Abstract
The aim of this study was to assess the predictive effect of the EBMT risk score on the outcomes of allogeneic stem cell transplantation in a relatively homogenous group of acute myelogenous leukemia (AML) patients regarding the occurrence of acute and chronic graft versus host disease (GVHD).
This historical cohort study included adult patients (≥ 15 years old) with AML (n=363) who received allogeneic peripheral blood stem cell transplantation from HLA-identical sibling donors in the first or higher complete remission following myeloablative conditioning regimens between 2004 and 2011.The patients recruited in this study were followed-up until January 2013. Patients with acute promyelocytic leukemia (APL) were excluded from the study. Early outcomes until day +100 and events after day +100 were regarded for acute and chronic GVHD, respectively. A multi state model for competing risks was applied.
We found that the EBMT risk score was a good predictor for overall survival (OS) and relapse incidence; however, it was not associated with transplant-related mortality (TRM). The EBMT risk score was not associated with acute and chronic GVHD. For early outcomes, the predictive effect of the EBMT risk score was not statistically significant in the presence of acute GVHD; however, in the presence of chronic GVHD, it was a significant predictor of relapse but not for TRM. It seems that the effect of EBMT risk score on OS and relapse incidence cannot be affected by GVHD. Although the results were insignificant, there was evidence that the EBMT risk score can predict early outcomes, while for late outcomes, it works well for relapse and OS but not for TRM.
Keywords: Acute myeloid leukemia, Peripheral blood stem cell transplantation, Graft versus host disease, Survival analysis, Competing risks, Multistate model
INTRODUCTION
Allogeneic stem cell transplantation (SCT) from an HLA-identical sibling donor following a myeloablative conditioning regimen is a powerful treatment in reducing the risk of relapse in patients with acute myelogenous leukemia (AML), especially in first complete remission (CR).1-4 However, the main part of this benefit is affected by complications due to allogeneic SCT. These complications which are related to toxicity, infections and graft-versus-host disease (GVHD) may cause transplant-related mortality (TRM).5, 6 Moreover, peripheral blood stem cells (PBSC), as a source of hematopoietic stem cells, has increasingly replaced bone marrow (BM) in allogeneic SCT for more than one decade7-10 which causes faster neutrophil and platelet recovery.3, 7-11 Lack of anemia, no anesthesia and hospitalization for donors and cost reduction are among the other advantages of PBSCT compared to BMT.12 Nevertheless, because of the greater number of T-cells in PBSCT, there is concern that the allogeneic PBSCT results in higher rates and severity of GVHD.7
Decision whether to go forward with allogeneic SCT can be improved by assessing the potential risks before allogeneic SCT.13-16 The European group of blood and marrow transplantation (EBMT) risk score for CML was introduced more than 10 years ago17 which was then extended to other malignancies, especially acute leukemias.15, 18 The EBMT risk score includes the recipient’s age, donor/recipient gender combinations, disease stage at the time of transplantation, donor type, and the time interval from diagnosis to transplant. As Gratwohl18 mentioned, pre-transplant factors can be influenced by transplant techniques, conditioning regimen, GVHD prevention and stem cell source.
The aim of the present study was to assess the predictive effect of the EBMT risk score on the results of AML patients who underwent allogeneic PBSCT from HLA-identical sibling donors with the same transplant technique, conditioning regimen and GVHD prophylaxis regarding the occurrence of acute and chronic GVHD.
PATIENTS AND METHODS
This historical cohort study was conducted on 377 patients who were transplanted at the Hematology- Oncology and Stem Cell Transplantation Research Center (Tehran, Iran), a tertiary referral center, from January 2004 to December 2011 and were followed up until January 2013. Patients’ and donors’ information including demographic characteristics and clinical data before and after transplant were collected from hospital archives, follow-up clinic records and database. Moreover, their disease and survival status were completed until Jan 2013. The eligibility criteria included AML adult patients (≥ 15 years old) other than acute promyelocytic leukemia in the first or higher CR who were transplanted from an HLA-identical sibling donor with PBSC. They were all administered a myeloablative conditioning regimen including oral BU 4 mg/kg/day for 4 days from day -6 to -3 and intravenous CY 60 mg/kg/day for 2 days from -3 to -2 before transplant. All patients received GVHD prophylaxis regimen containing intravenous cyclosporine 1.5 mg/kg/day from day -3 which increased to 3 mg/kg/day from day +8 plus a short course of methotrexate 10 mg/m2 on day +1 and 6 mg/m2 on days +3, +6, and +11. Cyclosporine (6 mg/kg/day) was continued orally as soon as oral tolerance until day +80. It was then followed by a tapering dose until 6 to 7 months after HSCT and discontinued in the absence of GVHD. The protocol of this study was approved by the Research Board of Tehran University of Medical Sciences.
The neutrophil recovery time was defined as the time to the first day of 3 consecutive post SCT days with a persistent ANC count ≥0.5 × 109/L, and the platelet recovery time was defined as the time to the first day of platelet count ≥20 × 109/L independent of platelet transfusions for at least the last 7 days.
Acute GVHD (aGVHD) was classified from grade 0 to 4 according to the Seattle criteria.19 Chronic GVHD (cGVHD) in types of de novo, progressive, and interrupted was graded as limited or extensive20 and was defined for patients who survived at least 100 days after transplant.
The EBMT risk score ranged from 0 for good to 7 for the worst with the following pre-transplant risk factors; recipient’s age below 20, 20 to 40, and above 40 years scored 0, 1, and 2, respectively. Female donor to male recipient scored 1, and 0 was given to other gender combinations. Patients transplanted in CR1, CR2, and CR3+ scored 0 to 2, respectively. A time interval more than 1 year from diagnosis to transplant received 1 score; however, this scoring was not applicable for patients in CR1. Donor types other than an HLA-identical sibling scored 1. As only transplants from HLA-identical siblings were considered, the latter item was zero in this study. The EBMT risk score was categorized into three groups of zero or 1, 2, and 3 or greater scores (3+) to ensure enough data in each group. In analysis of some states where no event was observed in group 3+, the EBMT risk score was categorized into two groups of 0 or 1, and scores 2 or higher (2+).
Overall survival (OS) was defined as the time from transplant to death from any cause. Leukemia-free survival (LFS) was defined for patients in remission as the time from transplant to hematologic relapse or death from any cause, whichever came first. Relapse incidence was defined for patients who were previously in disease remission as time from transplant to hematologic relapse. TRM was defined as death without relapse and was considered as a competing event for relapse. Patients who were alive without event at their last follow-up were considered as censored observations.
Events occurred in the first 100 days after transplant were defined as early outcomes and events after day +100 were considered as late outcomes.
Time to onset of aGVHD in the first 100 days after transplant was defined as a secondary outcome and either death or relapse before aGVHD was considered as its competing event. Likewise, time to onset of cGVHD was defined as another secondary endpoint after 100 days post-transplant and death or relapse before cGVHD was regarded as its competing event.
Statistical methods
Data were presented through median with range (minimum, maximum) and frequency with percentage for continuous and categorical variables, respectively. Survival curves were calculated using the Kaplan-Meier method and the 95% confidence interval (CI) for survival rates was calculated using the log-transformed method. The effect of covariates on OS and LFS was assessed by applying the Cox proportional hazards (PH) model and was reported through hazard ratio (HR) with 95% CI. Cumulative incidence functions were computed for relapse and TRM in a competing risks setting. The effect of covariates on relapse and TRM was evaluated using Fine & Gray competing risks regression, and sub-distribution hazard ratio (SHR) with 95% CI was reported.21 This method was also applied to assess the association of the EBMT risk score and its components with acute and chronic GVHD.
Although the outcomes can be influenced by GVHD, it’s incidence as a post-transplant risk factor is unknown at the time of transplant. Hence, a multistate approach was applied for competing risks22 to consider the information of GVHD as an intermediate event in addition to relapse and TRM in the analyses. The four final states considered in this model were the combination of two levels of GVHD (presence/absence) and two causes (relapse/TRM). The scheme of this model is shown in Figure 1. This model was used to assess the effect of the EBMT risk score on early outcomes with aGVHD as an intermediate event and on late outcomes with cGVHD as an intermediate event, separately. The statistical packages “survival”,23 “cmprsk”,24 and “mstate”25 in R software version 3.0.026 were used to perform the analyses.
Figure 1.
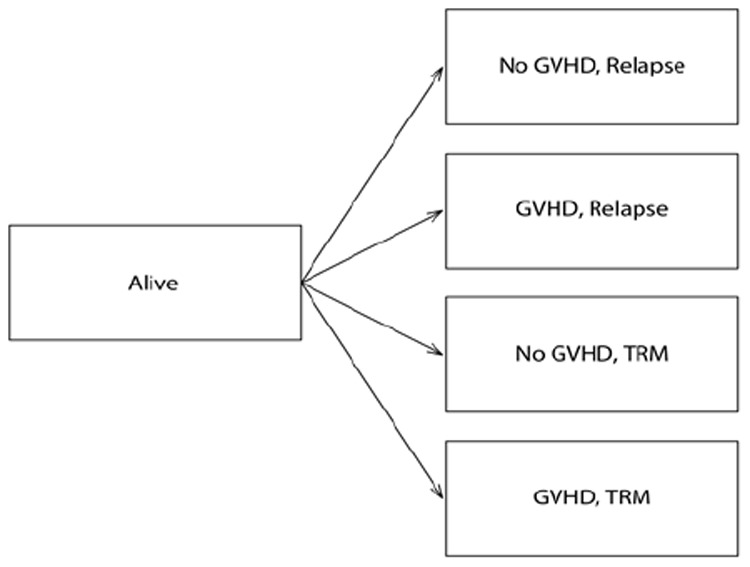
Multistate model for competing risks with four final states to take into account the information about the occurrence of GVHD
RESULTS
Three hundred and seventy-seven out of 426 patients who received allogeneic transplants between 2004 and 2011 met the study criteria, among whom, 14 (3.7%) were completely lost to follow-up after discharge and were excluded from the study. Out of the remaining 363 patients, 34 recipients (9.4%) had incomplete follow-up visits so that 11 had less than one year follow-up. However, the authors decided to include them in the analysis. The median follow-up time of the survivors was 52.3 months (range: 2.6 to 108.5). Demographic and baseline characteristics of the study patients are shown in Table 1.
Table 1.
Demographic and baseline characteristics of the study patients
| Characteristics | Number (%) |
|---|---|
| Recipient’s gender | |
| Male | 195 (53.7) |
| Female | 168 (46.3) |
| Recipient’s age at transplant (year)1 | 30 (15, 60) |
| Donor’s gender | |
| Male | 221 (60.9) |
| Female | 142 (39.1) |
| Donor’s age (year)1 | 29 (8, 63) |
| Donor/Recipient CMV serostatus | |
| +/+ | 335 (92.3) |
| +/- | 11 (3.0) |
| -/+ | 13 (3.6) |
| -/- | 4 (1.1) |
| Time interval between diagnosis and transplant (month)1 | 6.3 (1.3,114.6) |
| Status of disease at transplant | |
| CR1 | 293 (80.7) |
| CR2 | 61 (16.8) |
| CR3+ | 9 (2.5) |
| Karnofsky performance score | |
| > = 90 | 299/325 (92.0) |
| < 90 | 26/325 (8.0) |
Median (range); CMV, cytomegalovirus
Table 2 represents the characteristics of the outcomes and intermediate events. Ninety-nine percent of the recipients had neutrophil and 96% had platelet recovery. AGVHD occurred in about two-thirds of the recipients and nearly two-thirds of the survivors experienced cGVHD on day +100 after transplant (Table 2).
Table 2.
Characteristics of outcomes and intermediate events
| Event characteristics | Frequency (%) |
|---|---|
| Neutropenic fever | 281 (77.4) |
| Neutrophil recovery | |
| Yes | 360 (99.2) |
| Never dropped | 0 (0.0) |
| No | 3 (0.8) |
| Time to neutrophil recovery (day)1 | 12 (7, 42) |
| Platelet recovery | |
| Yes | 347 (95.6) |
| Never dropped | 12 (3.3) |
| No | 4 (1.1) |
| Time to platelet recovery (day)1 | 13 (7, 49) |
| Hospitalization days | 27 (7, 98) |
| Acute GVHD | 235 (64.7) |
| Acute GVHD grade | |
| I | 63/235 (26.8) |
| II | 64/235 (27.2) |
| III | 79/235 (33.6) |
| IV | 29/235 (12.3) |
| Time to acute GVHD (day)1 | 12 (7, 80) |
| Chronic GVHD | 216/328 (65.9) |
| De novo | 69/216 (31.9) |
| Progressive | 73/216 (33.8) |
| Interrupted | 74/216 (34.3) |
| Chronic GVHD extensity | |
| Limited | 127/216 (58.8) |
| Extensive | 89/216 (41.2) |
| Time to chronic GVHD (day)1 | 153 (101, 952) |
| Relapse | 88 (24.2) |
| Survival status | |
| Alive | 223 (61.4) |
| Dead | 140 (38.6) |
| Main causes of death | |
| Relapse | 80/140 (57.1) |
| GVHD | 29/140 (20.7) |
| Infection | 13/140 (9.3) |
| Other | 18/140 (12.9) |
| Follow-up (month)1 | 51.5 (2.6, 108.5) |
Median (range)
One-, two-, and five-year LFS were 72.8% (95% CI: 68.4-77.6%), 65.6% (95% CI: 60.8-70.8%) and 56.7% (95% CI: 51.3-62.6%), respectively (Figure 2.a). Additionally, one-, two-, and five-year OS were 76.4% (95% CI: 72.1-81.0%), 68.5% (95% CI: 63.8-73.6%), and 59% (95% CI: 53.6-64.9%, Figure 2.a), respectively. The most common causes of death were relapse, GVHD and infection.
Figure 2.
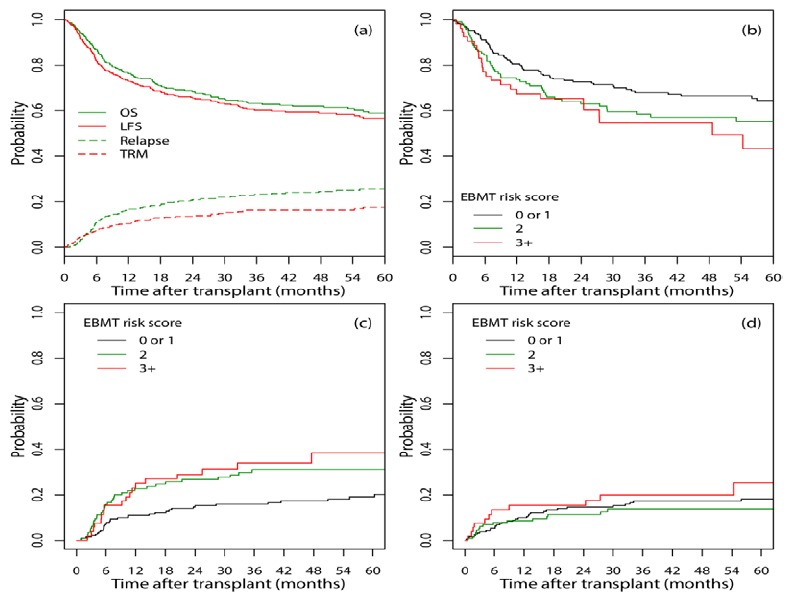
(a) OS, LFS, Relapse incidence, and TRM curves for all study patients; (b) OS, (c) cumulative incidence of relapse, and (d) cumulative incidence of TRM for different EBMT risk scores
The cumulative incidence of relapse at one, two, and five years after transplant was 16.8% (95% CI: 13.1-20.9%), 20.8% (95% CI: 16.7-25.3%), and 25.7% (95% CI: 21.0-30.7%), respectively while the cumulative incidence of TRM at one, two, and five years after transplant was 10.3% (95% CI: 7.4-13.7%), 13.6% (95% CI: 10.3-17.4%), and 17.6% (95% CI: 13.6-22.0%, Figure 2.a), respectively.
Characteristics of the EBMT risk score and its components are shown in Table 3. The correlation between the disease status at transplant and the time interval from diagnosis to transplant was 34.6%. This correlation increased to 60.2% between the disease stage and interval score. The status of the disease, and time interval longer than one year were the dominant pre-transplant risk factors of the EBMT risk score on outcomes.
Table 3.
Characteristics of the EBMT risk score and its components
| Variables | score | Frequency (%) |
|---|---|---|
| Age at transplant | ||
| < 20 | 0 | 45 (12.4) |
| 20 - 40 | 1 | 237 (65.3) |
| >40 | 2 | 81 (22.3) |
| Gender combination | ||
| Other | 0 | 296 (81.5) |
| Female donor to male recipient | 1 | 67 (18.5) |
| Disease status at transplant | ||
| CR1 | 0 | 293 (80.7) |
| CR2 | 1 | 61 (16.8) |
| CR3+ | 2 | 9 (2.5) |
| Time from diagnosis to transplant | ||
| < 1 year | 0 | 335 (92.3) |
| >1 year | 1 | 28 (7.7) |
| EBMT risk score | ||
| 0 | 22 (6.1) | |
| 1 | 171 (47.1) | |
| 2 | 117 (32.2) | |
| 3 | 45 (12.4) | |
| 4 | 7 (1.9) | |
| 5 | 1 (0.3) |
The univariate effect of covariates on OS, TRM and relapse are shown in Table 4. The EBMT risk score had a significant effect on OS (p=0.045) and relapse incidence (p=0.003). The higher the EBMT risk score, the higher the hazard of death (Table 4, Figure 2.b) and the incidence of relapse (Table 4, Figure 2.c). The effect of the EBMT risk score on TRM was not statistically significant (p=0.51, Table 4, Figure 2.d).
Table 4.
The univariate effect of covariates on OS, Relapse, and TRM
| Variables | Overall Survival |
Relapse |
TRM |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | SHR (95% CI) | p | SHR (95% CI) | p | |
| Gender (Male) | 1.20 (0.85, 1.67) | 0.298 | 1.22 (0.80, 1.87) | 0.346 | 1.30 (0.78, 2.18) | 0.311 |
| Age at transplant | 1.00 (0.99, 1.02) | 0.877 | 1.00 (0.98, 1.02) | 0.772 | 1.00 (0.98, 1.03) | 0.807 |
| Donor’s gender (Male) | 1.13 (0.80, 1.59) | 0.497 | 1.61 (1.02, 2.55) | 0.039 | 0.74 (0.45, 1.23) | 0.250 |
| Donor’s age | 1.00 (0.98, 1.01) | 0.573 | 0.99 (0.97, 1.01) | 0.241 | 1.01 (0.99, 1.03) | 0.475 |
| Time from diagnosis to transplant | 1.01 (1.00, 1.03) | 0.166 | 1.01 (1.00, 1.03) | 0.176 | 1.01 (0.98, 1.03) | 0.645 |
| Recipient’s CMV status (positive) | 0.85 (0.38, 1.93) | 0.700 | 0.63 (0.26, 1.52) | 0.300 | 2.51 (0.34, 18.72) | 0.370 |
| Donor’s CMV status (positive) | 1.25 (0.51, 3.05) | 0.630 | 0.95 (0.34, 2.65) | 0.918 | 2.93 (0.41, 21.23) | 0.286 |
| Karnofsky performance score (< 90) | 1.07 (0.61, 1.86) | 0.822 | 1.40 (0.73, 2.68) | 0.316 | 0.82 (0.33, 2.00) | 0.656 |
| Hospitalization days | 1.01 (0.99, 1.02) | 0.509 | 1.00 (0.98, 1.02) | 0.764 | 1.02 (1.00, 1.04) | 0.027 |
| Neutropenic fever | 1.13 (0.74, 1.72) | 0.571 | 1.04 (0.62, 1.74) | 0.877 | 1.22 (0.64, 2.33) | 0.553 |
| Age score | ||||||
| < 20 | 1.00 | 0.957 | 1.00 | 0.833 | 1.00 | 0.589 |
| 20-40 | 1.00 (0.60, 1.66) | 0.995 | 0.95 (0.50, 1.83) | 0.888 | 1.01 (0.48, 2.15) | 0.969 |
| >40 | 0.94 (0.52, 1.70) | 0.841 | 1.11 (0.53, 2.33) | 0.774 | 0.71 (0.28, 1.79) | 0.467 |
| Gender combination score F- > M | 1.12 (0.73, 1.70) | 0.606 | 0.66 (0.36, 1.21) | 0.183 | 1.91 (1.09, 3.35) | 0.024 |
| Disease stage | ||||||
| CR1 | 1.00 | < 0.001 | 1.00 | < 0.001 | 1.00 | 0.055 |
| CR2 | 2.27 (1.54, 3.33) | < 0.001 | 3.79 (2.41, 5.95) | < 0.001 | 0.76 (0.37, 1.59) | 0.472 |
| CR3 + | 3.51 (1.54, 8.04) | 0.003 | 2.61 (1.06, 6.46) | 0.037 | 3.71 (1.17, 11.82) | 0.026 |
| Interval score >1 year | 1.82 (1.07, 3.12) | 0.028 | 2.22 (1.20, 4.10) | 0.011 | 1.11 (0.43, 2.85) | 0.823 |
| EBMT risk score | ||||||
| 0 or 1 | 1.00 | 0.045 | 1.00 | 0.003 | 1.00 | 0.510 |
| 2 | 1.40 (0.97, 2.02) | 0.076 | 1.94 (1.21, 3.11) | 0.006 | 0.81 (0.45, 1.47) | 0.490 |
| 3 + | 1.70 (1.07, 2.70) | 0.024 | 2.31 (1.33, 4.00) | 0.003 | 1.29 (0.65, 2.56) | 0.480 |
SHR, sub-distribution hazard ratio; CMV, Cytomegalovirus
In the first 100 days after transplant, 13 (3.6%) patients experienced relapse, 18 (5.0%) died due to causes other than relapse, and 4 (1.1%) recipients were lost to follow-up from day 79 to 99. The EBMT risk score showed no statistically significant effect on aGVHD incidence (p=0.933).
Table 5 represents that the association between the EBMT risk score and its components with the incidence of aGVHD was not statistically significant.
Table 5.
The effects of the EBMT risk score and its components on acute and chronic GVHD
| Variables | acute GVHD |
chronic GVHD |
||
|---|---|---|---|---|
| SHR (95% CI) | p | SHR (95% CI) | p | |
| Age score | ||||
| < 20 | 1.00 | 0.570 | 1.00 | 0.778 |
| 20-40 | 1.12 (0.74, 1.69) | 0.598 | 1.00 (0.69, 1.45) | 0.989 |
| >40 | 0.96 (0.60, 1.53) | 0.859 | 1.11 (0.74, 1.68) | 0.614 |
| Gender combination score F- > M | 1.24 (0.91, 1.70) | 0.174 | 1.12 (0.82, 1.53) | 0.473 |
| Disease stage | ||||
| CR1 | 1.00 | 0.649 | 1.00 | 0.054 |
| CR2 | 0.89 (0.63, 1.26) | 0.516 | 0.72 (0.48, 1.07) | 0.106 |
| CR3 + | 1.23 (0.64, 2.36) | 0.544 | 1.94 (0.90, 4.18) | 0.090 |
| Interval score >1 year | 0.91 (0.56, 1.48) | 0.702 | 0.50 (0.26, 0.96) | 0.038 |
| EBMT risk score | ||||
| 0 or 1 | 1.00 | 0.933 | 1.00 | 0.904 |
| 2 | 0.96 (0.73, 1.26) | 0.760 | 0.94 (0.70, 1.25) | 0.660 |
| 3 + | 1.02 (0.71, 1.46) | 0.910 | 0.96 (0.66, 1.41) | 0.847 |
SHR, sub-distribution hazard ratio
By using a multistate model, it was resulted that the effect of the EBMT risk scores 2 and 3+ versus 0 or 1 in the absence of aGVHD on early relapse were 2.59 (95% CI: 0.43-15.51, p=0.297) and 3.84 (95% CI: 0.54-27.25, p=0.179), respectively (Figure 3.a). However, when aGVHD was present, the hazard of early relapse in patients with the EBMT risk score 2+ was 2.31 (95% CI: 0.42-12.63, p=0.333) times more than score 0 or 1 Figure 3.b). Likewise, for patients with scores 2 and 3+, the hazard of TRM in the absence of aGVHD was 0.84 (95% CI: 0.08-9.29, p=0.889) and 1.84 (95% CI: 0.17-20.33, p=0.618) times the hazard of patients with score 0 or 1 Figure 3.c). These measures were 2.00 (95% CI: 0.61-6.57, p=0.251) and 2.25 (95% CI: 0.54-9.43, p=0.266) in the presence of aGVHD, respectively Figure 3.d).
Figure 3.
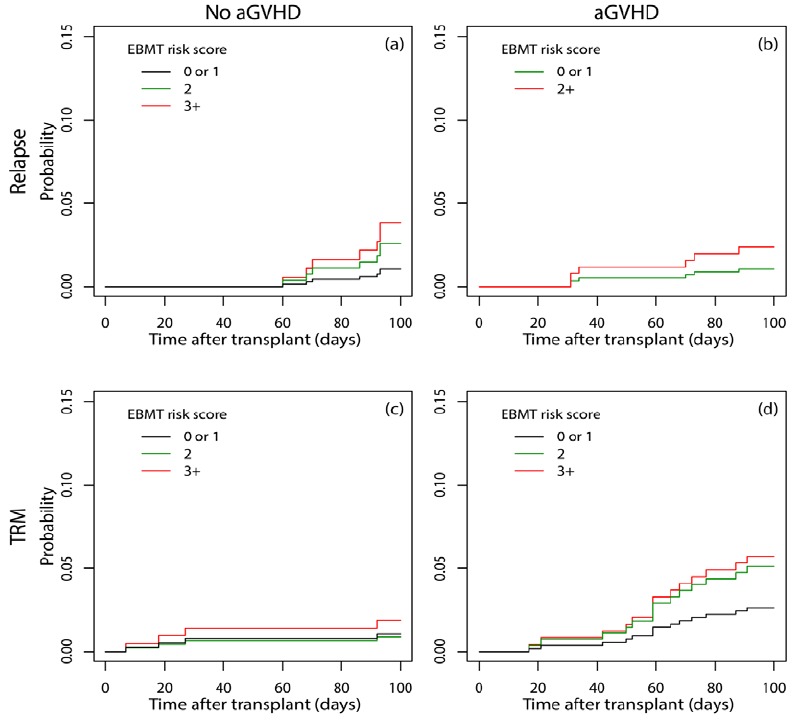
Cumulative incidence of early relapse (a, b) and TRM (c, d) considering the occurrence of aGVHD in the first 100 days after transplant in different EBMT risk scores using a multistate approach for competing risks
Among 328 survivors and event-free recipients, 75 (22.9%) relapsed and 42 (12.8%) died due to TRM on day +100 after transplant. Among 112 recipients who did not experience cGVHD, 65 (58%) had a history of aGVHD (21 grade I, 22 grade II, 17 grade III, and 5 grade IV). AGVHD [grade I = 18 (24.7%), grade II = 11 (15.1%), grade III = 32 (43.8%), grade IV = 12 (16.4%)] was observed in 73 patients with progressive cGVHD and 74 recipients [grade I = 22 (29.7%), grade II = 25 (33.8%), grade III = 20 (27.0%), grade IV = 7 (9.5%)] with interrupted cGVHD.
The predictive effect of the EBMT risk score on the incidence of cGVHD was not statistically significant (p=0.904). However, the interval score and status of disease, as the two components of the EBMT risk score, had statistically significant protective (p=0.038) and borderline (p=0.054) effect on the incidence of cGVHD (Table 5). All 269 patients who achieved CR1 were transplanted less than one year, while 25 out of 59 (42.4%) patients who were in CR2+ were transplanted after one year of diagnosis.
When the multistate model was applied, the hazard of late relapse in the absence of cGVHD in patients with scores 2 and 3+ were 2.20 (95% CI: 1.12-4.32, p=0.022) and 2.62 (95% CI: 1.15-5.96, p=0.021) times the hazard of patients with score 0 or 1, respectively (Figure 4.a); Similarly, when cGVHD was present, these measures were 1.54 (95% CI: 0.68-3.48, p=0.301) and 3.21 (95% CI: 1.33-7.76, p=0.010, Figure 4.b), respectively. The hazard of late TRM in patients with score 2+ in the absence of cGVHD was half (95% CI: 0.10-2.52, p=0.403) the hazard in patients with score 0 or 1 (Figure 4.c). When cGVHD was present, the hazard of late TRM in patients with the EBMT risk scores 2 and 3+ to those with scores 0 or 1 were 0.60 (95% CI: 0.25-1.43, p=0.251) and 1.37 (95% CI: 0.57-3.27, p=0.480), respectively (Figure 4.d).
Figure 4.
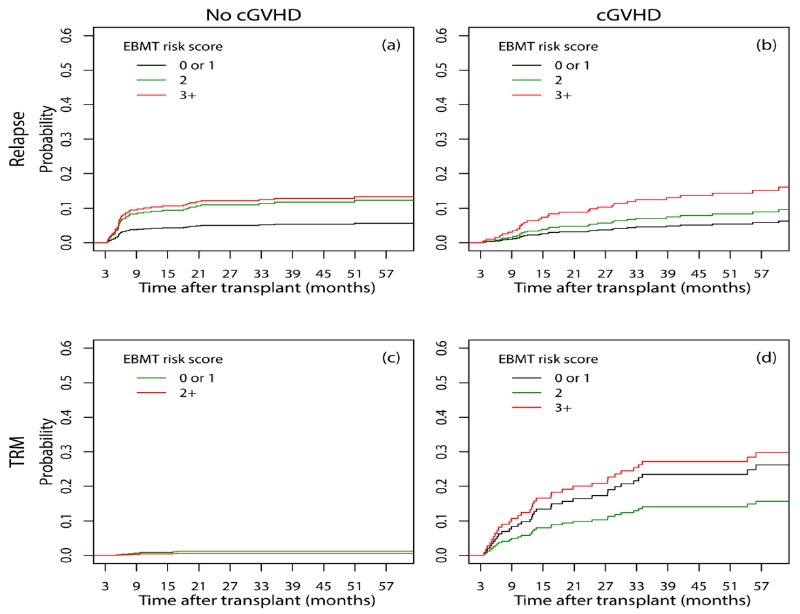
Cumulative incidence of relapse (a, b) and TRM (c, d) considering the occurrence of cGVHD for patients at risk on the day 100 post-transplant in different EBMT risk scores using a multistate approach for competing risks
DISCUSSION
The long-term estimates of LFS, OS, relapse incidence, and TRM of the whole study patients were very close to the 5-year report released by Keating et al from CIBMTR1 on AML patients (n=425) who received allogeneic PBSCT from HLA-identical sibling donors (54%, 59%, 26% and 20%, respectively).
Our results revealed that the main dominant pre-transplant risk factors of the EBMT risk score were the disease status and time interval between diagnosis and transplant which were moderately correlated. In general, we found that the EBMT risk score was a good predictor for OS and relapse incidence; however, it was not associated with TRM. Similarly, Hemmati et al.,27 reported a strong correlation between the time interval from diagnosis to transplant and disease status at transplant and found that their proposed modified EBMT risk score was highly predictive for OS and relapse.
Risk factors such as CMV serostatus and Karnofsky score did not have significant effect on outcomes in our study. This may be because of the high prevalence of CMV seropositive status among recipients and donors, and high frequency of Karnofsky performance score more than 90 in our data.
Our findings showed that the EBMT risk score did not have any association with the incidence of acute and chronic GVHD, but the time interval between diagnosis and transplant had significant protective effect on cGVHD. Since, all patients who were transplanted more than one year after diagnosis were in CR2+ the protective effect of time interval on cGHVD might be due to higher relapse and mortality in patients in CR2+ which precluded the chance of experiencing cGVHD.
In a multistate setting, the results demonstrated that in the presence or absence of aGVHD in the first 100 days, the association between the EBMT risk score and relapse incidence was not statistically significant; however, there was evidence that the higher the EBMT risk score, the higher the hazard of relapse incidence. This is also true for TRM with weaker evidence in the presence of aGVHD. After the day +100, in the presence or absence of cGVHD, the predictive effect of the EBMT risk score for relapse incidence was found to be statistically significant. However, this effect is not only statistically insignificant but also there exists no trend for the association of the EBMT risk score and TRM. Therefore, it seems that the effect of EBMT risk score on OS and relapse incidence cannot be affected by GVHD.
One of the advantages of this study was to better evaluation of the predictive effect of the EBMT risk score on post-transplant events in relatively homogenized patients by considering the influential factors such as conditioning regimen, source of stem cell and donor type as Gratwohl18 mentioned. This study suffered a small sample size since it was a single-left study and we did not have access to cytogenetic risk reports at diagnosis. Insufficient sample size resulted in a small number of events in the first 100 days after transplant which led to statistically insignificant results. Moreover, inaccessibility to cytogenetic risk reports at diagnosis, identified as the strongest predictor of relapse28, 29, made it impossible to distinguish high and intermediate risk in AML patients.
Gratwohl et al.,15 extended the EBMT risk score based on almost 50,000 allogeneic HSCT EBMT mega data from data registry, which is more heterogeneous than our single-left data. Therefore, it seems that the heterogeneous nature of the information helps the EBMT risk score to distinguish the patients much better in international data registries, as compared to homogeneous single-center data.
Despite these facts, although the EBMT risk score “explains at best 63% of the outcome” as Gratwohl et al mentioned, “the overall risk score retains its primary value as a rapid and instant tool for basic assessment”.18 Eventually, according to our data, it seems that the EBMT risk score predicts early TRM better than late TRM and works well for predicting relapse. However, the results of a single center study cannot be generalized with certainty.
Acknowledgments
The present study was supported by Tehran University of Medical Sciences as a part of a PhD thesis.
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
References
- 1.Keating A, DaSilva G, Perez WS, Gupta V, Cutler CS, Ballen KK, et al. Autologous blood cell transplantation versus HLA-identical sibling transplantation for acute myeloid leukemia in first complete remission: a registry study from the center for International Blood and Marrow Transplantation Research. Haematologica. 2013 Feb;98(2):185–92. doi: 10.3324/haematol.2012.062059. PubMed PMID: 22983587. Pubmed Central PMCID: 3561424. Epub 2012/09/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007 May 1;109(9):3658–66. doi: 10.1182/blood-2006-06-025627. PubMed PMID: 17213292. Epub 2007/01/11. eng. [DOI] [PubMed] [Google Scholar]
- 3.Craddock C. Full Intensity and Reduced Intensity Allogeneic Transplantation in AML. In: Lazarus HM, Laughlin MJ, editors. Allogeneic stem cell transplantation. Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 4.Gratwohl A, Baldomero H, Frauendorfer K, Urbano-Ispizua A. EBMT activity survey 2004 and changes in disease indication over the past 15 years. Bone marrow transplantation. 2006;37(12):1069–85. doi: 10.1038/sj.bmt.1705377. [DOI] [PubMed] [Google Scholar]
- 5.Valcarcel D, Martino R, Sureda A, Canals C, Altes A, Briones J, et al. Conventional versus reduced-intensity conditioning regimen for allogeneic stem cell transplantation in patients with hematological malignancies. European journal of haematology. 2005;74(2):144–51. doi: 10.1111/j.1600-0609.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 6.Cornelissen JJ, Löwenberg B. Role of allogeneic stem cell transplantation in current treatment of acute myeloid leukemia. ASH Education Program Book. 2005;2005(1):151–5. doi: 10.1182/asheducation-2005.1.151. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002 Aug 1;100(3):761–7. doi: 10.1182/blood-2001-12-0304. PubMed PMID: 12130483. Epub 2002/07/20. eng. [DOI] [PubMed] [Google Scholar]
- 8.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. The New England journal of medicine. 2001 Jan 18;344(3):175–81. doi: 10.1056/NEJM200101183440303. PubMed PMID: 11172139. Epub 2001/02/15. eng. [DOI] [PubMed] [Google Scholar]
- 9.Heldal D, Tjonnfjord G, Brinch L, Albrechtsen D, Egeland T, Steen R, et al. A randomised study of allogeneic transplantation with stem cells from blood or bone marrow. Bone marrow transplantation. 2000 Jun;25(11):1129–36. doi: 10.1038/sj.bmt.1702422. PubMed PMID: 10849524. Epub 2000/06/13. eng. [DOI] [PubMed] [Google Scholar]
- 10.Powles R, Mehta J, Kulkarni S, Treleaven J, Millar B, Marsden J et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet. 2000 Apr 8;355(9211):1231–7. doi: 10.1016/S0140-6736(00)02090-0. PubMed PMID: 10770306. Epub 2000/04/19. eng. [DOI] [PubMed] [Google Scholar]
- 11.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multileft comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100(5):1525–31. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 12.Ringden O. Allogeneic bone marrow transplantation for hematological malignancies-controversies and recent advances. Acta Oncologica. 1997;36(6):549–64. doi: 10.3109/02841869709001316. [DOI] [PubMed] [Google Scholar]
- 13.Wahlin A, Billström R, Björ O, Ahlgren T, Hedenus M, Höglund M, et al. Results of risk-adapted therapy in acute myeloid leukaemia. A long-term population-based follow-up study. European journal of haematology. 2009;83(2):99–107. doi: 10.1111/j.1600-0609.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 14.Meijer E, Cornelissen JJ, editors. Seminars in oncology. Elsevier; 2008. Allogeneic stem cell transplantation in acute myeloid leukemia in first or subsequent remission: weighing prognostic markers predicting relapse and risk factors for non-relapse mortality. [DOI] [PubMed] [Google Scholar]
- 15.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation. Cancer. 2009;115(20):4715–26. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 16.Lodewyck T, Cornelissen JJ. Allogeneic stem cell transplantation in acute myeloid leukemia: a risk-adapted approach. Blood reviews. 2008;22(6):293–302. doi: 10.1016/j.blre.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. The Lancet. 1998;352(9134):1087–92. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 18.Gratwohl A. The EBMT risk score. Bone marrow transplantation. 2012 Jun;47(6):749–56. doi: 10.1038/bmt.2011.110. PubMed PMID: 21643021. Epub 2011/06/07. eng. [DOI] [PubMed] [Google Scholar]
- 19.Glucksberg H, Storb R, Fefer A, Buckner C, Neiman P, Clift R, et al. Clinical Manifestations of Graft-Versus-Host Disease in Human Recipients of Marrow From Hl-A-Matched Sibling Donor, S. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. The American journal of medicine. 1980;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 22.Putter H, Fiocco M, Geskus R. Tutorial in biostatistics: competing risks and multi-state models. Statistics in medicine. 2007;26(11):2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 23.Therneau TM. A Package for Survival Analysis in S. 2.37-4 ed2013. [Google Scholar]
- 24.Gray B. cmprsk: Subdistribution Analysis of Competing Risks. 2.2-6 ed2013. [Google Scholar]
- 25.De Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non-and semi-parametric multi-state and competing risks models. Computer methods and programs in biomedicine. 2010;99(3):261–74. doi: 10.1016/j.cmpb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing. 3.0.0 ed. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 27.Hemmati PG, Terwey TH, le Coutre P, Vuong LG, Massenkeil G, Dorken B, et al. A modified EBMT risk score predicts the outcome of patients with acute myeloid leukemia receiving allogeneic stem cell transplants. European journal of haematology. 2011 Apr;86(4):305–16. doi: 10.1111/j.1600-0609.2011.01580.x. PubMed PMID: 21265883. Epub 2011/01/27. eng. [DOI] [PubMed] [Google Scholar]
- 28.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002 Dec 15;100(13):4325–36. doi: 10.1182/blood-2002-03-0772. PubMed PMID: 12393746. Epub 2002/10/24. eng. [DOI] [PubMed] [Google Scholar]
- 29.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. The New England journal of medicine. 2012 Oct 18;367(16):1487–96. doi: 10.1056/NEJMoa1203517. PubMed PMID: 23075175. Pubmed Central PMCID: PMC3816375. Epub 2012/10/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]


