, there was a trend for different survivorship probabilities based on BDNF GRS (p=0.134, Figure 1B), where those with a GRS=0 had the highest survival probability. In this case, multivariate Cox regression demonstrated that this trend for BDNF GRS did not survive covariate adjustment (Table 4B).
Figure 1. Kaplan-Meier curves show survivorship probabilities stratified by BDNF GRS.
Kaplan-Meier curves stratified by BDNF GRS at (A) 0–7d (GRS, p=0.144) and (B) 8–365d (GRS, p=0.134). GRS was significant in the Cox model for acute mortality, correcting for covariates. The post-acute (8–365d) is further examined in age cohorts split at age 45, (C) <45 (GRS, p=0.006) and (D) ≥45 (GRS, p=0.106). The GRS*age interaction was significant in the Cox model for post-acute mortality, correcting for covariates (Gene risk score, GRS: GRS=0, Val/Val, T/T; GRS=1, Val/Val, C-carriers or Met-carriers, T/T; GRS=2, Met-carriers, C-carriers).
Table 4.
Cox Model of BDNF Gene Risk Score (GRS)
| Variable | Parameter Estimate |
Standard Error |
Chi- Square |
p value |
Hazard Ratio |
95% Hazard Ratio CI# |
|---|---|---|---|---|---|---|
| A. BDNF GRS predicting time until death, 0–7d post-injury (n=279, 33 events) | ||||||
| Age | 0.02419 | 0.01118 | 4.681 | 0.031 | 1.024 | (1.002 – 1.047) |
| GCS* | −0.35982 | 0.08939 | 16.204 | <0.001 | 0.698 | (0.586–0.831) |
| NBS¥ | 0.47135 | 0.23476 | 4.031 | 0.045 | 1.602 | (1.011–2.538) |
| Pulmonary Complication |
−2.16656 | 0.43392 | 24.930 | <0.001 | 0.115 | (0.049–0.268) |
| Cardiac Complication |
1.69773 | 0.59712 | 8.083 | 0.005 | 5.462 | (1.695–17.603) |
| GRS (1 risk SNP)** | −1.04771 | 0.41069 | 6.508 | 0.011 | 0.351 | (0.157–0.784) |
| GRS (2 risk SNP)** | −1.05395 | 0.48134 | 4.794 | 0.029 | 0.349 | (0.136–0.895) |
| B. BDNF GRS predicting time until death, 8–-365d post-injury (n=246, 39 events) | ||||||
|---|---|---|---|---|---|---|
| Age | 0.07065 | 0.01109 | 40.594 | <0.001 | 1.073 | (1.050–1.097) |
| GCS* | −0.30681 | 0.07779 | 15.556 | <0.001 | 0.736 | (0.632–0.857) |
| Acute PTS | 0.74307 | 0.59263 | 1.572 | 0.210 | 2.102 | (0.658–6.717) |
| Intracranial Hemorrhage |
−0.74676 | 0.36615 | 4.160 | 0.041 | 0.474 | (0.231–0.971) |
| Wound Complication |
0.95654 | 0.46986 | 4.144 | 0.042 | 2.603 | (1.036–6.537) |
| GRS 1** | 0.38055 | 0.48137 | 0.625 | 0.429 | 1.463 | (0.570–3.758) |
| GRS 2** | 0.28517 | 0.52061 | 0.300 | 0.584 | 1.330 | (0.479–3.690) |
| C. BDNF GRS x Age interaction predicting time until death, 8–365d post-injury (n=246, with 39 events) | ||||||
|---|---|---|---|---|---|---|
| Age | 0.15128 | 0.02536 | 35.5716 | <.0001 | 1.163 | (1.107–1.223) |
| GCS* | 0.29814 | 0.07504 | 15.7837 | <.0001 | 0.742 | (0.641–0.860) |
| Acute PTS | 1.44824 | 0.61090 | 5.6202 | 0.0178 | 4.256 | (1.285–14.092) |
| Intracranial Hemorrhage |
−0.59617 | 0.36387 | 2.6845 | 0.1013 | 0.551 | (0.270–1.124) |
| Wound Complication |
1.10612 | 0.47471 | 5.4293 | 0.0198 | 3.023 | (1.192–7.664) |
| GRS 1** | 3.42870 | 1.07292 | 10.2123 | 0.0014 | 30.836 | (3.765–252.542) |
| GRS 2** | 6.25552 | 1.77560 | 12.4119 | 0.0004 | 520.881 | (16.05–16909.54) |
| GRS x Age Interaction |
−0.06964 | 0.01912 | 13.2643 | 0.0003 | 0.933 | (0.898–0.968) |
GCS (Glasgow Coma Scale, Best in 24 hours)
Neurological Burden Score
GRS (0 risk SNP) was the reference category
CI: Confidence Interval
Next, age*GRS interactions with survival probability were examined. There was no significant age*GRS interaction in 0–7d survivorship probability (data not shown). However, Table 4C shows a significant age*GRS interaction for 8–365d survivorship (Age*GRS interaction, p=0.0003, HR=0.933, 95% CI: 0.898–0.968). To increase the interpretability of this interaction, we tested the relationship of GRS with survivorship prediction in 8–365d post-injury across different age cut-points. The population was stratified, below and above Q3 (age=45), and GRS was examined in Kaplan-Meier curves for the two age strata. Among participants <45 years, GRS associations with survivorship demonstrated that those with a GRS=0 had the highest probability of survival (p=0.006), while participants >45 with a GRS=0 had the lowest survivorship probability (p=0.106) (Figure 1C–D).
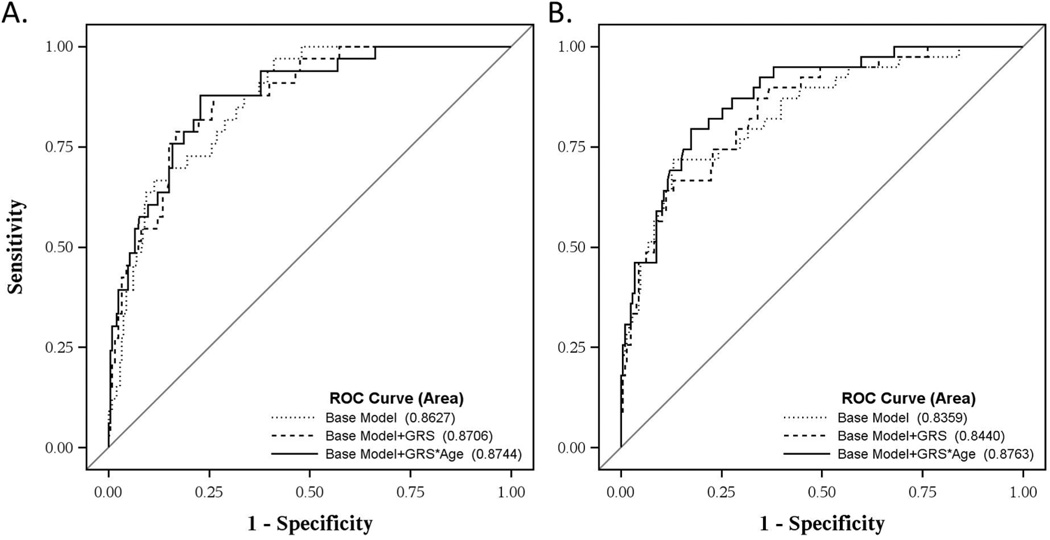
We further validated these models in multivariate ROC examining post-TBI mortality status. Using a similar censorship strategy and covariate selection as with our Cox models, we examined mortality status for acute (0–7d) and post-acute (8–365d) mortality. The 0–7d base model had an AUC=0.8412. The addition of BDNF GRS and a GRS*age interaction did not significantly improve this model. The 8–365d base model (excluding subjects who died in the acute phase) had an AUC=0.836. The addition of the GRS*age interaction increased the AUC above the base model (from 0.836 to 0.876, p=0.021, Figure 2).
Figure 2. Receiver operating curves of GRS models in acute (0–7d) and post-acute (8–365d) mortality.
(A) Mortality at 7d. The base model of age, GCS, pulmonary complications, cardiac complications, and neurological burden score did not differ from the base model+GRS (AUC=0.8412, 0.8532; p=0.501) or base model+GRS*age (AUC=0.8412, 0.8571; p=0.664). (B) Mortality at 365d (excluding subjects who died <7d). The AUC of the base model of age, GCS, wound complications and ICH plus the GRS*age interaction (base model+GRS*age, AUC=0.876) was increased from the base model (AUC=0.836, p=0.021) and the base model+GRS (AUC=0.844, p=0.248). (AUC, area under the curve; GCS, Glasgow Coma Score; Gene risk score, GRS: GRS=0, Val/Val, T/T; GRS=1, Val/Val, C-carriers or Met-carriers, T/T; GRS=2, Met-carriers, C-carriers).
Discussion
This study demonstrates important gene and gene*age interactions with BDNF in post-TBI mortality. First, we identified a dynamic temporal relationship between BDNF and survival. Second, we showed the hypothesized BDNF gene risk relationship to mortality is not supported acutely (0–7d post-injury), as the hypothesized no risk group had the lowest survival probability. Third, we demonstrated BDNF genetics interacts with age to inform survivorship predictions 8–365d post-injury. Here, the hypothesized risk relationship was supported in younger individuals, while older individuals maintained a similar risk pattern to that observed acutely. We speculate dynamic relationships between BDNF and mortality risk post-TBI may be attributable to age and injury effects on BDNF target receptors. Other actions of BDNF in autonomic function and other body systems may also impact its role in TBI-related mortality.
These unique relationships between BDNF and TBI mortality may be related to specific alterations in target receptor milieu with regard to TBI and age. BDNF, first synthesized as proBDNF, is processed either in the soma, extracellular space (after release), or dendrites (after endocytosis).37 If not cleaved, proBDNF targets the pro-apoptotic p75NTR receptor,38 while mature BDNF initiates pro-survival signaling through the full length TrkB receptor (TrkB.FL). Following experimental TBI, tissue plasminogen activator (tPA), an enzyme that cleaves proBDNF, shows increased activity ipsilateral to the injury39 and mice lacking tPA show reduced edema and cortical lesion volume40. Additionally, there are two truncated isoforms of TrkB, TrkB.T1 and TrkB.T2, which lack intracellular tyrosine signaling, but are implicated in other pathways.20,41 In experimental TBI, there are transient increases in hippocampal TrkB.FL; this study also showed regionally-specific p75NTR increases up to 8 weeks post-TBI.21 Although controversial, the ratio of TrkB.FL/Trk.T expressed has been suggested to influence cell survival during excitotoxic injury.20,41 Other studies report pre-incubation with BDNF prior to excitotoxic conditions/insults may be neuroprotective,42,43 but this effect is likely receptor-dependent. As studies demonstrate transient increases in hippocampal BDNF transcription immediately following experimental TBI,21,44 followed by chronically decreased levels,31 understanding temporal receptor expression changes may be critical in TBI.
Under the assumption that mature BDNF-TrkB.FL signaling is the primary action in adults,45 our risk allele assignment was based on the hypothesis that lower BDNF signaling would result in reduced pro-survival signaling and negatively impact survival. Thus, it would be reasonable to suggest lower BDNF signaling prior to/during an injury would exacerbate neuronal death post-TBI via reduced pro-survival signaling. However, the genetic variant hypothesized to increase BDNF activity-dependent secretion (Val/Val, rs6265) was associated with increased acute (0–7d) mortality risk. Consistent with animal literature,21 this relationship may be the result of an injury-specific balance of BDNF’s target receptors, with relative increases in TrkB.T or p75NTR compared to uninjured adults.
We show an important age*gene interaction with the BDNF GRS in post-acute mortality prediction. One explanation for age-specific risk profiles is differential expression patterns of target receptors across aging.19,46 Romanczyk et al reports dynamic prefrontal cortex TrkB expression across the lifespan, with a peak in young to middle adulthood that decreases with age.47 Webster et al reported similar TrkB expression patterns in the hippocampus and temporal lobe.48 Compared to young adult rats, aged rats have reduced TrkB.FL, but not TrkB.T,49 altering receptor ratios. Post-TBI, an age-specific shift in the balance of BDNF receptor ratios, from pro-survival to pro-apoptotic, could diminish recovery. Thus, older individuals with BDNF genotypes associated with higher baseline BDNF signaling may have a disadvantage.
This study focuses on two variants, rs6265 and rs7124442. While the rs6265 Met-allele impairs secretion and intracellular processing of mature BDNF in hippocampal neurons,22 there is currently no evidence that it alters proBDNF/BDNF ratios. The rs7124442 C-allele reduces BDNF mRNA trafficking from the soma to dendrites in hippocampal cultures.20 Given that studies suggest BDNF mRNA translated in dendrites are more likely to be secreted in the proBDNF form,50 it is possible rs7124442 alters proBDNF/BDNF ratios. While these variants are hypothesized to affect BDNF signaling, it is unclear if this remains true with age or injury. We suggest these variants are indicative of variability in neurotrophic support post-injury, and thus, may interact with receptor expression to produce TBI-specific dynamic risk profiles.
Previous studies with the BDNF gene suggest it interacts with age and environment to affect cognitive function.51 One study showed Met-carriers, despite theoretically lower activity-dependent BDNF secretion, have greater cognitive recovery following penetrating TBI decades after injury.25 These data are part of a growing literature demonstrating TBI-specific risk genetic relationships in TBI outcomes.52–54 However, in contrast to our findings, BDNF variation has also been examined in stroke, where the Met-allele was linked to poor recovery, regardless of age,55 though it is unclear if age interactions were explored in this relatively older population compared to our cohort. However, a preclinical study examining Val66Met in a rodent stroke model showed the Met-allele enhanced motor recovery chronically, further supporting the concept of injury-specific associations for this variant.56 Yet these studies focused on cognitive/plasticity effects of BDNF. It will be important to understand regional and temporal roles for BDNF in recovery or rehabilitation-based interventions compared to mortality risk.
In addition, BDNF can regulate energy metabolism and autonomic function. Evidence suggests that BDNF may be involved in brainstem regulation of cardiovascular function.12–14 Similarly, BDNF modulates the sympathetic/parasympathetic balance in cardiovascular function.57 Interestingly, rs6265 is associated with differences in heart rate variability29 and acute stress heart rate reactivity in healthy populations.27 In fact, one study showed that local BDNF administration following surgical sympathectomy induced hippocampal vascular changes and edema58. This study suggests BDNF effects during a state of compromised autonomic function (e.g. immediately following TBI59) could impact TBI pathology, particularly at early time-points when mortality rates are highest. There is a dearth of research about BDNF function outside of cognition or plasticity post-TBI, limiting speculation about how BDNF and CNS-peripheral modulation of autonomic function post-TBI might occur. It is also not clear how age may interact with BDNF in autonomic regulation.
Our data also suggest temporally specific prognostic factors for mortality across recovery. Many TBI survival studies use a cross-sectional approach to mortality. Our results suggest there are different factors contributing to mortality predictions over time that are not captured within the current literature. Acutely, a higher NBS significantly reduced survivorship probability, consistent with published studies showing the addition of neuroradiological findings improves mortality predictions.5 While pulmonary complications occur less frequently in survivors, subjects with pulmonary complications also were significantly younger, consistent with published studies in TBI.60 However, pulmonary complication effects were independent of age. One consideration for this finding is the possibility that there is a delayed onset for pulmonary complications, such as acute respiratory distress syndrome (ARDS), that may be secondary to TBI-specific ICP management, and thus, may not be a large negative factor within acute mortality models.61 Also, cardiac complications were a negative predictor of survival acutely. GCS was a significant factor in mortality predictions, consistent with previous studies on the relationship of injury severity to mortality post-TBI.5
Subjects in the post-acute survival model had a median time until death of 19d, suggesting that in this early post-acute time frame there are unique factors in mortality prediction. Early post-traumatic seizures were not related to mortality predictions in acute cohort. Yet, acute seizure occurrence negatively impacted survivorship post-acutely, consistent with epidemiological studies linking seizures to higher mortality risk after TBI62, suggesting the pathology associated with early seizures is also relevant to post-acute mortality. Subjects with wound complications had reduced survival probability, likely reflecting other important health/recovery factors, such as mobility, as contributing post-acute mortality risk.63 Wounds may also be a surrogate measure for infections or sepsis that could impact mortality post-TBI. Subjects with ICH had a higher probability of survival post-acutely compared to those without ICH. As these subjects survived acutely, they were likely monitored closely for surgical intervention, standard care that may have increased survival probability post-acutely.
This study also shows that, in our post-acute model, our GRS significantly added prognostic capacity to the base mortality model. While different cohorts, assessed over different time-frames, our AUC displays better mortality discrimination ability compared to previously published prediction models that include age, GCS, and pupil dilation only (AUC=0.7875). Further, the use of Cox models strengthens our survivorship predictions. These data suggest that, while clinical variables (eg. GCS) inform outcome, genetic factors can influence mortality predictions beyond what standard clinical variables are able to accomplish, particularly for the post-acute period when most primary neurological injury effects on mortality outcomes have already occurred. While novel and promising prognostic models, these findings need validation in independent studies with larger populations.
There are some limitations in interpretation and generalizability to consider. While BDNF is primarily expressed in the brain,33 BDNF is also synthesized and secreted from vascular endothelial cells and may have a peripheral action (see review, Caporali et al64). Also, there is a substantial peripheral store of BDNF in platelets,51 yet it is unclear if platelet release is altered in TBI. One study suggests plasma BDNF levels predict mortality in ICU patients without direct brain injury, but the mechanism of this association is unclear.16 Plasma BDNF levels were also related to all-cause mortality in a cohort of older women.17 With altered metabolic homeostasis immediately following TBI, there may be a vascular action of BDNF that could influence mortality.
There are some additional considerations in this study as it uses a candidate gene approach. The study findings are specific to a racially homogenous population, though models incorporating our small population with other racial backgrounds were stable with similar results as reported (data not shown). Future studies should evaluate BDNF risk in populations with diverse racial backgrounds to increase generalizability to the TBI population. The variants studied also had relatively low frequency for some genotypes, warranting a carrier approach, similar to published Val66Met studies.25,28 Similarly, these variants only cover the most well-studied isoform of BDNF, yet emerging research indicates that there are multiple isoforms for BDNF35. Future research may require additional genotyping to assess the relevance of these isoforms on TBI pathology and outcome prognostication.
Future studies may examine how these findings relate to other genetic variants such as apolipoprotein E(APOE) that have shown associations to TBI mortality.65 It was also difficult to examine genetic relationships to injury type given the severity of injury in this cohort, as many subjects showed multiple injury sub-types. As we do not have specific cause of deaths for this study, future studies may examine specific cause of death relationships to genetic risk.
Importantly, this study implicates BDNF signaling in TBI mortality prediction, supporting the need for validation studies and a better understanding of BDNF signaling post-TBI across body systems. This work supports the need for examination of specific regional TrkB.FL/TrkB.T/p75NTR receptor ratios in experimental TBI models and postmortem tissue. As serum BDNF is decreased acutely in clinical TBI,66 future studies may investigate BDNF as a biomarker, with age/gene variation as possible BDNF modifiers post-TBI. Future studies focusing on the dynamic roles of BDNF in mortality compared to rehabilitation and recovery following TBI are needed and may yield different associations reflective of unique pathology and/or recovery mechanisms.
ACKNOWLEDGEMENTS
This work was supported by the Department of Defense (W81XWH-07-1-0701, Wagner), National Institute on Disability and Rehabilitation Research (NIDRR H133A120087, Wagner), and National Institute of Health (R01HD048162, Wagner; R01NR008424 and R01NR013342, Conley)
References
- 1. [Accessed November 22, 2010];CDC - Injury - TBI - TBI in the US Report. Available at: http://www.cdc.gov/traumaticbraininjury/tbi_ed.html.
- 2.Wagner AK, Zitelli KT. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology: the official journal of the International Society for Pathophysiology/ISP. 2012 doi: 10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 4.Maas AIR, Murray GD, Roozenbeek B, et al. Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. The Lancet Neurology. 2013;12(12):1200–1210. doi: 10.1016/S1474-4422(13)70234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roozenbeek B, Chiu Y-L, Lingsma HF, et al. Predicting 14-Day Mortality after Severe Traumatic Brain Injury: Application of the IMPACT Models in the Brain Trauma Foundation TBI-trac ® New York State Database. Journal of Neurotrauma. 2012;29(7):1306–1312. doi: 10.1089/neu.2011.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Susman M, DiRusso SM, Sullivan T, et al. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53(2):219–223. doi: 10.1097/00005373-200208000-00004. discussion 223–224. [DOI] [PubMed] [Google Scholar]
- 7.Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. Journal of neurotrauma. 2004;21(2):125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- 8.Onyszchuk G, He Y-Y, Berman NEJ, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25(2):153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z-Y, Patel PD, Sant G, et al. Variant Brain-Derived Neurotrophic Factor (BDNF) (Met66) Alters the Intracellular Trafficking and Activity-Dependent Secretion of Wild-Type BDNF in Neurosecretory Cells and Cortical Neurons. J Neurosci. 2004;24(18):4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131(2):229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 11.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19(6):1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Zhou X-F. Injection of brain-derived neurotrophic factor in the rostral ventrolateral medulla increases arterial blood pressure in anaesthetized rats. Neuroscience. 2002;112(4):967–975. doi: 10.1016/s0306-4522(02)00085-4. [DOI] [PubMed] [Google Scholar]
- 13.Brady R, Zaidi SI, Mayer C, Katz DM. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J Neurosci. 1999;19(6):2131–2142. doi: 10.1523/JNEUROSCI.19-06-02131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem. 2014 doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264(1):49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritter C, Miranda AS, Giombelli VR, et al. Brain-derived neurotrophic factor plasma levels are associated with mortality in critically ill patients even in the absence of brain injury. Crit Care. 2012;16(6):R234. doi: 10.1186/cc11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krabbe KS, Mortensen EL, Avlund K, et al. Brain-derived neurotrophic factor predicts mortality risk in older women. J Am Geriatr Soc. 2009;57(8):1447–1452. doi: 10.1111/j.1532-5415.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 18.Halldén S, Sjögren M, Hedblad B, et al. Smoking and obesity associated BDNF gene variance predicts total and cardiovascular mortality in smokers. Heart. 2013;99(13):949–953. doi: 10.1136/heartjnl-2013-303634. [DOI] [PubMed] [Google Scholar]
- 19.Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812(1–2):200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- 20.Gomes JR, Costa JT, Melo CV, et al. Excitotoxicity downregulates TrkB.FL signaling and upregulates the neuroprotective truncated TrkB receptors in cultured hippocampal and striatal neurons. J Neurosci. 2012;32(13):4610–4622. doi: 10.1523/JNEUROSCI.0374-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rostami E, Krueger F, Plantman S, et al. Alteration in BDNF and its receptors, full-length and truncated TrkB and p75<sup>NTR</sup> following penetrating traumatic brain injury. [Accessed November 20, 2013];Brain research. 2013 doi: 10.1016/j.brainres.2013.10.047. Available at: http://www.sciencedirect.com/science/article/pii/S0006899313014571. [DOI] [PubMed]
- 22.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 23.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orefice LL, Waterhouse EG, Partridge JG, Lalchandani RR, Vicini S, Xu B. Distinct roles for somatically and dendritically synthesized brain-derived neurotrophic factor in morphogenesis of dendritic spines. J Neurosci. 2013;33(28):11618–11632. doi: 10.1523/JNEUROSCI.0012-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger F, Pardini M, Huey ED, et al. The role of the Met66 brain-derived neurotrophic factor allele in the recovery of executive functioning after combat-related traumatic brain injury. J Neurosci. 2011;31(2):598–606. doi: 10.1523/JNEUROSCI.1399-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostami E, Krueger F, Zoubak S, et al. BDNF Polymorphism Predicts General Intelligence after Penetrating Traumatic Brain Injury. PLoS ONE. 2011;6(11):e27389. doi: 10.1371/journal.pone.0027389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander N, Osinsky R, Schmitz A, Mueller E, Kuepper Y, Hennig J. The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology. 2010;35(6):949–953. doi: 10.1016/j.psyneuen.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Shalev I, Lerer E, Israel S, et al. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34(3):382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Yang AC, Chen T-J, Tsai S-J, et al. BDNF Val66Met polymorphism alters sympathovagal balance in healthy subjects. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(5):1024–1030. doi: 10.1002/ajmg.b.31069. [DOI] [PubMed] [Google Scholar]
- 30.Freedman ML, Reich D, Penney KL, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36(4):388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 32.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 33.Social Security Death Index. [Accessed December 9, 2013];GenealogyBank.com. Available at: http://www.genealogybank.com/gbnk/ssdi/
- 34.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruunsild P, Kazantseva1 A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90(3):397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B (Methodological) 1972:187–220. [Google Scholar]
- 37.Barker PA. Whither proBDNF? Nat Neurosci. 2009;12(2):105–106. doi: 10.1038/nn0209-105. [DOI] [PubMed] [Google Scholar]
- 38.Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Progress in neurobiology. 2000;61(2):205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 39.Sashindranath M, Samson AL, Downes CE, et al. Compartment- and context-specific changes in tissue-type plasminogen activator (tPA) activity following brain injury and pharmacological stimulation. Lab Invest. 2011;91(7):1079–1091. doi: 10.1038/labinvest.2011.67. [DOI] [PubMed] [Google Scholar]
- 40.Mori T, Wang X, Kline AE, et al. Reduced cortical injury and edema in tissue plasminogen activator knockout mice after brain trauma. Neuroreport. 2001;12(18):4117–4120. doi: 10.1097/00001756-200112210-00051. [DOI] [PubMed] [Google Scholar]
- 41.Vidaurre OG, Gascón S, Deogracias R, et al. Imbalance of neurotrophin receptor isoforms TrkB-FL/TrkB-T1 induces neuronal death in excitotoxicity. Cell Death Dis. 2012;3:e256. doi: 10.1038/cddis.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida RD, Manadas BJ, Melo CV, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12(10):1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 43.Lindvall O, Kokaia Z, Bengzon J, Elme´r E, Kokaia M. Neurotrophins and brain insults. Trends in Neurosciences. 1994;17(11):490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 44.Hicks RR, Numan S, Dhillon HS, Prasad MR, Seroogy KB. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res Mol Brain Res. 1997;48(2):401–406. doi: 10.1016/s0169-328x(97)00158-7. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto T, Rauskolb S, Polack M, et al. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11(2):131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 46.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Research Reviews. 2008;59(1):201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Romanczyk TB, Weickert CS, Webster MJ, Herman MM, Akil M, Kleinman JE. Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. European Journal of Neuroscience. 2002;15(2):269–280. doi: 10.1046/j.0953-816x.2001.01858.x. [DOI] [PubMed] [Google Scholar]
- 48.Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expression Patterns. 2006;6(8):941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132(3):613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 50.An JJ, Gharami K, Liao G-Y, et al. Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134(1):175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan GB, Vasterling JJ, Vedak PC. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol. 2010;21(5–6):427–437. doi: 10.1097/FBP.0b013e32833d8bc9. [DOI] [PubMed] [Google Scholar]
- 52.Failla MD, Burkhardt JN, Miller MA, et al. Variants of SLC6A4 in depression risk following severe TBI. Brain Inj. 2013;27(6):696–706. doi: 10.3109/02699052.2013.775481. [DOI] [PubMed] [Google Scholar]
- 53.Wagner AK, Hatz LE, Scanlon JM, et al. Association of KIBRA rs17070145 polymorphism and episodic memory in individuals with severe TBI. Brain injury: [BI] 2012 doi: 10.3109/02699052.2012.700089. [DOI] [PubMed] [Google Scholar]
- 54.Graham DP, Helmer DA, Harding MJ, Kosten TR, Petersen NJ, Nielsen DA. Serotonin transporter genotype and mild traumatic brain injury independently influence resilience and perception of limitations in Veterans. J Psychiatr Res. 2013;47(6):835–842. doi: 10.1016/j.jpsychires.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siironen J, Juvela S, Kanarek K, Vilkki J, Hernesniemi J, Lappalainen J. The Met Allele of the BDNF Val66Met Polymorphism Predicts Poor Outcome Among Survivors of Aneurysmal Subarachnoid Hemorrhage. Stroke. 2007;38(10):2858–2860. doi: 10.1161/STROKEAHA.107.485441. [DOI] [PubMed] [Google Scholar]
- 56.Qin L, Jing D, Parauda S, et al. An Adaptive Role for BDNF Val66Met Polymorphism in Motor Recovery in Chronic Stroke. J Neurosci. 2014;34(7):2493–2502. doi: 10.1523/JNEUROSCI.4140-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang B, Slonimsky JD, Birren SJ. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat Neurosci. 2002;5(6):539–545. doi: 10.1038/nn0602-853. [DOI] [PubMed] [Google Scholar]
- 58.Kasselman LJ, Sideris A, Bruno C, et al. BDNF: A missing link between sympathetic dysfunction and inflammatory disease? Journal of Neuroimmunology. 2006;175(1–2):118–127. doi: 10.1016/j.jneuroim.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Goldstein B, Toweill D, Lai S, Sonnenthal K, Kimberly B. Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am J Physiol. 1998;275(4 Pt 2):R1287–R1292. doi: 10.1152/ajpregu.1998.275.4.R1287. [DOI] [PubMed] [Google Scholar]
- 60.Rincon F, Ghosh SM, Dey SM, et al. Impact of Acute Lung Injury and Acute Respiratory Distress Syndrome After Traumatic Brain Injury in the United States. [Miscellaneous Article] Neurosurgery October. 2012;71(4):795–803. doi: 10.1227/NEU.0b013e3182672ae5. [DOI] [PubMed] [Google Scholar]
- 61.Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001;95(4):560–568. doi: 10.3171/jns.2001.95.4.0560. [DOI] [PubMed] [Google Scholar]
- 62.Harrison-Felix C, Whiteneck G, Devivo MJ, Hammond FM, Jha A. Causes of death following 1 year postinjury among individuals with traumatic brain injury. J Head Trauma Rehabil. 2006;21(1):22–33. doi: 10.1097/00001199-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Zhan C, Miller MR. EXcess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 64.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89(1):279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teasdale GM, Murray GD, Nicoll JAR. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005;128(Pt 11):2556–2561. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- 66.Kalish H, Phillips TM. Analysis of neurotrophins in human serum by immunoaffinity capillary electrophoresis (ICE) following traumatic head injury. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(2):194–200. doi: 10.1016/j.jchromb.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]