Abstract
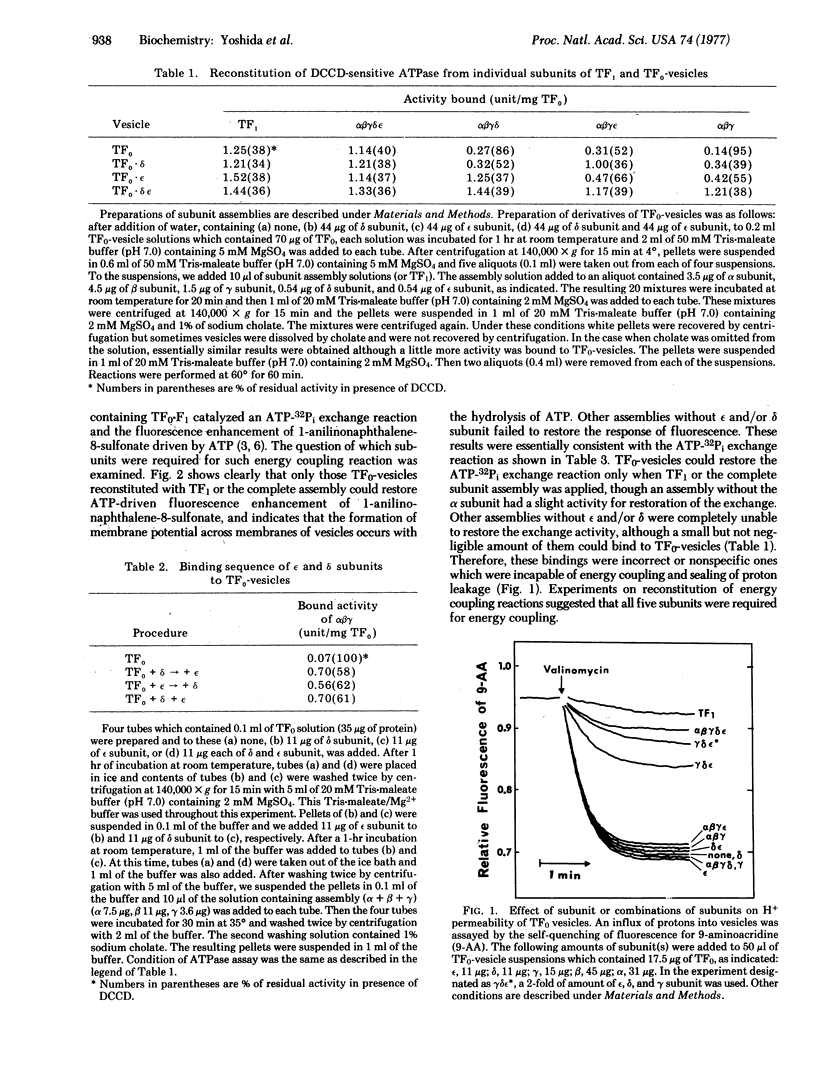
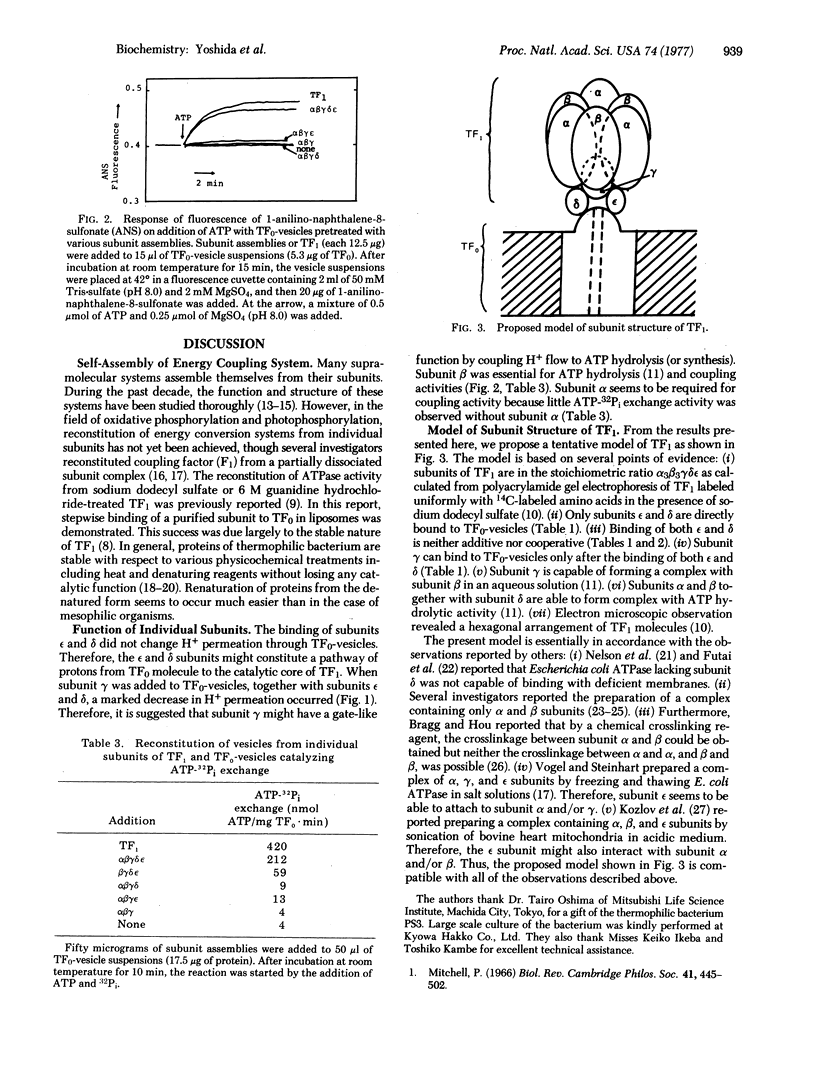
Purified dicyclohexylcarbodiimide-sensitive ATPase (TF0-F1) from thermophilic bacterium PS3 is composed of a water soluble part with ATP hydrolytic activity (TF1) and a water insoluble moiety (TF0). All of the five subunits (alpha, beta, gamma, delta, and epsilon) of TF1 were isolated. TF1 was reconstituted from the five subunits, which catalyzed an ATP-32Pi exchange and an ATP-driven enhancement of fluorescence of 1-anilinonaphthalene-8-sulfonate, when adsorbed on proteoliposome inlaid with TF0 (TF3-vesicles). Subunit epsilon and/or delta became firmly bound to TF0-vesicles and there was no preferential sequence in the binding. Both subunits were required for binding of the remaining subunits of TF1 to TF0-vesicles, but they did not modify the high H+ -permeability of TF0-vesicles. The addition of gamma but they did not modify the high H+-permeability of TFO-vesicles. The addition of gamma subunit together with epsilon and delta subunits caused a marked decrease of H+ -permeability of TF0-vesicles, similar to that induced by TF1. We conclude tentatively that the epsilon and delta subunits connect TF0 and the other subunits forming a part of a proton pathway, gamma is a gate of proton flow coupled to ATP hydrolysis (or synthesis), and alpha and beta subunits contain the active site for energy transformation. A possible model of subunit structure of TF1 is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bragg P. D., Hou C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium. Arch Biochem Biophys. 1975 Mar;167(1):311–321. doi: 10.1016/0003-9861(75)90467-1. [DOI] [PubMed] [Google Scholar]
- Deters D. W., Racker E., Nelson N., Nelson H. Partial resolution of the enzymes catalyzing photophosphorylation. XV. Approaches to the active site of coupling factor I. J Biol Chem. 1975 Feb 10;250(3):1041–1047. [PubMed] [Google Scholar]
- Drachev L. A., Kondrashin A. A., Samuilov V. D., Skulachev V. P. Generation of electric potential by reaction center complexes from Rhodospirillum rubrum. FEBS Lett. 1975 Feb 1;50(2):219–222. doi: 10.1016/0014-5793(75)80492-3. [DOI] [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Energy coupling in membrane vesicles of Escherichia coli. I. Accumulation of metabolites in response to an electrical potential. J Biol Chem. 1974 May 10;249(9):2939–2945. [PubMed] [Google Scholar]
- Höckel M., Hulla F. W., Risi S., Dose K. Me2+-(13 S) ATPase from Micrococcus sp. ATCC 398E. The effect of trypsin on the purified enzyme. Biochim Biophys Acta. 1976 May 13;429(3):1020–1028. doi: 10.1016/0005-2744(76)90346-6. [DOI] [PubMed] [Google Scholar]
- Ishihama A., Ito K. Subunits of RNA polymerase in function and structure. II. Reconstitution of Escherichia coli RNA polymerase from isolated subunits. J Mol Biol. 1972 Dec 14;72(1):111–123. doi: 10.1016/0022-2836(72)90073-3. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Kandrach A., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J Biol Chem. 1973 Jan 25;248(2):676–684. [PubMed] [Google Scholar]
- Kagawa Y., Sone N., Yoshida M., Hirata H., Okamoto H. Proton translocating ATPase of a thermophilic bacterium. Morphology, subunits, and chemical composition. J Biochem. 1976 Jul;80(1):141–151. doi: 10.1093/oxfordjournals.jbchem.a131246. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- Kozlov I. A., Kondrashin A. A., Kononenko V. A., Metelsky S. T. Functional role of soluble mitochondrial ATPase subunits. J Bioenerg. 1976 Feb;8(1):1–7. doi: 10.1007/BF01559385. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nelson N., Kanner B. I., Gutnick D. L. Purification and properties of Mg2+-Ca2+ adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2720–2724. doi: 10.1073/pnas.71.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Assembly of bacterial ribosomes. Science. 1973 Mar 2;179(4076):864–873. doi: 10.1126/science.179.4076.864. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T. Subunit structure and properties of two forms of adenosine triphosphatase released from Micrococcus lysodeikticus membranes. Biochem Biophys Res Commun. 1972 Oct 17;49(2):350–357. doi: 10.1016/0006-291x(72)90417-2. [DOI] [PubMed] [Google Scholar]
- Serrano R., Kanner B. I., Racker E. Purification and properties of the proton-translocating adenosine triphosphatase complex of bovine heart mitochondria. J Biol Chem. 1976 Apr 25;251(8):2453–2461. [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C. Restoration of coupling factor activity to Escherichia coli ATPase missing the delta subunit. Biochem Biophys Res Commun. 1975 Feb 3;62(3):764–771. doi: 10.1016/0006-291x(75)90465-9. [DOI] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Purification and properties of a dicyclohexylcarbodiimide-sensitive adenosine triphosphatase from a thermophilic bacterium. J Biol Chem. 1975 Oct 10;250(19):7917–7923. [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Okamoto H., Kagawa Y. Electrochemical potential of protons in vesicles reconstituted from purified, proton-translocating adenosine triphosphatase. J Membr Biol. 1976 Dec 28;30(2):121–134. doi: 10.1007/BF01869663. [DOI] [PubMed] [Google Scholar]
- Vogel G., Steinhart R. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry. 1976 Jan 13;15(1):208–216. doi: 10.1021/bi00646a032. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Oshima T., Imahori K. Fructose-1, 6-bisphosphatase of an extreme thermophile. J Biochem. 1973 Dec;74(6):1183–1191. doi: 10.1093/oxfordjournals.jbchem.a130346. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. A highly stable adenosine triphosphatase from a thermophillie bacterium. Purification, properties, and reconstitution. J Biol Chem. 1975 Oct 10;250(19):7910–7916. [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. ATP synthesis catalyzed by purified DCCD-sensitive ATPase incorporated into reconstituted purple membrane vesicles. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1295–1300. doi: 10.1016/0006-291x(75)90167-9. [DOI] [PubMed] [Google Scholar]


