Abstract
Objectives
This study aimed to investigate the effect of water-pipe (WP) smoking on hematological parameters of Wistar rats.
Methods
Thirty-five young male rats (200-250 g) were randomly assigned to five groups (n=7). The control group was exposed to room air and the experimental groups were exposed to WP smoking, using a special apparatus designed to have the ability to keep the rats for 40 minutes every day for 4, 8, 12 and 12 weeks; moreover, one of the two groups of 12 weeks of WP exposer had four following weeks of rest. Blood samples were collected to evaluate red blood cell count, hemoglobin, hematocrit, white blood cell and platelet counts.
Results
The results showed that RBC count, Hb and Hct parameters were significantly higher in WP smoking rats than the control group (P< 0.001). We found that WBC counts insignificantly increased (P < 0.39) but Plt counts insignificantly decreased (P < 0.13) in WP smoking rats compared with control group.
Conclusion
The findings may help to raise awareness of tobacco smokers about the potential toxicities of WP; likewise, the results can be used by physicians and public health officials in tobacco prevention programs.
Keywords: Hematological parameters, Health, Water-Pipe, Smoke, Rat
INTRODUCTION
Tobacco is a commercial product obtained from dried and processed yellow-brown leaves collected from Nicotiana tobacum, the plant that is widely cultivated and grown in many countries around the world. According to data reported from the World Health organization (WHO), there is about 2.4 billion people worldwide that have consumed tobacco in the forms of smoking, chewing, snuffing or dipping. WHO also estimates that tobacco-related deaths will amount to 6.4 million in 2015, 8.3 million in 2030 and one billion deaths during the 21st century.1-3
Water-pipe (WP) is a classical device used for tobacco smoking attached with water bowl. The WP usage has a history about 400 years with the different names like as a shisha, narghile, hookah chillum and arghile.4, 5 WP is often linked with social activity where two or more people may share the same pipe. In some cultures, children may smoke with their parents. It is estimated that approximately 100 million people use WP smoke throughout the world.6 Due to the lack of awareness, there is a viewpoint in different cultures that WP is less dangerous than cigarette, so its prevalence is increasing, particularly among adolescent and young adults.7 This increasing trend can be attributed to the popular beliefs that the smoke is “filtered” by the water where harmful effect is believed to be reduced by the so-called “filtering” process.8, 9 However the research findings highlight that WP smoking carries similar or higher risks than other forms of tobacco exposure. The research studies also indicate that WP has led to increase the risk of infectious diseases,10 cardiovascular disease,11 pulmonary illness,12 cancers13-15 and low fetal birth weight in pregnant women.16 The hematologic index alterations are used as physiological markers of organ and tissue damage. Therefore, the various pharmacological actions of nicotine and other materials led to change the status of hematologic and hemostatic parameters.
However, there are few studies on the effect of WP smoking on hematological parameters in both human and animals. This study aimed to investigate the effect of WP smoking on hematological parameters such as red blood cells (RBCs), hemoglobin (Hb), hematocrit (Hct), white blood cells (WBCs) and platelet counts in Wistar rats between four and twelve weeks of exposure.
MATERIALS AND METHODS
We studied 35 young male Wistar rats weighing between 200 and 250g. They were all obtained from the animal care unit of Zahedan University of Medical Sciences, Iran. All procedures involving the animals were performed in accordance with the regulations defined by guides and protocols approved by Ethics Committee of the Deputy of Research in Zahedan University of Medical Sciences. Before and during the experiment, all animals were maintained on the standard feeding and were allowed to access to tap water and libitum throughout the period of experiment. The experimental environmental was maintained at a temperature range of 21±2°C and animals were kept under a schedule of 12-h light/dark cycle. They were held in polypropylene cages in small groups of 1 or 2 rats per cage during the study. The animals were then randomly divided into five groups, each consisting of seven rats. Group A (control group) was exposed to room air. Groups B (sub-acute), C (sub-chronic) and D (chronic) were exposed to WP smoking for 4, 8 and 12 weeks, respectively. Group E was similarly exposed to WP smoking for 12 weeks and was held in control situation for 30 days.
Rats of experimental groups were put in an isolated box during daily test in a corner of the experimental room and after being exposed to WP smoking, the rats were returned to their own cages. To do this,a special apparatus was designed to have the ability to keep the rats for 40 minutes in a situation very similar to WP smoking by human.It had a vacuum suction for condensation of the smoke, a glass box in a cube shape (aquarium shape) with the size of 60 × 100 × 200 cm for keeping the animals and a hood over the aquarium-shaped box to evacuate the extra smoke from the environment. A speed-control ventilator was connected to the air outflow of the smoke chamber for controlling the inner air velocity. The speed of air exchange was determined before the experiment so that a high enough smoke concentration within the smoke box was always guaranteed. The animals were placed in the box and exposed to hookah smoking (10gr) for 10 minutes. The procedure was repeated 4 times daily with adequate time interval (10 min).
At the end of each experimental period, the animals were sacrificed by ether inhalation method. The blood samples were then collected, using a 5-ml syringe attached to a needle (21 SWG) via cardiac puncture into EDTA-contained tubes.
All samples were analyze within 24 hours and in that time were kept in refrigerator at 4° C. Complete blood counts (CBC) were determined using the automated cell counter (Sysmex K1000, Supplied by Sysmex Corporation, Japan) at Ali-Asghar Hospital affiliated to Zahedan University of Medical Sciences. The complete blood count included WBCs, RBCs, platelets, Hct and Hb. All statistical analyses were performed using SPSS version 11.0. The data were expressed as the mean + SD, statistical significant was determined using ANOVA Test followed by post-hoc test for parametric values. A P-value less than 0.05 was considered statistically significant.
RESULTS
The results indicated that RBC count in sub-chronic and chronic, amount of Hb in sub-acute, sub-chronic and Hct in sub-acute, sub-chronic and chronic were significantly higher in WP smoking rats in the control group (P< 0.001).Details are shown in Table 1 and Figures 1 -3. We found that WBC counts insignificantly increased (P < 0.39) and Plt counts insignificantly decreased (P < 0.13) in WP smoking rats compared with control group (Table 1 and Figures 4-5).
Table 1.
Hematologic parameters in different groups [values are Mean ± SD (range)]
| Group | RBC (× 106/mm3) | WBC(× 103/mm3) | Hb (g/dl) | Hct (%) | Plt (× 103/mm3) |
|---|---|---|---|---|---|
| Sub- acute | 9.4 ± 0.8 (8.6-10.1) | 5.2±2.4(2.9-7.4) | 18.4±2.5 (16.1-20.8) | 56.6 ± 6.4 (50.7-62.5) | 770 ± 199 (586-955) |
| Sub- chronic | 10.1±1.4 (8.7-11.4) | 5.9± 1.2 (4.8-6.9) | 19.8± 3.1(16.9-22.7) | 61.9± 8.4 (54.1-69.7) | 787± 206 (597-978) |
| Chronic | 9.8± 0.8 (8.9-10.6) | 5.4± 1 (4.4-6.4) | 17.1±0.9(16.2-18) | 58.9±4.4 (4.2-63.5) | 741±128 (607-876) |
| After -Treatment | 8.5±0.4(8.2-8.8) | 5.7±1.7(4.6-6.9) | 14.6± 0.8(14-15.1) | 51.1 ± 2 (49.7-52.4) | 916±121 (834-997) |
| Control | 8.8 ± 0.4 (8.6-9) | 4.6± 1.8 (3.6-5.6) | 15.1±1.3(14.4-15.8) | 50.2 ± 2.3 (49-51.4) | 838±115 (777-900) |
| P | 0.001 | 0.39 | 0.001 | 0.001 | 0.13 |
Figure 1.
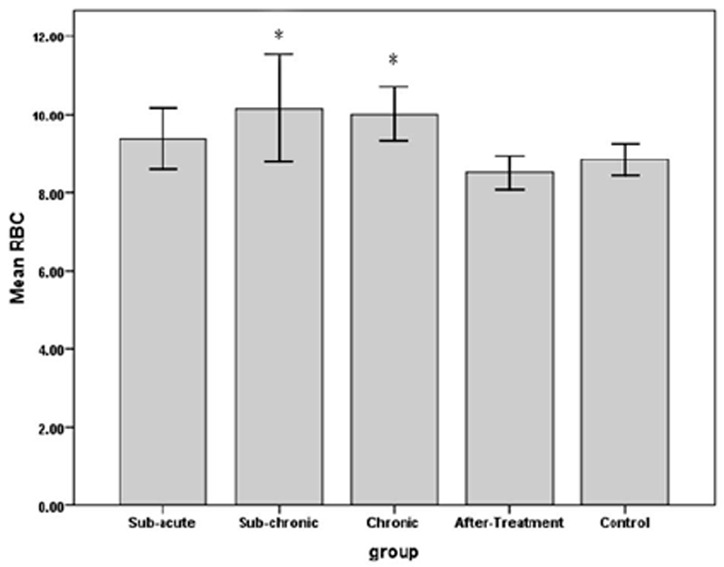
The effects of water-pipe smoking on red blood cell count (RBC) in different groups of rats exposed at different times.
*: P < 0.05 vs. control group
Figure 3.
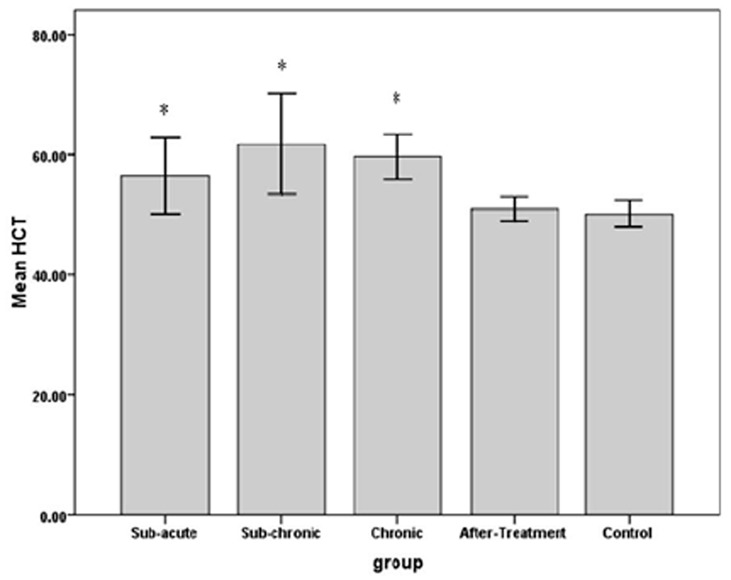
The effects of water-pipe smoking on hematocrite (Hct) in different groups of rats exposed at different times.
*: P < 0.05 vs. control group.
Figure 4.
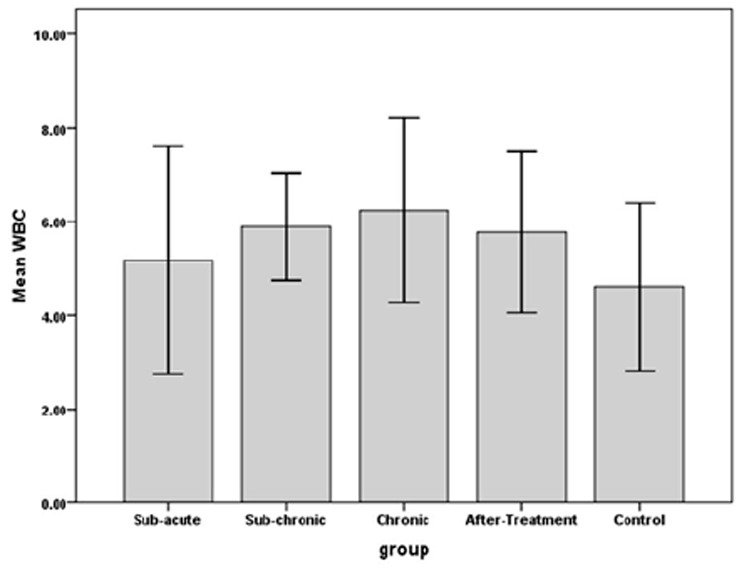
The effects of water-pipe smoking on white blood cell (WBC) parameter in different groups of rats exposed at different times.
Figure 5.
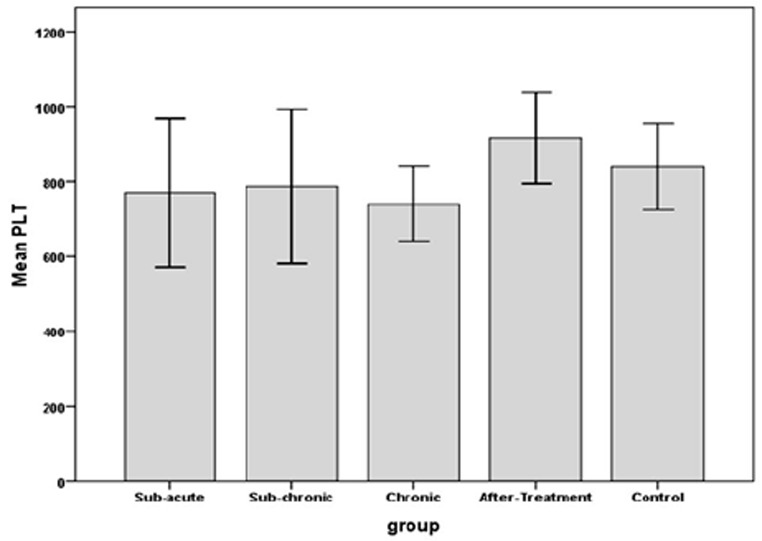
The effects of water-pipe smoking on platelet (Plt) count parameter in different groups of rats exposed at different times.
DISCUSSION
Since cigarette smoking causes several health problems in people, the findings clearly show that WP smoking has severe adverse effects on hematological parameters (e.g. Hb, Hct, WBC, RBC, Plt count) among rat models.
Results indicated that WP smoking led to increasing WBC count compared to control group. Kurtoglu reported that the mean leukocyte counts were significantly greater in smokers compared to nonsmokers.17 Friedman observed that WBC counts in smokers were about 20-25% higher than non-smokers and also they increased with intensity of smoking.18 Watanabe found that not only WBC counts but also tumour necrosis factor (TNF) system activities increased in current smokers than non-smokers. Light smoking associated with WBC counts, while heavy smoking related to TNF system activities.19 A group of researchers suggested that the increased leukocyte count might be due to nicotine-induced release of catecholamines, resulting in an increase in blood lymphocyte counts. In addition, the irritant effect of cigarette smoke on respiratory tree with resultant inflammation might be a contributory factor for higher WBC count. Also, it has been suggested that inflammatory stimulation of the bronchial tract induces an increase in inflammatory markers in the blood circulation.20 Another study showed that nicotine increased soluble Kit ligand, consistent with stem cell activation, followed by increase WBC count.21 The high WBC count may be a marker of smoking-induced tissue damage, and can be a risk factor for development of cardiovascular diseases through multiple pathologic mechanisms such as mediate inflammation, plug the microvasculature, induce hypercoagulability and promote infarct expansion.22, 23
The results indicated that RBC count values were significantly high in WP smoking (P=0.001). Elevated levels of Hb and Hct are correlated with increased numbers or sizes of RBCs (P=0.011). A normal carbon monoxide (CO) level in the blood stream is less than 8 PPM. A person that smokes one or two pack of cigarettes per day raises a blood CO level to 20 PPM. When a smoker stops smoking, the CO level in their blood stream typically returns to normal level within a few days (www.carbon-monoxide-survivor.com). Hakim et al. compared the acute effects of a single 30-min session of WP smoking on concentration of carboxyhemoglobin (COHb) before and after smoking. The survey showed that COHb concentration significantly increased after WP smoking (1.47% ± 0.57% to 9.47% ± 5.52%).24 COHb is the molecule formed from the combination of CO and hemoglobin The affinity of Co is 200-fold more than O2 to Hb.25 Thus, CO displaces oxygen from Hb in RBC to produce COHb, which reduces the transfer of oxygen to tissues.26 When body tissues do not receive a continuous and adequate supply of oxygen, they starve and begin to suffocate, malfunction, and finally die. Oxygen affects RBC membranes because they have more polyunsaturated fatty acids than other body tissues. Cigarette smoke increased 2, 2’-azo-bis-(2-amidinopropane) dihydrochloride-induced erythrocyte hemolysis by 281.7%. Nicotine leads to 13.8% increase in erythrocytes membrane peroxidation at the highest concentration and its effects are dose-dependent.27 The combination of CO in tobacco with effects of nicotine disrupts oxygen delivery to tissue and stimulates the bone marrow to produce more RBCs and thereby increase HCT and Hb. Stopping smoking should cause a slow return to pre-smoking levels.28 In people who smoked WP secondary polycythemia caused by CO poisoning and all blood parameters returned to normal ranges within six weeks after stopping smoking.29
Also, our results indicated that Plt count values were reduced between 10-15% in WP smokers compared to non-smokers (P=0.13). This finding is consistent with Narayanrao Gitte’s report in which the mean Plt counts were 257,325 and 215,483 per mm3 in 120 male smokers and healthy non-smokers, respectively.30 Tell studied Plt count among Norwegian adolescent population and found that the mean Plt counts for smokers and non-smokers were 300,000 and 275,000 per mm3, respectively. The results of Tell study showed that Plt counts were increased in adolescent who started smoking relatively early ages. Elevation of Plt counts in adolescent smokers indicates that these blood components may have an early role in the pathogenesis of arteriosclerosis.31 It has been proposed that fibrinogen links Plt receptors, which are preconditions for Plt aggregation and also promote hypercoagulable state as well as causing endothelial damage, disorganization and dysfunction. Aghaji showed that the Plt counts were higher for the regular WP smokers than non-smokers (P value= 0.0046). WBC count in smokers was slightly higher than non-smokers (P = 0.94).32 Al-Dahr found that there was no significant difference between the Plt count and mean Plt volume in smokers compared to nonsmokers (in control group 278,000 vs WP smoking 306,000 and cigarette smoking 264,000 per mm3), but the expression of Plt CD 40 and CD 62 were higher in smokers than non-smokers. Recent evidence indicates that Plt CD62 is rapidly expressed on the surface of activated Plts, where it mediates adhesion of Plts to neutrophils and monocytes. Plt activate and the consequent increased expression of Plt CD62 as direct mediators of vascular inflammation and atherosclerosis. Also, an increased expression of CD40 markers in smoking group may indicate induction of chemokine secretion and up-regulation of adhesion molecules. This process leads to recruitment and extravasation of leukocytes at the site of injury and thereby immediately links homeostasis to the inflammatory system. The increased cardiovascular morbidity and mortality among cigarette smokers is also mediated in part by enhanced Plt reactivity and activation.33 Research has shown that smoking may increase Plt aggregation and cause atherothrombotic cardiovascular events as well as Plt aggregation in patients with ischemic congenital heart disease in an aspirin non-responsive manner.34 A strong correlation has been found between cigarette smoking and atherosclerosis and cardiovascular disease.35, 36 Potential mechanisms for smoking-induced damage include increased Plt reactivity and agreeability,34 change in lipids and lipoprotein levels,37, 38 alteration in homeostasis system39 and increased WBC count40 WP smoking is not a safe alternative to cigarettes. The researchers found that the smoke from one simulated WP session produces much more tar (100-fold), nicotine (10-fold), CO (30-fold) and polycyclic aromatic hydrocarbons (2- to 5-fold) than a single cigarette.41, 42 Estimates of the equivalence between cigarette smoking and WP smoking could vary, where daily 200 puffs of WP are equal to 100 cigarettes. They observed that a 30-min single session of WP Smoking significantly increased the median COHb concentration from 1.4% (1.47% ± 0.56%) to 7.4% (9.49% ± 5.52%) (P < .001). The post-WP smoking COHb concentration was between 10% and 15% in 10 subjects, 15% to 20% in one subject and more than 20% in three subjects.24
In sum, RBC, Hb, HCT and WBC counts are higher in WP smokers, and these biomarkers might be associated with a greater risk for developing different diseases. Reduction in smoking improves some of these biomarkers. Additional research is necessary to determine which biomarkers are more sensitive to measure improved health and to what extent smoking needs to be reduced to obtain health benefits. We hope that these findings will be used by physicians and public health officials to inform WP tobacco smokers of the risk of tobacco-induced nicotine addiction and cardiovascular disease.
Figure 2.
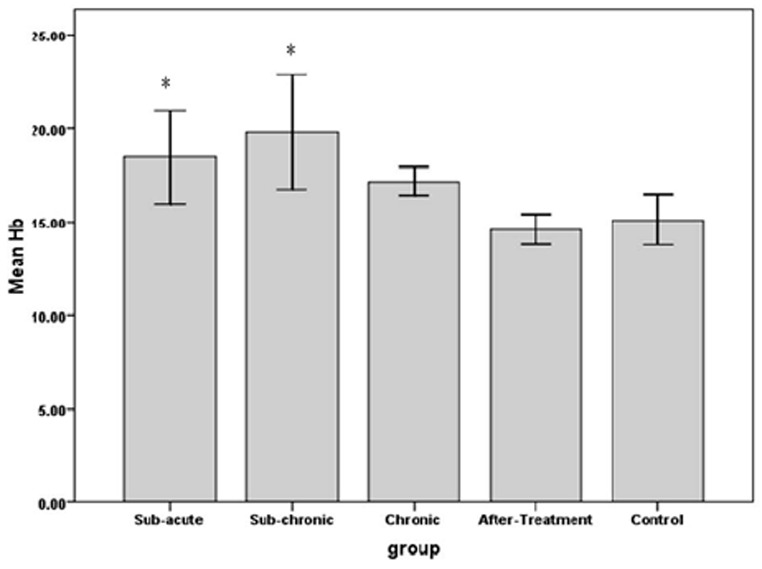
The effects of water pipe smoking on hemoglobin (Hb) in different groups of rats exposed at different times.
*: P < 0.05 vs. control group
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
References
- 1.World Health Organization. Report on the Global Tobacco Epidemic, 2009: Implementing smoke-free environments. WHO Press; Geneva; Switzerland: 2009. [Google Scholar]
- 2.World Health Organization. The Global Burden of Disease:2004 Update. WHO Press; 2008. [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009;37(6):518–23. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maziak W. The global epidemic of waterpipe smoking. Addict Behav. 2011;36(1-2):1–5. doi: 10.1016/j.addbeh.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maziak W, Ward KD, Afifi Soweid RA, et al. Tobacco smoking using a waterpipe: a re-emerging strain in a global epidemic. Tob Control. 2004;13(4):327–33. doi: 10.1136/tc.2004.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakkash R, Khalil J. Health warning labelling practices on narghile (shisha, hookah) waterpipe tobacco products and related accessories. Tob Control. 2010;19(3):235–9. doi: 10.1136/tc.2009.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maziak W, Eissenberg T, Rastam S, et al. Beliefs and attitudes related to narghile (waterpipe) smoking among university students in Syria. Ann Epidemiol. 2004;14(9):646–54. doi: 10.1016/j.annepidem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Aljarrah K, Ababneh ZQ, Al-Delaimy WK. Perceptions of hookah smoking harmfulness: predictors and characteristics among current hookah users. Tob Induc Dis. 2009;5(1):16. doi: 10.1186/1617-9625-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noonan D, Kulbok PA. New tobacco trends: waterpipe (hookah) smoking and implications for healthcare providers. J Am Acad Nurse Pract. 2009;21(5):258–60. doi: 10.1111/j.1745-7599.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- 11.Cobb CO, Sahmarani K, Eissenberg T, et al. Acute toxicant exposure and cardiac autonomic dysfunction from smoking a single narghile waterpipe with tobacco and with a “healthy” tobacco-free alternative. Toxicol Lett. 2012;215(1):70–5. doi: 10.1016/j.toxlet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammad Y, Kakah M, Mohammad Y. Chronic respiratory effect of narguileh smoking compared with cigarette smoking in women from the East Mediterranean region. Int J Chron Obstruct Pulmon Dis. 2008;3(3):405–14. doi: 10.2147/copd.s1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaouachi K, Sajid KM. A critique of recent hypotheses on oral (and lung) cancer induced by water pipe (hookah, shisha, narghile) tobacco smoking. Med Hypotheses. 2010;74(5):843–6. doi: 10.1016/j.mehy.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Fouad FM, Rastam S, Al Moustafa AE. Involvement of water pipe smoking in the development of human pancreatic cancer. Int J Cancer. 2010;127(2):497–8. doi: 10.1002/ijc.25061. [DOI] [PubMed] [Google Scholar]
- 15.Mfoumou E, Li Z, Al Moustafa AE. Current tobacco and water-pipe smoking enhance human cancer invasion and metastasis. Int J Cancer. 132(4):990–1. doi: 10.1002/ijc.27744. [DOI] [PubMed] [Google Scholar]
- 16.Nuwayhid IA, Yamout B, Azar G, et al. Narghile (hubble-bubble) smoking, low birth weight, and other pregnancy outcomes. Am J Epidemiol. 1998;148(4):375–83. doi: 10.1093/oxfordjournals.aje.a009656. [DOI] [PubMed] [Google Scholar]
- 17.Kurtoglu E, Akturk E, Korkmaz H, et al. Elevated red blood cell distribution width in healthy smokers. Turk Kardiyol Dern Ars. 2013;41(3):199–206. doi: 10.5543/tkda.2013.42375. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Siegelaub AB, Seltzer CC, et al. Smoking habits and the leukocyte count. Arch Environ Health. 1973;26(3):137–43. doi: 10.1080/00039896.1973.10666241. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N, Fukushima M, Taniguchi A, et al. Smoking, white blood cell counts, and TNF system activity in Japanese male subjects with normal glucose tolerance. Tob Induc Dis. 2011;9(1):12. doi: 10.1186/1617-9625-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calapai G, Caputi AP, Mannucci C, et al. Cardiovascular biomarkers in groups of established smokers after a decade of smoking. Basic Clin Pharmacol Toxicol. 2009;104(4):322–8. doi: 10.1111/j.1742-7843.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang E, Forsberg EC, Wu J, et al. Cholinergic activation of hematopoietic stem cells: role in tobacco-related disease? Vasc Med. 2010;15(5):375–85. doi: 10.1177/1358863X10378377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loimaala A, Rontu R, Vuori I, et al. Blood leukocyte count is a risk factor for intima-media thickening and subclinical carotid atherosclerosis in middle-aged men. Atherosclerosis. 2006;188(2):363–9. doi: 10.1016/j.atherosclerosis.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Madjid M, Awan I, Willerson JT, et al. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Hakim F, Hellou E, Goldbart A, et al. The acute effects of water-pipe smoking on the cardiorespiratory system. Chest. 2011;139(4):775–81. doi: 10.1378/chest.10-1833. [DOI] [PubMed] [Google Scholar]
- 25.Carallo C, Pujia A, Irace C, et al. Whole blood viscosity and haematocrit are associated with internal carotid atherosclerosis in men. Coron Artery Dis. 1998;9(2-3):113–7. [PubMed] [Google Scholar]
- 26.Cronenberger C, Mould DR, Roethig HJ, et al. Population pharmacokinetic analysis of carboxyhaemoglobin concentrations in adult cigarette smokers. Br J Clin Pharmacol. 2008;65(1):30–9. doi: 10.1111/j.1365-2125.2007.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asgary S, Naderi G, Ghannady A. Effects of cigarette smoke, nicotine and cotinine on red blood cell hemolysis and their -SH capacity. Exp Clin Cardiol. 2005;10(2):116–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Roethig HJ, Koval T, Muhammad-Kah R, et al. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regul Toxicol Pharmacol. 2010;57(2-3):333–7. doi: 10.1016/j.yrtph.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Bonadies N, Tichelli A, Rovo A. When water does not clear the smut from the smoke. BMJ Case Rep. 2013:2013. doi: 10.1136/bcr-2013-200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitte RN. Effect of Cigarette Smoking on Plasma Fibrinogen and Platelet Count. Asian Journal of Medical Sciences. 2011;2:181–184. [Google Scholar]
- 31.Tell GS, Grimm RH, Jr, Vellar OD, et al. The relationship of white cell count, platelet count, and hematocrit to cigarette smoking in adolescents: the Oslo Youth Study. Circulation. 1985;72(5):971–4. doi: 10.1161/01.cir.72.5.971. [DOI] [PubMed] [Google Scholar]
- 32.Aghaji M, Nnabuko R, Uzuegbunam C, et al. The relationship of white blood cell and platelet counts to cigarette smoking in adult Nigerians. Cent Afr J Med. 1990;36(11):273–8. [PubMed] [Google Scholar]
- 33.Al-Dahr MHS. Impact of Smoking on Platelet, Coagulation and Lipid Profile in Young Male Subjects. World Applied Sciences Journal. 2010;11(1):118–123. [Google Scholar]
- 34.Pamukcu B, Oflaz H, Onur I, et al. Effect of cigarette smoking on platelet aggregation. Clin Appl Thromb Hemost. 2011;17(6):E175–80. doi: 10.1177/1076029610394440. [DOI] [PubMed] [Google Scholar]
- 35.Venn A, Britton J. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation. 2007;115(8):990–5. doi: 10.1161/CIRCULATIONAHA.106.648469. [DOI] [PubMed] [Google Scholar]
- 36.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. Bmj. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talmud PJ, Stephens JW, Hawe E, et al. The significant increase in cardiovascular disease risk in APOEepsilon4 carriers is evident only in men who smoke: potential relationship between reduced antioxidant status and ApoE4. Ann Hum Genet. 2005;6(Pt 6):613–22. doi: 10.1111/j.1529-8817.2005.00205.x. [DOI] [PubMed] [Google Scholar]
- 38.Humphries SE, Talmud PJ, Hawe E, et al. Apolipoprotein E4 and coronary heart disease in middle-aged men who smoke: a prospective study. Lancet. 2001;358(9276):115–9. doi: 10.1016/S0140-6736(01)05330-2. [DOI] [PubMed] [Google Scholar]
- 39.Tsiara S, Elisaf M, Mikhailidis DP. Influence of smoking on predictors of vascular disease. Angiology. 2003;54(5):507–30. doi: 10.1177/000331970305400501. [DOI] [PubMed] [Google Scholar]
- 40.Al-Awadhi AM, AlFadhli SM, Mustafa NY, et al. Effects of cigarette smoking on hematological parameters and von Willebrand factor functional activity levels in asymptomatic male and female Arab smokers. Med Princ Pract. 2008;17(2):149–53. doi: 10.1159/000112970. [DOI] [PubMed] [Google Scholar]
- 41.Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Toxicol. 2005;43(5):655–61. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Schubert J, Hahn J, Dettbarn G, et al. Mainstream smoke of the waterpipe: does this environmental matrix reveal as significant source of toxic compounds? Toxicol Lett. 2011;205(3):279–84. doi: 10.1016/j.toxlet.2011.06.017. [DOI] [PubMed] [Google Scholar]


