Abstract
Background
Immune thrombocytopenic purpura (ITP) is an autoimmune disease that can cause bleeding disorders in patients, and presents in acute and chronic forms. The acute form is frequently seen in children, but the chronic form mainly inflicts adults. There are differences and similarities in clinical and laboratory findings of the disease between children and adults. We study these differences and similarities in these two groups of patients with ITP.
Methods
In this study, we retrospectively evaluated the clinical and laboratory data of 323 ITP cases within three years. None of our patients had a history of thrombocytopenia. Patients were classified into two groups of children (3 months to 16 years of age) and adults (≥ 16 years). Data analysis was conducted using SPSS software, and the analysis results were compared between the two age groups.
Results
Overall, the disease prevalence was higher in women than men, but the prevalence of childhood ITP was higher in males than females. The prevalence of initial symptoms including petechiae, purpura and ecchymosis was 60.5% and 61%, respectively in all patients, but severe bleeding rarely occurred in patients (28.8%). 30.5% of patients had a history of infection before developing ITP, and the children had a higher frequency of infection (80.8%). Before treatment, the mean platelet count in adults and children was 33000/μL and 35000/μL, respectively.
Conclusion
Comparison of data in children and adults with ITP indicated similarities and differences in clinical and laboratory findings between the two groups with differences in prevalence, bleeding symptoms, initial platelet count and infection history.
Keywords: Immune thrombocytopenic purpura, Autoimmune disease, Bleeding
INTRODUCTION
Immune Thrombocytopenic Purpura (ITP) is an autoimmune disorder characterized by an increased rate of platelet destruction caused by autoantibody binding to platelet surface glycoproteins, especially GpIIb/IIIa and GpIb/IX complexes, leading to platelet clearance by reticuloendothelial system (RES) macrophages in the spleen.1 ITP is a disease that affects both children and adults. There are two forms of ITP: acute and chronic. The acute form is a seasonal disease that usually occurs following a mild viral infection or vaccination, and is transient in most cases. Children are mainly affected by acute but benign and self-limited forms of the disease, which requires minimal intervention.2, 3 The chronic form is defined by persistent thrombocytopenia for more than 12 months, while it mostly occurs in young women.4-7 Nearly 20% of children and the majority of adults have chronic ITP, which is associated with recurrence in adults and usually requires some forms of therapy.8, 9 Based on presence or absence of background disease, ITP is classified to primary and secondary ITP. Primary ITP is defined as isolate thrombocytopenia in the absence of a definite etiology or disease, while secondary ITP is defined by presence of a background disease (such as systemic lupus erythematosus, lymphoproliferative disorders and chronic infections like Helicobacter pylori, HIV or HCV), disrupting the immune system and ultimately leading to thrombocytopenia.10 The prevalence of ITP in children is estimated to be about 1.9 to 6.4 cases per 100,000 per year and 3.3 cases per 100,000 per year in adults.11 ITP diagnosis is based on clinical findings and exclusion of other cases of thrombocytopenia which occur during HIV infection, SLE and lymphoproliferative disorders with absence of splenomegaly along with normal or increased megakaryocytes in BM test.12, 13 Platelet production is defective in ITP because antibodies against platelets can also damage megakaryocytes. Although there is possibility of severe thrombocytopenia due to ITP, the symptoms of acute bleeding usually have a low incidence.14, 15 Patients usually show symptoms of petechiae or purpura, and the symptoms progress in those with platelet counts lower than 20,000/μL.16 Severe skin bleeding, prolonged epistaxis, vaginal bleeding or menorrhagia may occur in individuals with platelet counts lower than 10,000/μL. The most important symptom in patients with platelet count of 30000-50000/μL may be easy bruising.17, 18 The role of a number of viral and bacterial agents has been well recognized in ITP, and mimicking human antigens by infectious agents triggers the mechanism of ITP.19 Various strategies have been used for ITP treatment including corticosteroids, immunosuppressive drugs, splenectomy and high-dose IVIg. Approximately 20% of ITP cases are resistant to treatment.5, 20, 21 IVIg and glucocorticoids rapidly increase platelet count by decreasing the rate of platelet destruction, and IVIg is the treatment choice for severe bleeding cases (clinically “severe” ITP). Although IVIg is a popular drug, up to 75% of children show complications such as headache and fever.15, 22 Although most guidelines recommend treatment for adult patients with platelet counts lower than 30,000, ICIS group recommends that children without bleeding require no treatment regardless of their platelet count.10 In this article, we reviewed the clinical and laboratory findings of ITP in children and adults upon diagnosis and compared the differences in symptoms between the two groups. Based on our findings, the differences observed were not the same as we expected.
MATERIALS AND METHODS
In this retrospective study, we assessed the ITP patients who were referred to Shafa Hospital, Ahvaz, from March 21, 2010 to March 19, 2013. None of the patients in this study had a history of ITP, and all were new cases. The diagnosis of ITP was based on patient history, physical examination, platelet counts lower than 100,000 μ/L, normal concentration of hemoglobin and WBC, peripheral blood smear examination and the absence of other diseases causing thrombocytopenia, including infection HIV, SLE and lymphoproliferative disorders confirmed by BM examination.
Patient selection
Patients with thrombocytopenia were identified by their medical records. ITP patients were selected among them, and personalized data including age, sex, disease symptoms from petechiae or purpura to acute bleeding, platelet count upon diagnosis and after treatment, bone marrow smear examination, Coomb’s test results, underlying diseases such as infections, presence or absence of organomegaly, selected treatment based on platelet count (PLT< 20000/μL and PLT≥ 20000/μL with bleeding require to treatment) were extracted.
The response to treatment is classified as follows:
Patients with platelet counts lower than 50,000/μL after treatment did not respond to treatment and were known as “No response” (NR, < 50000/μL)
If the patient had a platelet count between 50000/μL and 150000/μL, they were grouped as “Incomplete response” (IR, 50-150/109)
Patients with platelet counts over 150000/μL were classified as “Complete response” (CR,> 15000/μL).23
Statistical analysis
Descriptive data analysis was conducted using SPSS software. Chi-square test was used to assess the correlation between variables, and a value of p<0.05 was considered significant.
RESULTS
In this retrospective study, medical records of 323 ITP patients were evaluated. The patients were classified based on the age into childhood ITP (3 months to 16 years of age) and adult ITP (16≤ years) groups. The results were as follows:
Prevalence
Among the total thrombocytopenia patients, 223 patients (69%) had childhood ITP with a mean age of 3.6 years, and 100 patients (31%) had adult ITP with a mean age of 34.3 years. The prevalence of disease in females and males under the age of 16 and older was 57.1% (n=93), 81.2% (n=130), 42.9% (n=70) and 18.8% (n=30), respectively.
The overall prevalence was 49.5% (n=160) in males and 50.5% (n=163) in females. Childhood ITP was more frequent in males than females, while the ratio of females was higher in adult ITP and the whole number of patients (p=0. 0001). Of the total patients, 66 were infants (3 months to 1 year of age), among whom 43 (65.2%) were male and 23 (34.8%) were female. Among all patients, 32 (9.9%) were born from consanguineous marriages, of whom 22 (68.7%) were male and 10 (31.3%) were female. 21 male and 9 female patients developed childhood ITP, while one male and female patient had adult ITP. There was only one male infant with a history of thrombocytopenia in a family whose parents were not relatives. A summary of patients’ characteristics is given in Table 1.
Table 1.
Characteristics of 323 patients with ITP as initial diagnosis
| Characteristics | All patients(n=323) |
|---|---|
| Sex, no. of patients (%) | |
| Male | 160(49.5) |
| Female | 163(55.5) |
| Mean age, y(rang) | |
| Male | 9 (4m-55) |
| Female | 17(4m-85) |
| Thrombocytopenia | |
| Moderate | 126(39) |
| Sever | 197(61) |
| Presenting symptoms, no. of patients (%) | |
| Petechiae | 194 (60.5) |
| Ecchymosis | 197 (61) |
| Sever bleeding | 93(28.8) |
| Splenomegaly | 15 (4.6) |
Moderate thrombocytopenia = platelet count between 30000/μL and 100000/μL; sever thrombocytopenia = platelet count below 30000/μL.
Primary symptoms including petechiae or purpura, ecchymosis and severe bleeding including epistaxis, urinary system and severe bleeding in mouth, gums and brain
60.5% of patients (94 males and 100 females) had petechiae or purpura on the incidence of disease (p=0.004). 61% of total patients (98 males and 99 females) had ecchymosis at disease onset (p=0.035) (Table 2).
Table 2.
Frequency of petechiae or purpura and ecchymosis upon the onset of disease
| Gender | Type ITP | Petechiae or purpura, n (%) | Ecchymosis, n (%) |
|---|---|---|---|
| Male | Childhood | 79(61.2) | 81(62.8) |
| Adult | 15(48.4) | 17(54.8) | |
| Female | Childhood | 69(64.5) | 60(75.8) |
| Adult | 31(44.3) | 39(56.5) |
Total patients (28.8%) including 41 male (25.6%) and 52 female patients (31.9%) had severe bleeding, among whom 58.1% (n=54) had childhood ITP and 41.9% (n=39) had adult ITP. None of our patients had bleeding in cerebral veins. In general, bleeding signs were more frequent in children than adults (p=0.0001). 46 (49.5%) of 93 patients with severe bleeding had platelet counts lower than 20000, and 24 patients (25.8%) had platelet counts between 20000 and 30000.
Splenomegaly
15 (4.8%) patients, 9 males (5.8%) and 6 females (3.8%), had splenomegaly, among whom 4.2% had childhood ITP and 6.2% had adult ITP.
Infection history
60 males (37.5%) and 39 females (25.3%) had a history of infection about one to three weeks prior to ITP, and overall 30.5% of patients had a history of infection, among whom 80.8% (n=80) had childhood ITP and 19.2% (19 patients) had adult ITP. Among male patients with a history of infection before ITP, 52 (86.7%) had a history of common cold and fever, 7 (11.7%) had a history of nausea and vomiting and only 1 patient (1.6%) had a history of variola before the incidence of ITP. Among female patients with a history of infection before ITP, 32 (82%) had a history of cold and fever, 6 (15.4%) had a history of nausea and vomiting and only 1 patient (2.6%) had a history of hepatitis A before the incidence of ITP. 10 female patients had a history of parturition about two to three weeks before the incidence of ITP.
80 childhood ITP patients had a history of infection, among whom 68 patients (85%) had a history of cold and fever, 10 (12.5%) had a history of nausea and vomiting, 1 (1.2%) had a history of hepatitis A and 1 patient had a history of variola. 19 patients with adult ITP had a history of infection before ITP incidence, among whom 16 patients (84.2%) had a history of cold and fever and 3 patients (15.8%) had a history of nausea and vomiting (Table 3).
Table 3.
Prevalence of infection history among childhood and adult ITP patients
| Childhood ITP, n (%) | Adult IT, n (%) | |
|---|---|---|
| Cold and fever | 68 (85%) | 16 (84.2%) |
| Male, n : 52 | ||
| Female, n : 32 | ||
| Nausea and vomiting | 10 (12.5%) | 3 (15.8%) |
| Male, n : 6 | ||
| Female, n : 6 | ||
| Variola | 1 (1.2%) | 0 |
| Male, n : 1 | ||
| Hepatitis A | 1 (1.2%) | 0 |
| Female, n : 1 |
Presence of concurrent disease
7 patients had a concurrent disease with ITP, among whom 1 had childhood ITP and 6 had adult ITP (p=0.013) There were three women aged 26, 33 and 55 years with ovarian cysts, a 49-year-old woman with psychiatric disease, a 25-year-old man with fatty liver, a 20-year-old man with type I diabetes and finally a male newborn with thyroid disease. According to our results, there was no significant correlation between age and sex with organomegaly, infection and bleeding.
Platelet counts before and after treatment
Mean platelet counts before treatment in childhood and adult ITP patients were 33000/μL and 35000/μL, respectively. Platelet counts less than 20000/μL were more frequent in children (n=79, 35.6%) compared to adults at diagnosis (n=25, 24.8%) (p=0.053). The distribution of the platelet counts before and after treatment is shown in Table 4 and Table 5, respectively
Table 4.
Platelet count of children and adults at the time of ITP diagnosis
| PLT<50000/μL, n (%) | 50000≤ PLT ≤ 100000/μL, n (%) | |
|---|---|---|
| Male | 103(64.6) | 57(35.6) |
| Female | 115(70.6) | 48(29.4) |
| Childhood ITP | 152(68.2) | 71(31.8) |
| Adult ITP | 66(66) | 34(34) |
Table 5.
Platelet count of children and adults after treatment
| PLT<50000/μL, n (%) | 50000≤ PLT <150000, n (%) | PLT ≥150000/μL | |
|---|---|---|---|
| Male | 27(20) | 50(37) | 58(43) |
| Female | 29(23) | 59(46.8) | 38(30.2) |
| Childhood ITP | 29(16.1) | 64(35.6) | 87(48.3) |
| Adult ITP | 27(33.3) | 45(55.6) | 9(11.1) |
BM and Coombs’ tests
196 out of 323 patients underwent BM biopsy, of whom 65 (33.2%) had hypercellular BM with increased megakaryocytes, 124 (63.3%) had normocellular BM with increased megakaryocytes and 7 (3.6%) had normocellular BM without increased megakaryocytes. Based on our data, nobody had secondary ITP. The treatment plan for 196 patients with childhood and adult ITP who underwent BM test is shown in Table 6.
Table 6.
Drug administration in patients subject to BM test
| Drug | Children, n (%) | Adult, n (%) |
|---|---|---|
| Methylprednisolone | 28 (16.4) | 10 (40) |
| IVIg | 3 (1.8) | 1 (4) |
| Methylprednisolone + IVIg | 139 (81.3) | 9 (36) |
| No treatment | 1 (0.6) | 5 (20) |
Indirect and direct Coombs’ tests were done for 139 patients, of whom 133 (95.7%) were negative for both tests, 5 (3.6%) were positive for both tests and only 1 patient (0.7%) was positive for direct Coombs’ test.
Treatment
Available treatment options including IVIg and corticosteroids such as methylprednisolone and rarely splenectomy are used to treat ITP in Shafa Hospital, Ahvaz, but since patients usually do not respond to corticosteroids or IVIg alone, the first-line treatment in this center is a combination of the two drugs. We specifically deal with the effects of IVIg and methylprednisolone administration. Among our patients, 86 (26.6%) received only methylprednisolone, 15 (4.6%) received IVIg, 207 (64.1%) received both drugs and 15 (4.6%) received none. Among childhood ITP patients, 7 (3.2%) were only treated with IVIg, 36 (16.2%) were only given prednisolone (16.2%), 174 (78.4%) received both drugs and 5 (2.3%) were given none. Among adult ITP patients, 8 (7.9%) were only treated with IVIg, 50 (49.5%) received only prednisolone, 33 (32.7%) were given both drugs and 10 (9.9%) received none (Figure 1). There were 10 adults with a mean age of 36.9 years and 5 children with a mean age of 2.1 years among 15 patients who received no medication. 5 of these 15 patients [1 child (2.7%) and 4 adults (19.6%)] had bleeding from mouth, gum or nose (p=8). Among these patients, 3 had platelet counts lower than 20000/μL, and their mean platelet count with childhood and adult ITP was 7300/μL and 5500/μL, respectively. Only three patients in our study (0.9%) were subject to splenectomy, of which platelet count was dropped in two patients.
Figure 1.
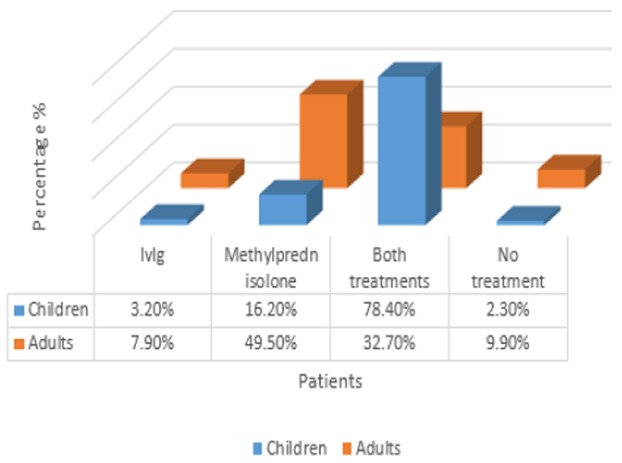
Diagram evaluating various treatments in ITP patients in different age groups: IVIg and methylprednisolone therapy in childhood and adult ITP patients
DISCUSSION
In this retrospective study, we have practically identified the clinical features of Immune Thrombocytopenic Purpura (ITP). For the first time, we have set a basis for recognizing important clinical features of this disease and comparing the clinical and laboratory signs in children and adults. According to previous studies, there are differences in clinical and laboratory aspects of ITP between children and adults, including clinical outcome, variable disease presentation and response to treatment.11 Like our results (in which general prevalence was higher in females and those aged above 16 compared to males of the same age), Doan et al., concluded that females are typical adults with ITP, generally occurring between 18 to 40 years of age.18 The following features distinguish childhood ITP from ITP in older children:
High M/F ratio (12, 24) in infants (this article)
Low prevalence of infection in infants compared to children (1-10 years) due to less contact between infants and other children. In this article, we obtained similar results. 17 male infants had a history of infection, among whom 14 (82.4%) had a history of cold plus fever and 3 (17.6%) had a history of nausea and vomiting. 6 female infants had a history of infection, among whom 4 (66.7%) had a history of cold plus fever and 2 (33.3%) had a history of nausea and vomiting. Similar to our results, upper respiratory tract infections were more prevalent in newborn males and children.
Low prevalence of chronic ITP.25 According to previous studies, 56% of ITP patients had infection before the incidence of ITP, but in this study, we obtained a much lower rate (30.5%). This may indicate negligible effects of infection in Iranian patients or problems in detection of infectious agents, as the tests for infectious agents like HCV, HIV and H.pylori are not routinely performed in Iran. Infection was highly prevalent before ITP diagnosis in children 1 to 10 years old, and occurred less frequently in infants as well as 10- to 16-year-old children (41% and 52%, respectively). In this paper, children were classified as the age group under 16 and ,similar to the results of other studies, the highest prevalence of infection (80.8%) was observed in this group.9
A number of studies suggest that long-term refractory ITP increases the risk of severe bleeding in adults. In another study, like our own, it was concluded that there was no correlation between severity of bleeding and age plus sex.6, 26 Management of childhood ITP is usually based on platelet count, as childhood ITP patients with severe thrombocytopenia upon diagnosis (PLT count<20000/μL) are at high risk of hemorrhage, and drug therapy is often performed in children with restriction in physical activity. We adopted this criteria in this study and performed drug therapy in children with platelet counts lower than 20000.27 According to previous studies, megakaryocyte count was normal in 65% of ITP patients and increased in 33% of the cohort. These results are not consistent with our findings since 96.5% of our patients showed increased megakaryocytes in BM Biopsy.28 Immune thrombocytopenia during pregnancy is specifically caused by ITP although it can be caused by other secondary factors such as SLE. There is 2:1000 frequency of ITP in pregnancy, but there was no pregnant woman amongst our patients, and 10 patients had given birth 2 to 3 weeks before the incidence of ITP.29
In summary, ITP is a disease characterized by reduced platelet count due to autoimmune disorders, autoantibody production against platelets and defective platelet production. Compared to previous studies, there are similarities and differences between disease symptoms in children and adults. Prevalence of disease in both sexes, bleeding signs and initial platelet count were similar to other studies, but there was a significant difference in the effect of infection in disease onset between Iranian and non-Iranian patients. Further studies are required to detect infectious agents as predisposing factors in Iranian patients.
Acknowledgments
This paper forms part of Sajedeh Saeidi’s M.Sc. thesis. We extend special thanks to Ahvaz Jundishapur University of Medical Science for the financial support. We specifically thank colleagues in Shafa Hospital for their support.
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
References
- 1.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. New England Journal of Medicine. 2002;346(13):995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 2.Provan D, Newland A. Fifty years of idiopathic thrombocytopenic purpura (ITP): management of refractory ITP in adults. British journal of haematology. 2002;118(4):933–44. doi: 10.1046/j.1365-2141.2002.03669.x. [DOI] [PubMed] [Google Scholar]
- 3.George JN, Woolf SH, Raskob GE, Wasser J, Aledort L, Ballem P, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3–40. [PubMed] [Google Scholar]
- 4.Yang R, Han ZC. Pathogenesis and management of chronic idiopathic thrombocytopenic purpura: an update. International journal of hematology. 2000;71(1):18–24. [PubMed] [Google Scholar]
- 5.Berchtold P, McMillan R. Therapy of chronic idiopathic thrombocytopenic purpura in adults [see comments] Blood. 1989;74(7):2309–17. [PubMed] [Google Scholar]
- 6.Vianelli N, Valdrè L, Fiacchini M, de Vivo A, Gugliotta L, Catani L, et al. Long-term follow-up of idiopathic thrombocytopenic purpura in 310 patients. haematologica. 2001;86(5):504–9. [PubMed] [Google Scholar]
- 7.Imbach P. Treatment of immune thrombocytopenia with intravenous immunoglobulin and insights for other diseases. A historical review. Swiss Med Wkly. [Historical Article Research Support, Non-U.S. Gov’t Review] 2012;142:w13593. doi: 10.4414/smw.2012.13593. [DOI] [PubMed] [Google Scholar]
- 8.DeHart K, Witfill K, Miller R. IDIOPATHIC THROMBOCYTOPENIC PURPURA. Journal of the American Osteopathic College of Dermatology. 22 [Google Scholar]
- 9.Kühne T, Buchanan GR, Zimmerman S, Michaels LA, Kohan R, Berchtold W, et al. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. The Journal of pediatrics. 2003;143(5):605–8. doi: 10.1067/s0022-3476(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 10.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 11.Kühne T, Berchtold W, Michaels LA, Wu R, Donato H, Espina B, et al. Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. haematologica. 2011;96(12):1831–7. doi: 10.3324/haematol.2011.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kühne T, Imbach P, Bolton-Maggs PH, Berchtold W, Blanchette V, Buchanan GR. Newly diagnosed idiopathic thrombocytopenic purpura in childhood: an observational study. The lancet. 2001;358(9299):2122–5. doi: 10.1016/S0140-6736(01)07219-1. [DOI] [PubMed] [Google Scholar]
- 13.Brighton T, Evans S, Castaldi P, Chesterman C, Chong B. Prospective evaluation of the clinical usefulness of an antigen-specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88(1):194–201. [PubMed] [Google Scholar]
- 14.Gernsheimer T, Stratton J, Ballem PJ, Slichter SJ. Mechanisms of response to treatment in autoimmune thrombocytopenic purpura. New England Journal of Medicine. 1989;320(15):974–80. doi: 10.1056/NEJM198904133201505. [DOI] [PubMed] [Google Scholar]
- 15.Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New England Journal of Medicine. 2007;357(22):2237–47. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 16.Aledort LM, Hayward CP, Chen MG, Nichol JL, Bussel J. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. American journal of hematology. 2004;76(3):205–13. doi: 10.1002/ajh.20104. [DOI] [PubMed] [Google Scholar]
- 17.Cortelazzo S, Finazzi G, Buelli M, Molteni A, Viero P, Barbui T. High risk of severe bleeding in aged patients with chronic idiopathic thrombocytopenic purpura. Blood. 1991;77(1):31–3. [PubMed] [Google Scholar]
- 18.Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP) Blood. 2005;106(7):2244–51. doi: 10.1182/blood-2004-12-4598. [DOI] [PubMed] [Google Scholar]
- 19.Kohda K, Kuga T, Kogawa K, Kanisawa Y, Koike K, Kuroiwa G, et al. Effect of Helicobacter pylori eradication on platelet recovery in Japanese patients with chronic idiopathic thrombocytopenic purpura and secondary autoimmune thrombocytopenic purpura. British journal of haematology. 2002;118(2):584–8. doi: 10.1046/j.1365-2141.2002.03612.x. [DOI] [PubMed] [Google Scholar]
- 20.Imbach P, d’Apuzzo V, Hirt A, Rossi E, Vest M, Barandun S, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. The lancet. 1981;317(8232):1228–31. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 21.Nomura S, Dan K, Hotta T, Fujimura K, Ikeda Y. Effects of pegylated recombinant human megakaryocyte growth and development factor in patients with idiopathic thrombocytopenic purpura. Blood. 2002;100(2):728–30. doi: 10.1182/blood.v100.2.728. [DOI] [PubMed] [Google Scholar]
- 22.Bolton-Maggs P. Idiopathic thrombocytopenic purpura. Archives of disease in childhood. 2000;83(3):220–2. doi: 10.1136/adc.83.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi CW, Kim BS, Seo JH, Shin SW, Kim YH, Kim JS, et al. Response to high-dose intravenous immune globulin as a valuable factor predicting the effect of splenectomy in chronic idiopathic thrombocytopenic purpura patients. American journal of hematology. 2001;66(3):197–202. doi: 10.1002/1096-8652(200103)66:3<197::aid-ajh1044>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Choi E, Lee K, Ahn H. Childhood acute immune thrombocytopenic purpura: Multicenter study of Korean pediatric hematology and oncology group. Int J Hematol. 2002;76(Suppl 1):5. [Google Scholar]
- 25.Monto AS. Epidemiology of viral respiratory infections. The American journal of medicine. 2002;112(6):4–12. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 26.Schattner E, Bussel J. Mortality in immune thrombocytopenic purpura: report of seven cases and consideration of prognostic indicators. American journal of hematology. 1994;46(2):120–6. doi: 10.1002/ajh.2830460212. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. The Journal of pediatrics. 2002;141(5):683–8. doi: 10.1067/mpd.2002.128547. [DOI] [PubMed] [Google Scholar]
- 28.Houwerzijl EJ, Blom NR, van der Want JJ, Esselink MT, Koornstra JJ, Smit JW, et al. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood. 2004;103(2):500–6. doi: 10.1182/blood-2003-01-0275. [DOI] [PubMed] [Google Scholar]
- 29.Kelton J. Idiopathic thrombocytopenic purpura complicating pregnancy. Blood reviews. 2002;16(1):43–6. doi: 10.1054/blre.2001.0181. [DOI] [PubMed] [Google Scholar]


