Abstract
Few behavioral indices of risk for autism spectrum disorders (ASD) are present before 12 months, and potential biomarkers remain largely unexamined. This prospective study of infant siblings of children with ASD (n=16) and low-risk comparison infants (n= 15) examined group differences in event-related potentials (ERPs) indexing processing of facial positive affect (N290/P400, Nc) at 9 months and their relation to joint attention at 15 months. Group differences were most pronounced for subtle facial expressions, in that the low-risk group exhibited relatively longer processing (P400 latency) and greater attention resource allocation (Nc amplitude). Exploratory analyses found associations between ERP responses and later joint attention, suggesting that attention to positive affect cues may support the development of other social competencies.
Keywords: autism, ERP, face, infant, joint attention, positive affect, risk
Social-communicative impairments are a salient and defining feature of autism spectrum disorders (ASD; Osterling & Dawson 1994; Wetherby et al. 2004). In recent years, there has been a burgeoning of neuroimaging studies with older children and adults with ASDs that demonstrate atypical patterns of neural activation and connectivity during the processing of social information, including emotional expressions (Dawson et al. 2012; Pelphrey et al. 2011). However, the relationship between later phenotypes and atypical early behaviors is unclear.
Prospective longitudinal studies of high-risk siblings provide an optimal developmental design for understanding the emergence of ASD-related behavioral characteristics. A recent study indicated that almost 1 in 5 infant siblings of children with ASD (high-risk siblings; Ozonoff et al. 2011) will receive an ASD diagnosis, which is a markedly higher rate than in the general population where the most recent estimates of diagnosis are 1 in 68 (Centers for Disease Control 2014). Although the majority of high-risk siblings will not receive a diagnosis, like other first-degree relatives of individuals with ASD, younger siblings often display subclinical deficits in social and communicative behaviors. The developmental patterns and eventual outcomes of high-risk siblings are variable, representing a continuum from typical, to mild social and communicative difficulties, to clinically significant deficits in all three domains included in the ASD diagnosis (Bolton et al. 1994; Dereu, Roeyers, Raymaekers, & Warreyn 2012; Landa & Garrett-Mayer 2006; Messinger et al. 2012; Zwaigenbaum et al. 2005).
Disturbances in social-communicative behaviors can be evident by the end of the first year of life in infants at risk for later ASD based on family history. Several studies have indicated that, as a group, high-risk siblings show lower levels of social-communication behaviors, including initiating joint attention and responding to joint attention (Ibanez, Grantz, & Messinger 2012; Presmanes et al. 2007; Rozga et al. 2011; Stone et al. 2007; Yirmiya et al. 2006), which are hallmark deficits in older children with ASD.
It has been suggested that the deficits in social-communicative behaviors exhibited by children with ASD may be rooted in diminished social motivation (Chevallier, Kohls, Troiani, Brodkin, & Schultz 2012; Mundy & Sigman 2006; Dawson et al. 2005). In examining factors that contribute to and promote social motivation, processing changes in emotional expressions appears to be key as it allows infants to receive feedback from a social partner when engaging in social-communicative behaviors such as joint attention and social referencing (Boccia & Campos 1989; Tomasello 1992). This feedback, in turn, guides and promotes infants’ attention allocation, social responsiveness, and sense of intersubjectivity (Rosen, Adamson, & Bakeman 1992; Trevarthen & Hubley 1978). Hence, the ability to detect changes in facial expressions of emotion is an important early step in the development of social cognition (Campos & Stenberg 1981; Hornik & Gunnar 1988). Less interest in and value allocation to a social partner’s positive affect has been cited as a factor that contributes to attenuated social motivation in children with ASD. A recent study examining pupillary response suggested that children with ASD exhibit reduced reactivity to the reward value of smiling faces (Sepeta et al. 2012).
Electrophysiological (EEG/ERP) studies using passive paradigms that do not require an overt response provide the unique opportunity to study the early neural correlates of social information processing and identify subtle differences in neural processing that may precede any obvious behavioral markers of ASD risk. Although there are no clear behavioral indicators of eventual ASD risk before one year of age, a variety of differences have been noted in neural processing of social information using infant sibling samples. For example, ERPs have been recorded in 9- to 12-month-old high-risk siblings during a variety of face processing tasks to examine sensitivity to faces versus objects (e.g., McCleery et al. 2009), familiar versus unfamiliar faces (Luyster et al. 2011; Key & Stone 2012a), facial features (Key & Stone 2012b), and faces varying in gaze direction (Elsabbagh et al. 2009). Of particular interest are the N290 and P400 responses, which are two consecutive ERP peaks recorded over the occipito-temporal region that index face-specific perceptual processing in infants, much like the N170 in older children and adults (see de Haan, Johnson, & Halit 2003, for a review; Csibra et al. 2008; de Haan & Nelson 1999). In addition, the frontal Nc response, thought to broadly index attention allocation in infants, tends to be reliably elicited during processing of novel social and non-social stimuli (de Haan et al. 2004; Reynolds & Richards 2005).
The existing studies show that as a group, high-risk infants show few differences in face-elicited N290 and P400 responses compared to infants at lower risk, although some evidence indicates less hemispheric lateralization (suggestive of a possible delay in neural maturation or atypical connectivity) in the responses of high-risk siblings (e.g., McCleery et al. 2009). Elsabbagh and colleagues (2009) reported that high-risk siblings showed a longer latency P400 to unfamiliar faces compared to low-risk siblings but only in response to direct gaze (vs. averted gaze) conditions, suggesting less automatic processing of faces with direct gaze. Differences in Nc amplitude also suggest that high-risk siblings might show atypical patterns of attention allocation during face processing. For example,McCleery et al. (2009) reported that high-risk siblings showed a smaller (less negative) Nc response to faces (both familiar and novel) than low-risk siblings, suggesting less overall attention allocation. Similarly,Luyster et al. (2011) reported less differentiation in Nc amplitude in high-risk siblings in response to familiar versus unfamiliar faces at midline and right hemisphere locations. However, high- and low-risk siblings do not appear to differ in their ability to detect novel faces among familiar faces (Key & Stone 2012a; Luyster et al. 2011).
No ERP study to date has examined emotional face processing in infants siblings of children with ASD. The number of such ERP studies in typical infants is limited, and they typically focus on the contrast between negative (fear, anger) vs. neutral or happy facial expressions (Nelson & de Haan 1996; de Haan et al. 2004; Leppänen et al. 2007). In 7-month-olds, Leppänen et al. (2007) reported a larger P400 response to fearful than neutral faces, while others identified Nc amplitudes as sensitive to the emotional content of the stimuli, with larger amplitudes recorded to fearful than happy faces (Nelson & de Haan 1996; de Haan et al. 2004).
The primary aim of the current study was to extend prior findings by using an ERP paradigm to study differences between high-risk and low-risk siblings in processing of positive affect cues as measured by the N290/P400 and Nc responses. A passive (no behavioral response required) oddball paradigm was used to elicit both the perceptual N290/P400 and the attentional Nc responses while infants viewed color photographs of young female adult faces with neutral and various degrees of positive facial expressions. Based on the existing behavioral evidence that children with ASD experience a particular difficulty processing mild affective expressions compared to the prototypical emotions (Wong et al. 2012), we anticipated greater group differences in response to faces with a less intense positive affect expression (i.e., small smiles) rather than stronger, full smile faces.
Furthermore, while concurrent relations between emotion processing and socialcommunicative behavior have been reported, the early developmental associations between these constructs remain unexamined in infants at risk for ASD. Therefore, the secondary aim of the current study was to explore whether the extent of processing of more subtle expressions of positive affect at 9 months, as indexed by differences in the amplitudes of N290/P400 and Nc responses to small smiles vs. neutral faces, was associated with joint attention in the second year of life. We anticipated that more extensive processing of facial affect cues evidenced by higher ERP amplitudes would be associated with higher levels of initiating and responding to joint attention at 15 months.
Method
Participants
Thirty-one infants were examined at 9 and 15 months as part of a larger multi-site longitudinal study1. Infant ERPs were recorded at age 9 months (M age= 9.09, range = 8.60 – 9.50), while behavioral data were collected at 15 months (M age= 15.32, range = 14.30–15.90).
Informed consent was obtained from parents prior to participation in the research procedures. Infants in the high-risk sibling group (n = 16) had at least one older sibling with a community diagnosis of an ASD that was confirmed via administration of the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000), Autism Diagnostic Interview-Revised (Rutter, LeCouteur, & Lord 2003), and a DSM-IV-based (APA 2000) clinical diagnosis from a licensed psychologist experienced in ASD. Infants in the low-risk sibling group (n = 15) had an older sibling with no ASD concerns (as verified by parental information and the Social Communication Questionnaire; SCQ; Berument, Rutter, Lord, Pickles, & Bailey 1999) and no family history of autism in 1st, 2nd, or 3rd degree relatives. Older siblings in both groups were at least 36 months old upon study entry. Exclusion criteria for both groups included severe sensory or motor impairments, presence of identified metabolic, genetic, or progressive neurological disorders, gestational age less than 37 weeks and birth weight below 2500 grams. Chi-square tests revealed no significant differences between high-risk and low-risk siblings on ethnicity, gender, or maternal education, ps = .11 – .55 (see Table 1). Independent t-tests indicated that there were no group differences in age at the two assessments, ps= .43 – .54. An additional 35 infants (20 high-risk, 15 low-risk siblings) were not included in this sample due to insufficient amount of usable ERP data. A chi-square indicated that risk group was unrelated to the likelihood of an infant having usable ERP data (p = .65).
Table 1.
Participant Characteristics and Demographics
| Demographics | High-Risk Siblings % / (n) |
Low-Risk Siblings % / (n) |
|---|---|---|
| Gender | ||
| Male | 56.3 / (9) | 66.7 / (10) |
| Female | 43.7 / (7) | 33.3 / (5) |
| Ethnicity | ||
| White/Non-Hispanic | 81.2 / (13) | 80 / (12) |
| African-American | 6.2 / (1) | 0 / (0) |
| Multi-racial | 12.5 / (2) | 20 / (3) |
| Parent Education | ||
| Some College | 37.5 / (6) | 6.7 / (1) |
| 2-year College | 0 / (0) | 6.7 / (1) |
| 4-year College | 37.5 /(6) | 33. 3/ (5) |
| Advanced Professional Degree | 25 /(4) | 53.3 / (8) |
Stimuli
The stimuli comprised six color photographs using two unfamiliar female models. Each model was photographed in three conditions (1) neutral expression, (2) small smile expression, and (3) full Duchenne smile that included eye constriction in addition to the smiling mouth shape (Figure 1). The photographs subtended a visual angle of 16.44° (w) × 18.62°(h). Thus, the on-screen stimuli were close to life-size and all facial features were clearly visible. Each infant saw only one of the models. The inclusion of two different models reduced the likelihood of ERP effects being attributed to unique features of a particular face.
Figure 1.
Stimulus faces.
Electrodes
A high-density array net of 124 Ag/AgCl electrodes embedded in soft sponges (Geodesic Sensor Net without the lower eye channels, EGI, Inc., Eugene, OR) was used to record infant ERPs. Electrode impedance levels were adjusted to less than 40 kOhm. Data were sampled at 250Hz with a high pass filter of 0.1Hz and a low pass filter of 100 Hz. During data collection, all electrodes were referred to Cz and then later re-referenced offline to an average reference configuration.
Procedure
Each participant was tested while seated in the parent’s lap in a darkened quiet room. One research assistant remained in the collection room throughout the testing protocol to monitor the infant’s attention and compliance. ERPs were obtained using a passive oddball paradigm with two blocks of 100 trials. The neutral face served as the standard stimulus in both blocks and was presented for 5 consecutive trials in the beginning of the experiment (“face introduction” stage) and on 70% of the trials in each block. The small or full smile stimuli served as the deviants and were presented on 30% of the trials within their respective blocks. Each stimulus was presented for 750 ms against a black background in the center of the computer screen positioned 90 cm in front of the participant. Interstimulus interval varied randomly between 1000–1300 ms to prevent habituation to stimulus onset. Stimulus sets (i.e., model face) and the order of the trial blocks were counterbalanced across the participants.
EEG recording was controlled by Net Station software (v. 4.3; EGI, Inc., Eugene, OR). Stimulus presentation was controlled by E-Prime (v. 2.0, PST, Inc., Pittsburgh, PA). During the entire test session, infants’ electroencephalogram (EEG) and behavior were continuously monitored and stimulus presentation occurred only when the infant was quiet and looking at the monitor. During periods of inattention and/or motor activity, stimulus presentation was suspended, and the researcher in the testing room redirected infants to the computer screen using a wand with flashing spinning lights or verbal directions (e.g., “Look! Who is that?”).
ERP Data Analysis
Recorded EEG files were low-pass filtered at 30Hz, and individual ERPs were derived by segmenting the ongoing EEG on each stimulus onset to include a 100-ms prestimulus baseline and a 900 ms post-stimulus interval. To avoid biasing the results due to a largely uneven number of standard and deviant trials (Thomas, Grice, Najm-Briscoe, & Miller 2004), only the standard trials immediately preceding a deviant stimulus were selected for the analysis. Individual trials were screened for artifacts first using NetStation tools; the results were then verified by a manual review. Trials contaminated by eye or movement artifacts (eye channel voltage in excess of 140 μV) or containing more than 15 bad channels (12% of the electrodes) were excluded from the analysis. For the remaining trials, data from bad channels (voltage shifts exceeding 200 μV) were reconstructed using spherical spline interpolation procedures. After artifact rejection, the remaining ERPs were referenced to an average reference and baseline corrected. For an infant to be included in the final analyses, a minimum of 10 usable trials had to be available for each condition (neutral, small smile, big smile). Trial retention rates were similar for the small (M= 12.39, SD=3.64) and full smile (M= 11.94, SD=2.93) conditions, pair-wise t (30) =.569, p =.574, but more trials were retained in the neutral (M = 22.39, SD=10.30) condition, pair-wise t-tests vs. small smile t(30)=6.812, p <.001, vs. full smile t(30)=6.378, p <.001, as would be expected since separate neutral trials preceded small vs. full smile trials. One-way ANOVA indicated that there were no risk group differences in the number of trials retained for the small (MHigh Risk = 13.31, SD=4.38, MLow Risk = 11.40, SD=2.41, F(1,29)=2.225, p = .147) or full smiles (MHigh Risk = 12.31, SD=2.98, MLow Risk = 11.53, SD=2.92, F(1,29)= 0.538, p = .469). More neutral trials were retained for high-risk (M = 26.56, SD=11.61) than low-risk siblings (M = 17.93, SD=6.46, F(1,29)= 6.413, p = .017); however, individual differences in the number of neutral trials retained did not correlate with ERP responses to neutral faces.
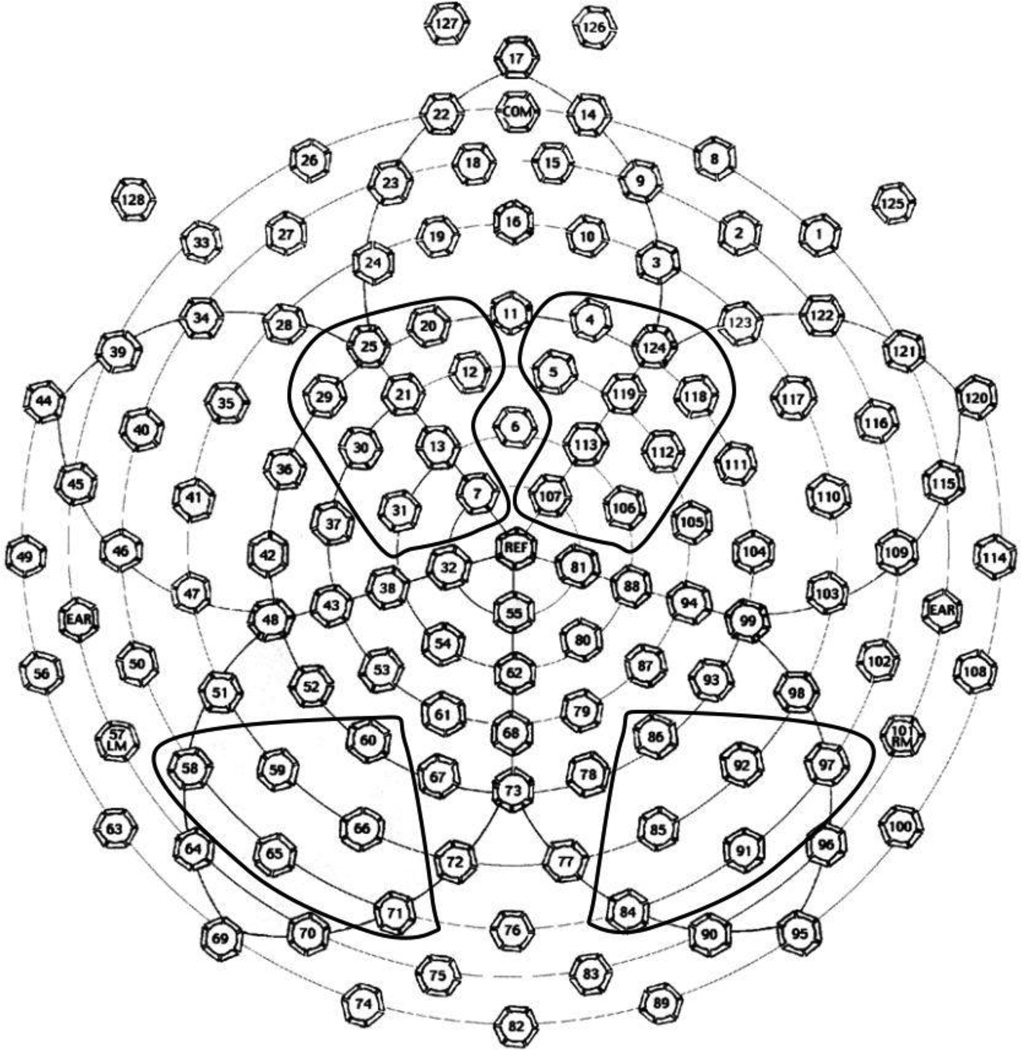
To reduce the number of variables in the statistical analyses, data from 124 electrodes were limited to a priori selected sets of electrode clusters (Figure 2) corresponding to scalp locations previously used to examine face processing in 9-month-old infants (Key, Stone, & Williams 2009) and known as the optimal sites for face-sensitive occipito-temporal N290/P400 response (Halit, de Haan, & Johnson 2003; Scott & Nelson 2006) and the novelty-sensitive frontal-central Nc peak (de Haan & Nelson 1997; de Haan et al. 2003). Next, within each electrode cluster, peak latency (ms) and mean amplitude (μV) measures were obtained for N290 (250–350ms), P400 (350–500ms), and Nc (500–800ms) peaks using NetStation statistical extraction tool. Latency windows were determined based on the examination of the grand-averaged waveform and in line with previously published studies (e.g., Key & Stone 2012b).
Figure 2.
128-electrode net layout and the electrode clusters used in the analyses.
Behavioral assessments
Early Social Communication Scales (ESCS; Mundy et al. 2003; Mundy et al. 2007)
The ESCS was used to measure responding to joint attention (RJA) and initiating joint attention (IJA). The ESCS is a 15–25 minute procedure that can be used with children with a verbal age of 8–30 months during which an examiner engages the child in a semi-structured interaction with a standardized toy set. Tasks are designed to elicit triadic attention with the examiner through the use of high-interest objects such as wind-up toys (for IJA) and colorful posters (for RJA). The ESCS was scored from video by coders who were blind to the risk status of the participants. For this study, measures of IJA and RJA were used to assess social-communicative functioning at 15 months of age.
RJA was coded when infants followed the examiner’s point combined with a vocalization (i.e., the child’s name) to a distal stimulus. IJA was coded when infants shared interest in an object or event by either: (1) directing eye contact toward the examiner with or without the use of gestures (e.g., pointing or showing); or (2) pointing to an object with or without eye contact. Instances in which the experimenter’s overt behaviors (e.g., talking or moving) may have elicited the infant’s attention were not coded. RJA was tabulated as the number of correctly followed trials (out of 8). The total number of IJA acts was indexed as a rate per minute. Descriptive statistics are presented separately by risk group, as well as for the full sample, in Table 2. Twenty percent of ESCSs were double coded to assess interobserver reliability. Mean absolute intra-class correlations indicated that reliability was high was across both ages for RJA (M = .95) and IJA (M = .91).
Table 2.
Means and Standard Deviations for scores on behavioral assessments of social-communicative functioning at 15 months of age.
| High-Risk siblings | Low-Risk siblings | Full Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | n | M | SD | |
| ESCS IJA Total (rpm) | 12 | 1.29 | 0.69 | 9 | 1.28 | 0.96 | 21 | 1.29 | .79 |
| ESCS RJA (correct looks) | 12 | 3.08 | 2.23 | 8 | 3.88 | 2.75 | 20 | 3.40 | 2.41 |
Note. ESCS - Early Social Communication Scales; IJA – initiating joint attention; RJA – responding to joint attention; rpm – rate per minute. There were no significant differences between High-Risk siblings and Low-Risk siblings on IJA or RJA, ps= .49–.97.
Results
ERP Responses
In order to examine risk group differences in processing of positive affect in facial expressions, latency and mean amplitude data were examined in separate repeated measures ANOVAs with Risk Group (2: high-risk, low-risk siblings) as the between-subject factor and Stimulus (3: neutral, small smile, full smile) and Electrode (2: left/right posterior occipital clusters for N290/P400 or left/right fronto-central locations for Nc) as the within-subject factors. Huynh-Feldt corrections for violations of sphericity were used. Significant main effects and interactions were followed by planned comparisons using one-way ANOVAs and within-group paired t-tests to compare ERP responses to the smile targets relative to each other and the neutral face. Means and standard deviations for amplitude and latency are presented separately for stimulus type and risk group in Table 3.
Table 3.
Means and Standard Deviations for N290, P400, and Nc Mean Amplitudes and Latencies
| Neutral Face | Small Smile | Full Smile | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-risk siblings |
Low-risk siblings |
High-risk siblings |
Low-risk siblings |
High-risk siblings |
Low-risk siblings |
||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| N290 | |||||||||||||
| mean amplitude |
L H |
3.90 | 5.26 | 1.28 | 5.80 | 2.93 | 5.47 | 6.19 | 7.58 | 3.68 | 5.42 | 5.23 | 10.55 |
| R H |
2.65 | 7.95 | −0.85 | 7.05 | 2.56 | 4.79 | 4.03 | 7.05 | 1.70 | 7.01 | 1.44 | 9.91 | |
| latency | L H |
296.88 | 30.26 | 285.11 | 15.28 | 299.77 | 31.99 | 281.42 | 25.59 | 301.37 | 20.39 | 290.62 | 25.10 |
| R H |
294.15 | 32.84 | 285.09 | 26.85 | 297.38 | 23.91 | 282.71 | 24.91 | 303.92 | 33.25 | 293.04 | 29.38 | |
| P400 | |||||||||||||
| mean amplitude |
L H |
6.68 | 3.91 | 7.50 | 4.73 | 5.25 | 8.12 | 12.80 | 10.82 | 7.25 | 7.76 | 9.92 | 10.83 |
| R H |
5.96 | 5.34 | 3.35 | 6.96 | 5.05 | 7.06 | 9.31 | 7.50 | 4.47 | 7.48 | 4.13 | 10.03 | |
| latency | L H |
421.69 | 35.14 | 446.22 | 27.14 | 414.06 | 36.66 | 444.62 | 40.23 | 451.75 | 41.12 | 429.89 | 40.81 |
| R H |
429.06 | 40.20 | 422.64 | 44.63 | 420.81 | 37.53 | 436.69 | 31.65 | 438.31 | 34.05 | 427.44 | 42.12 | |
| Nc | |||||||||||||
| mean amplitude |
L H |
−0.17 | 5.61 | 3.83 | 4.88 | 0.38 | 7.78 | −1.51 | 5.74 | −1.40 | 5.70 | 1.46 | 4.53 |
| R H |
−1.63 | 3.86 | −1.93 | 6.77 | −0.95 | 5.58 | −5.23 | 6.97 | −3.12 | 5.46 | −3.12 | 8.17 | |
| latency | L H |
557.97 | 51.29 | 563.90 | 57.20 | 594.68 | 63.55 | 611.99 | 85.02 | 622.15 | 51.50 | 602.53 | 82.44 |
| R H |
559.61 | 38.31 | 577.13 | 69.17 | 605.24 | 55.78 | 640.00 | 79.89 | 595.81 | 77.86 | 612.67 | 68.37 | |
Note. LH= Left Hemisphere; RH= Right Hemisphere
N290/P400
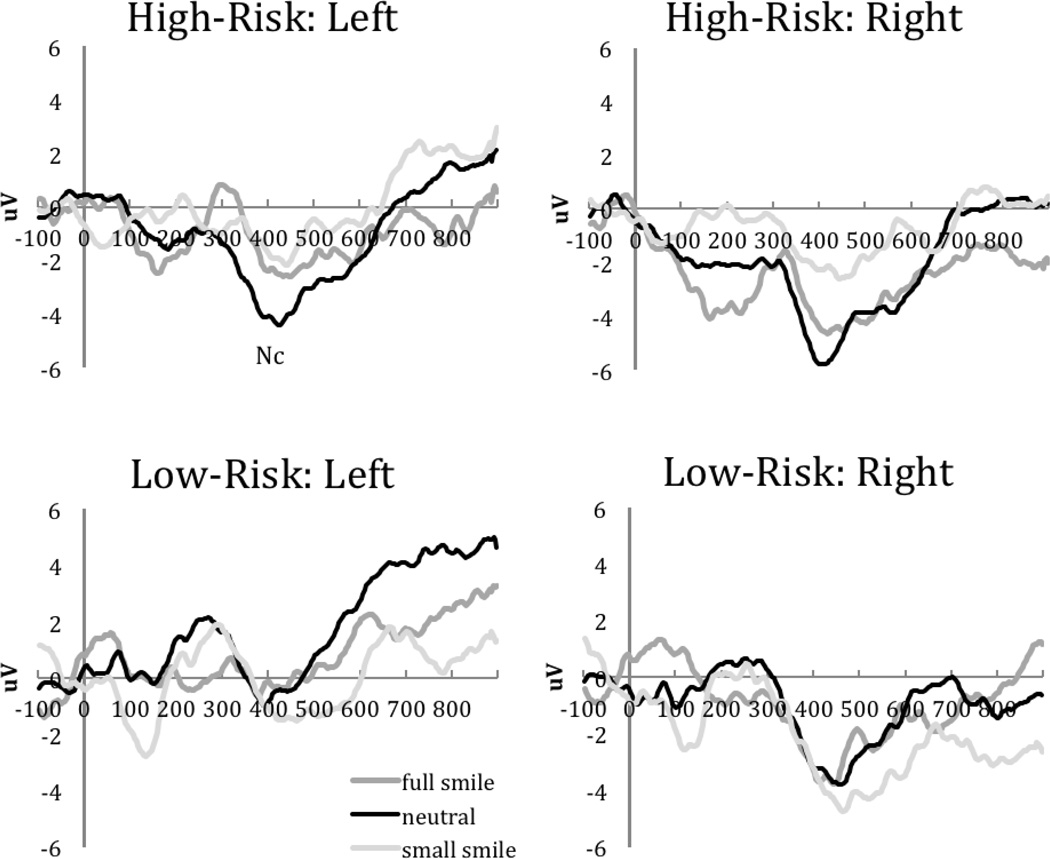
There were no significant main effects or interactions involving Risk Group or Stimulus for posterior N290 amplitude and latency or P400 amplitude, ps=.095-.960. A Stimulus × Risk Group interaction was present for the latency of the P400, F(2, 58) = 4.10, p =.027, η2 =.124. Post hoc between-group analyses indicated that high-risk siblings (M = 417.44 ms, SD=31.30) had a shorter P400 latency to small smiles than low-risk siblings (M= 440.66 ms, SD = 29.06), F(1, 30)= 4.57, p =.041 (Figure 3). In addition, within-group comparisons revealed that only high-risk siblings showed a significantly shorter latency to small smiles (M = 417.44 ms, SD=31.30) than to full smiles (M = 445.03 ms, SD=30.71), t(15)= 2.36, p =.032, Cohen’s d =.591. Low-risk siblings did not show this stimulus discrimination, p = .239.
Figure 3.
Posterior N290/P400 ERP responses for high-risk (top row) and low-risk (bottom row) siblings.
Nc
Mean amplitude of the Nc was characterized by a Stimulus × Risk Group interaction, F(2, 58) = 3.089, p =.053, η2 =.096. Post hoc between-group analyses revealed no group differences in Nc amplitude based on stimulus type, p = .14–.42. However, within-group comparisons revealed that only low-risk siblings showed a larger Nc response to small smiles (M= −3.37 μV, SD=5.03) compared to the neutral face (M =.94 μV, SD=4.69), t(14)=2.78, p =.015, Cohen’s d =.718 (Figure 4). High-risk siblings did not show this stimulus discrimination, p = .742.
Figure 4.
Fronto-central Nc responses for high-risk (top row) and low-risk (bottom row) siblings.
Across all participants, there was a main effect of stimulus on the Nc latency, F(2, 58) = 6.41, p =.003, η2 =.181. Infants had shorter Nc latencies to neutral faces (M= 564.46 ms, SD=45.32) compared to both small smiles (M= 612.56 ms, SD=64.62) and full smiles (M = 608.31 ms, SD=56.49), t(30)=3.23, p =.003, Cohen’s d =.581 and t(30) = 3.56, p=.001, Cohen’s d =.640, respectively. There were no significant group differences in the latency of Nc response (p=.267).
Brain-Behavior Associations
In an exploratory analysis, hierarchical linear regressions were conducted using the full sample to investigate whether individual differences in facial affect processing at 9 months were associated with joint attention as measured by ESCS at 15 months, and whether these associations were moderated (i.e., differed in strength) by risk group membership. Processing of facial affect was quantified as differences between the small smile and neutral face for N290, P400, and Nc amplitudes. To reduce the number of ERP variables, we created composite scores by averaging ERPs across hemispheres since there were no significant stimulus-related effects that varied by hemisphere. Across the full sample, there were no univariate or multivariate outliers on the ERP composites, IJA, or RJA variables, and each variable had acceptable values of skewness and kurtosis as indicated by Tabachnick and Fidell (2001).
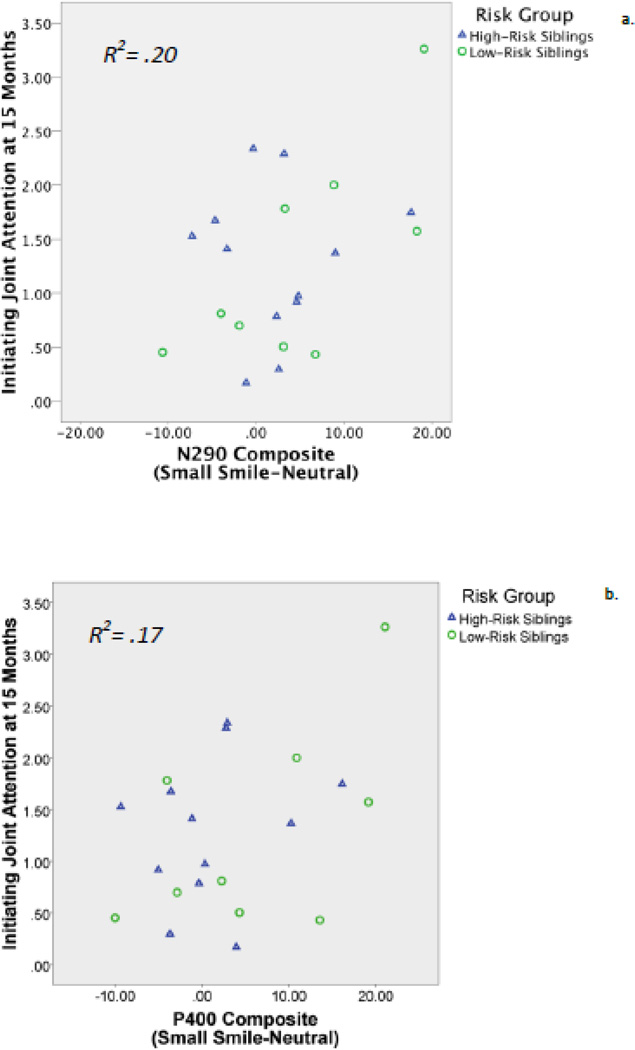
Results revealed that initiating joint attention at 15 months was predicted by both the N290 and P400 responses. A linear regression indicated that larger N290 to small smiles than to neutral faces predicted higher IJA scores, R2 = .20, F(1, 19) = 4.68, β = .45, p = .04 (Figure 5a). This association was not moderated by risk group, as including risk group and its interaction with the N290 composite score as predictors in the regression did not significantly improve the model’s prediction of IJA scores, R2Δ = .11, p = .09. A linear regression also indicated that there was a tendency for larger P400 to small smiles compared to neutral faces to predict higher IJA scores, R2 = .17, F(1, 19) = 3.90, β = .41, p = .06 (Figure 5b). This marginally significant association was not moderated by risk group, as including risk group and its interaction with the P400 composite score as predictors in the regression did not significantly improve the model’s prediction of IJA scores, R2Δ = .04, p = .25. There were no significant associations between Nc responses and IJA scores, βs= −.24 – −.25, or between any of the ERP amplitudes and RJA scores, βs= −.26–.29.
Figure 5.
Associations between ERP composite scores and later initiating joint attention.
Discussion
The purpose of this study was to examine individual differences in positive affect processing in infants at high and low risk for ASD, and to determine whether such differences were associated with later social-communicative functioning. Our results revealed that risk group differences were most pronounced for subtle facial expressions, in that compared to the high-risk group, the low-risk group exhibited relatively longer processing (P400 latency) and greater attention resource allocation (Nc amplitude) to small smiles relative to neutral expressions. Additionally, regression analyses provided preliminary evidence that individual differences in ERPs at 9 months may be associated with social-communicative functioning at 15 months, suggesting that more extensive processing of positive affect cues may support the development of other social competencies.
Findings related to the amplitude and latency of ERP components associated with affective face processing and attention allocation were generally in line with results from older, nonclinical participants (Eimer & Holmes 2002) and with other studies of face processing in infants at high risk for ASD (Key & Stone 2012a; Luyster et al. 2011). There were no significant condition or risk group differences in the amplitude or latency of the N290 response. The infant N290 is considered to be a developmental precursor of the adult N170 response, and prior studies have demonstrated that N170 is usually not affected by emotional content (Eimer & Holmes 2002). The lack of high-risk versus low-risk group differences in the N290 response is also consistent with prior infant sibling studies (Luyster et al. 2011; McCleery et al. 2009) that reported no group differences in the amplitude or latency of the N290 response to faces (but see McCleery et al. for evidence of group differences in the N290 latency to objects).
The current study did not reveal amplitude modulation of the P400 by facial expression. This finding is unsurprising as in combination with the N290, this peak shares characteristics of the adult N170 (Halit, deHaan & Johnson 2003). Although P400 amplitude may modulate based on familiarity, with novel stimuli eliciting larger P400 responses (Key & Stone 2009; Scott et al. 2006), such novel vs. familiar face contrasts typically involve significant changes in facial features (e.g., placing the eyes from one face into a more familiar face). Larger P400 responses to fearful vs. neutral faces (Leppänen et al. 2007) could also be attributed to greater physical change in facial features. In the present study, physical stimulus differences were more subtle, because the same face was presented in neutral and emotional conditions, resulting in increased physical similarity and subjective familiarity across conditions.
In typically developing individuals, affective differences in facial expressions are typically observed in responses later than the N170. Consistent with this, our results indicated that the P400 latency and the Nc amplitude and latency were sensitive to differences in the degree of positive affect, although they were manifested differently across the two risk status groups. Compared to low-risk siblings, high-risk infants evidenced significantly shorter P400 latencies for small smiles, and within the high-risk sample, P400 latencies were significantly shorter to the small smiles than to the full smile faces. Accelerated P400 response could reflect less extensive perceptual analysis of or memory search in response to the small smile stimulus. Similar faster than typical P400 latencies to infrequent novel faces were recently reported in 9-month-old siblings of children with ASD during a mother-stranger face discrimination task (Key & Stone, 2012a). The authors attributed such differences to increased reliance on the general perceptual properties (vs. distinguishing features) of faces in the high-risk infants. Our proposed interpretation of differences in processing of facial details relevant to the detection of the smile expression (e.g., the extent of lip corner retraction, presence of infra-orbital furrowing beneath the cheeks, and eye constriction) suggests that more exaggerated facial expressions may better support social information processing in the high-risk infant sibling group. This is also consistent with the results of a recent behavioral study in older children with ASD that noted a particular difficulty processing mild affective expressions compared to more pronounced emotional displays (Wong et al. 2012).
The amplitude of the Nc was also sensitive to risk group differences. Specifically, only low-risk siblings generated a larger Nc response to small smiles relative to neutral faces. This finding suggests that infants allocated more processing resources and/or recognized the subtle small smile expression as less familiar relative to both the frequently presented neutral faces and the less subtle (but equally rare) full smile target faces. The absence of a comparable response in high-risk siblings may suggest differences in the depth of processing of the small smile face. Possibly, high-risk siblings did not pursue additional cognitive evaluation of the faces beyond the perceptual categorization indexed by P400 latency.
All participants in our sample showed shorter Nc latencies to the neutral face compared to both the small and large smile faces. This finding suggests that regardless of risk status, infants perceived the emotional faces as a different from neutral and allocated more time to processing the rare emotional faces.
Brain-Behavior Associations
Our exploratory analyses suggest that there may be developmental associations between brain measures of facial affect processing at 9 months and initiating joint attention at 15 months. Specifically, greater resource allocation to processing of small smile vs. neutral faces, as indexed by increased ERP amplitude differences, predicted higher levels of initiating joint attention at 15 months of age. This pattern of results may provide support for the suggested role of emotional processing in the early development of social-communicative behaviors. Although future studies with larger samples will be required to replicate these findings, the results suggest that attention to positive affect may play a role in the early development and continued maintenance of social motivation, which in turn may lead to more opportunities for joint attention episodes and subsequent joint attention bids (Dawson et al. 2004).
Limitations
Although many of our findings are consistent with the existing literature, the present study has several limitations. While comparable to other ERP studies, our attrition rate due to insufficient number of artifact-free ERP trials was relatively high. The attrition is thought to be due mainly to infants’ general fatigue, as the ERP sessions were preceded by a number of behavioral assessments, combined with potential boredom with the stimuli given the repetitive nature of the oddball design. In the future, recording ERPs during a separate visit or prior to extensive behavioral testing may be more optimal. Using a more visually diverse paradigm could also increase participant retention. There appears to be no systematic relationship between ASD risk status and the likelihood of completing the EEG protocol, and future studies will need to address the minimum amount of electrophysiological data and the optimal sample size needed to obtain reliable group comparisons and predictive associations. In addition, the sample of high-risk siblings was relatively small, and given the estimated prevalence rates for these infants, only a few infants will receive a later ASD diagnosis. A follow-up diagnostic assessment at 36 months of age is scheduled for infants in our sample and will allow us to examine the relation between individual differences in brain responses to faces with various degrees of positive affect and later developmental outcomes. However, it is important to note that results from previously published studies of high-risk siblings suggest that early group differences may be due to the elevated genetic vulnerability of the group as a whole and are not necessarily driven by the minority who receive a later diagnosis of ASD (Stone et al. 2007; Georgiades et al. 2012). Thus, even though most high-risk siblings will not receive an ASD diagnosis, the group as a whole may evidence altered brain mechanisms underlying face processing (e.g., see Dawson et al. 2005 for evidence of atypical face processing in unaffected parents of children with ASD). Further, recent studies suggest that tracking individual trajectories of growth (or lack thereof) in children’s behavioral or physiological processing may provide an important index of relative risk for ASD (e.g., Dereu et al. 2012; Ibanez et al. 2012; Tierney, Gabarb-Durnam, Vogel-Farely, Tager-Flusberg, & Nelson 2012). Therefore, repeated assessments of both neural and behavioral markers of affective information processing and language and social-communicative behaviors may be more sensitive to the mechanisms underlying relative levels of risk and resilience among infants with a family history of ASD.
Conclusion
In sum, our present findings suggest that high-risk and low-risk siblings may differ in their processing of positive affect prior to 12 months of age. These differences are most pronounced for the more subtle, small smiles, which required longer processing time and/or more processing resources. The ability to detect the subtle changes in facial expressions may allow an infant to better process the social input and feedback provided by his or her social partner. The individual differences in brain activity observed at 9 months were predictive of social-communicative functioning at 15 months of age, suggesting that early ability to process various degrees of positive affect cues may support the development of other social competencies. Future studies will need to examine the relative contributions of neural responses, behavioral performance, and genetic vulnerability in predicting diagnostic outcomes of ASD.
Acknowledgments
This research was supported by National Institute of Child Health and Human Development Grant R01 HD057284 to Wendy L. Stone and Daniel S. Messinger.
We would like to thank the participants and their families for their support of the study. We are grateful to Amber Vinson, Dorita Jones, Michelle Fong, Kim Ono, Devon Gangi, and Nicole Coman for their help with acquisition and processing of the ERP data.
Footnotes
Consistency in the recruitment procedures and inclusion/exclusion criteria as well as in data acquisition procedures was ensured by joint in-person training on all study procedures for the research staff at the three sites. Standardized behavioral assessments at each site were administered by trained clinical psychologists, ensuring strict adherence to the established procedural guidelines. All EEG data were processed and analyzed by the single lab, ensuring consistency in artifact detection procedures.
Contributor Information
Alexandra P. Key, Email: sasha.key@vanderbilt.edu, Vanderbilt Kennedy Center for Research on Human Development and Department of Hearing and Speech Sciences, Vanderbilt University, 230 Appleton Place / PMB 74, Nashville, TN 37203, Phone: 615-322-3498.
Lisa V. Ibanez, Email: lisavibanez@gmail.com, Department of Psychology, University of Washington, CHDD Box 357920, Seattle, WA 98195-7920, USA.
Heather A. Henderson, Email: h.henderson@miami.edu, University of Miami, Department of Psychology, P.O. Box 248185, Coral Gables, FL 33124-0751, Phone: 305-284-2814.
Zachary Warren, Email: zachary.e.warren@vanderbilt.edu, Treatment and Research Institute for Autism Spectrum Disorders (TRIAD), Vanderbilt Kennedy Center, 230 Appleton Place / PMB 74, Nashville, TN 37203-5721, Phone: 615.936.0267.
Daniel S. Messinger, Email: dmessinger@miami.edu, University of Miami, P.O. Box 249229, Coral Gables, FL 33101-0721, Phone: 305-284-8443.
Wendy L. Stone, Email: stonew@u.washington.edu, Department of Psychology, University of Washington, CHDD Box 357920, Seattle, WA 98195-7920, USA.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision. Washington, DC: Author; 2000. [Google Scholar]
- Berument S, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. The British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Boccia M, Campos JJ. Maternal emotional signals, social referencing, and infants' reactions to strangers. New Directions for Child Development. 1989;44:25–49. doi: 10.1002/cd.23219894404. [DOI] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case-control family history study of autism. Journal of Child Psychology and Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Campos J, Stenberg C. Perception, appraisal and emotion: The onset of social referencing. In: Lamb ME, Sherrod LR, editors. Infant Social Cognition. Hillsdale, NJ: Erlbaum; 1981. [Google Scholar]
- Centers for Disease Control and Prevention. Autism. 2014 Retrieved from http://www.cdc.gov/ncbddd/autism/addm.html.
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G, Kushnerenko E, Grossmann T. Electrophysiological methods in studying infant cognitive development. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2nd Ed. MIT Press; 2008. pp. 247–262. [Google Scholar]
- Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17(3):679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, Kamara D, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(11):1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- de Haan M, Belsky J, Reid V, Volein A, Johnson MH. Maternal personality and infants' neural and visual responsivity to facial expressions of emotion. Journal of Child Psychology and Psychiatry. 2004;45:1209–1218. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother's face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68(2):187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35(4):1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- Dereu M, Roeyers H, Raymaekers R. Exploring individual trajectories of social communicative development in toddlers at risk for autism spectrum disorders. Research in Autism Spectrum Disorders. 2012;6(3):1038–1052. [Google Scholar]
- Eimer M, Holmes A. An ERP study on the time course of emotional face processing. NeuroReport. 2002;13(4):427–431. doi: 10.1097/00001756-200203250-00013. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, Krljes S, et al. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65(1):31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, Zwaigenbaum L, Bryson S, Brian J, Roberts W, Smith I, et al. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. Archives of General Psychiatry. 2012:E1–E7. doi: 10.1001/2013.jamapsychiatry.1. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Cortical specialization for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage. 2003;19(3):1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Hornik R, Gunnar MR. A descriptive analysis of infant social referencing. Child Development. 1988;59:626–634. [PubMed] [Google Scholar]
- Ibanez LV, Grantz CJ, Messinger DS. The development of referential communication and autism symptomatology in high-risk infants. Infancy. 2012 doi: 10.1111/j.1532-7078.2012.00142.x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A, Stone W. Processing of novel and familiar faces in infants at average and high risk for autism. Developmental Cognitive Neuroscience. 2012a;2:244–255. doi: 10.1016/j.dcn.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A, Stone W. Same but different: Nine-month-old infants at low and high risk for autism look at the same facial features but process them using different brain mechanisms. Autism Research. 2012b;5:253–266. doi: 10.1002/aur.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A, Stone W, Williams S. What do infants see in faces? ERP evidence of different roles of eyes and mouth for face perception in 9-month-old infants. Infant and Child Development. 2009;18:149–162. doi: 10.1002/icd.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Leppänen J, Moulson M, Vogel-Farley V, Nelson C. An ERP study of emotional face processing in the adult and infant brain. Child Development. 2007;78(1):232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Luyster R, Wagner J, Vogel-Farley V, Tager-Flusberg H, Nelson C. Neural correlates of familiar and unfamiliar face processing in infants at risk for autism spectrum disorders. Brain Topography. 2011 doi: 10.1007/s10548-011-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery J, Akshoomoff N, Dobkins K, Carver L. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry. 2009;66:950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger D, Young G, Ozonoff S, Zwaigenbaum L, Dobkins K, Carter L, Charman T, Landa R, Strauss M, Constantino J, Hutman T, Bryson S, Iverson J, Carver L, Rogers S, Sigman M, Stone W. Beyond ASD: Developmental outcomes of high risk siblings; Paper presented at the International Meeting for Autism Research; Toronto, Canada. 2012. [Google Scholar]
- Mundy P, Fox N, Card J. EEG coherence, joint attention and language development in the second year. Developmental Science. 2003;6:48–54. [Google Scholar]
- Mundy PC, Henderson HA, Inge AP, Coman DC. The modifier model of autism and social development in higher functioning children with autism. Research & Practice for Persons with Severe Disabilities. 2007;32:124–139. doi: 10.2511/rpsd.32.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Gwaltney M, Henderson H. Self-referenced processing, neurodevelopment and joint attention in autism. Autism. 2010;14(5):408–429. doi: 10.1177/1362361310366315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M. Joint attention, social competence, and developmental psychopathology. In: Mundy P, Sigman M, editors. Developmental psychopathology, Vol 1: Theory and method. 2nd ed. Hoboken, NJ: US:John Wiley & Sons Inc.; 2006. pp. 293–332. [Google Scholar]
- Nelson CA, de Haan M. Neural correlates of infants' visual responsiveness to facial expressions of emotion. Developmental Psychobiology. 1996;29:577–595. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–57. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Rogers SJ, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128(3):488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Constraining heterogeneity: The social brain and its development in autism spectrum disorder. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(6):631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, Yoder PJ. Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Reynolds G, Richards J. Familiarization, attention, and recognition memory in infancy: An event-related potential and cortical source localization study. Developmental Psychology. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WD, Adamson LB, Bakeman R. An experimental investigation of infant social referencing: Mother's messages and gender differences. Developmental Psychology. 1992;28:1172–1178. [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, Sigman M. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders. 2011;41(3):287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview–Revised (ADI–R) Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Scott L, Nelson C. Featural and configural face processing in adults and infants: A behavioral and electrophysiological investigation. Perception. 2006;35:1107–1128. doi: 10.1068/p5493. [DOI] [PubMed] [Google Scholar]
- Sepeta L, Tsuchiya N, Davies MS, Sigman M, Bookheimer SY, Dapretto M. Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. Journal of Neurodevelopmental Disorders. 2012;4:17. doi: 10.1186/1866-1955-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WL, Ousley OY, Littleford CD. Motor imitation in young children with autism: What's the object? Journal of Abnormal Child Psychology. 1997;25(6):475–485. doi: 10.1023/a:1022685731726. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social communicative and cognitive development of younger sibling of children with autism spectrum disorders. Archive of Pediatrics and Adolescent Medicine. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, Osterlind SJ. Using multivariate statistics. Allyn and Bacon; 2001. [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: An endophenotype of autism spectrum disorder. PLoS ONE. 2012;7(6):e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Grice J, Najm-Briscoe R, Miller J. The influence of unequal numbers of trials on comparisons of average event-related potentials. Developmental Neuropsychology. 2004;26:753–774. doi: 10.1207/s15326942dn2603_6. [DOI] [PubMed] [Google Scholar]
- Tomasello M. The social bases of language acquisition. Social Development. 1992;1:67–87. [Google Scholar]
- Trevarthen C, Hubley P. Secondary intersubjectivity: confidence, confiding and acts of meaning in the first year. In: Lock A, editor. Action, Gesture and Symbol: The Emergence of Language. New York and London: Academic Press; 1978. [Google Scholar]
- Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. Journal of Autism and Development Disorders. 2004;34(5):473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- Wong N, Beidel DC, Sarver DE, Sims V. Facial emotion recognition in children with high functioning autism and children with social phobia. Child Psychiatry and Human Development. 2012;43(5):775–794. doi: 10.1007/s10578-012-0296-z. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]