Abstract
We have recently developed a novel and highly efficient strategy that exclusively employs the purine analog 6-thioguanine (6TG) for both pre-transplant conditioning and post-transplant chemoselection of hypoxanthine-guanine phosphoribosyltransferase (HPRT)-deficient bone marrow (BM). In a mouse BM transplant model, combined 6TG preconditioning and in vivo chemoselection consistently achieved >95% engraftment of HPRT-deficient donor BM and long-term reconstitution of histologically and immunophenotypically normal hematopoiesis in both primary and secondary recipients, without significant toxicity and in the absence of any other cytotoxic conditioning regimen. In order to translate this strategy for combined 6TG conditioning and chemoselection into a clinically feasible approach, it is necessary to develop methods for genetic modification of normal HSC to render them HPRT-deficient and thus 6TG-resistant. Here we investigated a strategy to reduce HPRT expression and thereby confer protection against 6TG myelotoxicity to primary murine bone marrow cells by RNA interference (RNAi). Accordingly, we constructed and validated a lentiviral gene transfer vector expressing short-hairpin RNA (shRNA) that targets the murine HPRT gene. Our results showed that lentiviral vector-mediated delivery of HPRT-targeted shRNA could achieve effective and long-term reduction of HPRT expression. Furthermore, in both an established murine cell line as well as in primary murine bone marrow cells, lentiviral transduction with HPRT-targeted shRNA was associated with enhanced resistance to 6TG cytotoxicity in vitro. Hence this represents a translationally feasible method to genetically engineer HSC for implementation of 6TG-mediated preconditioning and in vivo chemoselection.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is a mainstay of treatment for hereditary disorders and lymphohematopoietic malignancies, and is the most commonly employed modality for ex vivo gene therapy. However, this procedure often entails myeloablative conditioning by irradiation and/or cytotoxic drugs which incur significant morbidity [1, 2], and low engraftment rates frequently limit efficacy in the absence of a selective advantage that amplifies genetically modified donor HSC [3]. In order to enhance HSC engraftment, several in vivo selection strategies employing various drug resistance genes such as dihydrofolate reductase (DHR) [4] or multiple drug-resistance gene 1 (MDR1) [5] have been tested previously, but have been associated with unacceptable toxicity or insufficient selection efficiency. One of the most successful examples of this approach to date employs the P140K mutant form of human O(6)-methylguanine-DNA-methyltransferase (MGMT (P140K)), which confers resistance to O6-benzylguanine (6BG) and DNA damaging agents, such as 1,3-bis (2-chloroethyl)-1-nitrosurea (BCNU) [6, 7]. MGMT(P140K) expressed from retroviral or lentiviral vectors enables selection of transduced HSC in mice [8], non-human primates [9, 10] and is being tested in clinical trials for myeloprotection in glioblastoma patients [11]. However, high level MGMT(P140K) expression has been reported to cause cytotoxicity in itself [12], and more generally, the potential immunogenicity of exogenous drug resistance transgene protein products is a concern for wider application.
In this context, we have recently developed a novel strategy for combined conditioning and chemoselection predicated on the following observations: (i) 6-thioguanine (6TG) myelotoxicity is largely dependent on its enzymatic conversion to thioguanine nucleotide by hypoxanthine-guanine phosphoribosyltransferase (HPRT), (ii) HPRT expression is highest in hematopoietic cells in humans as well as mice, and (iii) HPRT deficiency is not associated with major hematopoietic abnormalities in either humans or mice [13]. Our initial results demonstrated that, at appropriate doses, 6TG induces selective myeloablation in HPRT-wild type (wt) mice without detectable adverse effects on extra-hematopoietic tissues; in contrast, HPRT-deficient mice were highly resistant to its cytotoxic effects. Accordingly, we developed an optimized protocol employing 6TG as a single agent, both for myeloablative conditioning of 6TG-sensitive HPRT-wt recipients and for in vivo chemoselection of 6TG-resistant HPRT-deficient donor BM after transplantation, which achieved highly efficient engraftment (~95%) without significant toxicity and in the absence of any other cytotoxic conditioning regimen [13]. Long-term reconstitution of histologically and immunophenotypically normal BM was achieved after combined 6TG preconditioning and chemoselection in both primary and secondary transplant recipients, indicating amplification of the self-renewing, pluripotent HSC population from HPRT-deficient donor BM.
In order to translate this combined 6TG conditioning and chemoselection strategy into a clinically feasible procedure, it is necessary to develop methods for genetic modification of normal HSC to render them HPRT-deficient and thus resistant to 6TG cytotoxicity. In particular, RNA interference (RNAi) has recently become an important genetic approach for post-transcriptional silencing of gene expression by triggering degradation of homologous transcripts through a complex multi-step enzymatic process involving sequence-specific double-stranded small interfering RNA (siRNA) [14]. Here we employed a lentiviral vector system for delivery of precursor short hairpin RNA (shRNA) constructs, which are intracellularly processed to generate siRNA duplexes. Lentiviral vectors have emerged as potent and versatile tools for this purpose as they offer the ability to efficiently infect a wide variety of primary cell types, whether dividing or non-dividing, and can achieve stable vector integration into the target cell genome, thereby enabling long-term modification of the cellular phenotype [15–19]. Therefore, in the present study, we developed a novel third-generation self-inactivating (SIN) lentiviral vector expressing shRNA that specifically targets the HPRT gene, and examined whether this vector could mediate effective and stable knockdown of HPRT expression, as well as confer protection from 6TG cytotoxicity in both an established cell line and primary murine BM cells in vitro.
MATERIAL AND METHODS
Lentiviral vector production and titration
Lentiviral vector preparations were produced with a standard third-generation packaging system as previously described [20]; briefly, packaging plasmids (pMD.G encoding VSV-G envelope, pMDLg/p encoding HIV gag-pol, and pRSV-REV encoding HIV rev) and vector plasmid expressing HPRT shRNA (pRRL-HPRTshRNA) or no shRNA (control construct; pRRL-ctr) were co-transfected into HEK293T cells (ATCC# 1573) by calcium phosphate precipitation, and 48 hours later the virus-containing supernatant medium was collected, filtered, and concentrated by ultracentrifugation. Vector transducing unit (TU) titers were determined by infection of K562 cells (ATCC# CCL-243) with serial dilutions of the concentrated virus preparation, followed by flow cytometric analysis of GFP expression 48 hours later using a BD FACSCanto II running BD FACSDiva (BD Biosciences, San Jose, CA). Alternatively, viral p24 antigen concentration measured by ELISA (Alliance® HIV-I p24 ELISA Kit, Perkin Elmer, Waltham, MA) was used as a surrogate assay for virus particle titer.
Cell culture and lentiviral transduction
Murine neuroblastoma cell line Neuro-2a (ATCC# CCL-131) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin-streptomycin (pen-strep). Neuro-2a cells were transduced with lentiviral vectors at different multiplicities of infection (MOI; i.e., virus-to-cell ratio) in DMEM supplemented with 10% FBS, 1% pen-strep, and 4 µg protamine sulfate overnight. For primary murine bone marrow cells, lineage depletion was performed with the Mouse Lineage Cell Depletion Kit (Miltenyi Biotec, Auburn, CA) according to manufacturer’s instructions. Briefly, whole BM was isolated from C57BL/6 mice by flushing femurs and tibia with a solution containing PBS pH 7.2, 0.5% BSA, and 2 mM EDTA (Miltenyi Biotec), and incubated with a cocktail of biotin-conjugated lineage specific rat anti-mouse monoclonal antibodies (CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7/4, and Ter-119) and anti-biotin conjugated magnetic beads, and separated on an AutoMACS device (Miltenyi Biotec). For lentiviral transduction, non-tissue culture treated plates were coated with Retronectin (Takara, Mountain View, CA) according to manufacturer’s instructions. Virus was added at a MOI of 100 in X-Vivo 15 medium (Lonza, Allendale, NJ) with 5 mM L-glutamine, 0.5 % pen-strep, 100 µM β-mercaptoethanol (MeOH) supplemented with cytokines (100 ng/ml rmSCF, 100 ng/ml rmFlt3 ligand, and 50 ng/ml rmThrombopoietin (R&D Systems, Minneapolis, MN)), and the plates centrifuged for 90 min at 2200 rpm and 32°C. Lin- BM cells were then added, and following overnight incubation at 37°C, transduced cells were collected and resuspended in Iscove’s Modified Dulbecco’s medium (IMDM) containing 30% FBS, 1% BSA, 5 mM L-glutamine, 0.5% pen-strep, 100 µM β-MeOH, and cytokines for long-term culture.
Western blot analysis
Whole cell lysates were separated in 12% SDS polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to PVDF membranes (Immobilon-P, Millipore Corporation, Billerica, MA). The following antibodies were used: rabbit anti-HPRT (ab 109021, Abcam, Cambridge, MA) and rabbit anti-goat-HRP secondary antibody (R&D Systems). Equal protein transfer and loading was routinely controlled by incubating the filters with a monoclonal β-actin antibody (ab 8227, Abcam).
Cell viability analysis
Cell viability was measured by MTS assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, CellTiter 96 Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI). Percentage of viable cells (% viability) was calculated by normalizing the absorbance at OD420 observed in cells treated with 6TG (Sigma-Aldrich, Saint Louis, MO) to the absorbance at OD420 in non-6TG treated cells for each group.
Flow cytometry
Flow cytometric data were acquired on a BD FACSCanto II cytometer running BD FACSDiva (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistical analysis
Data were analyzed using the QuickCalcs statistical software program (http://www.graphpad.com/quickcalcs/index.cfm). Unpaired t-tests were used to calculate P values, and P values of less than 0.05 were considered statistically significant.
RESULTS
Development of a lentiviral vector expressing HPRT-targeted shRNA
Different shRNA candidate sequences targeting HPRT mRNA were screened by analyzing the level of HPRT mRNA knockdown with quantitative real-time PCR (data not shown). The shRNA sequence showing the most efficient knockdown, which targets the HPRT sequence 5’-AAAGTTGAGAGATCATCTC-3’ (Clone V2LHS_82406, Open Biosystems, Pittsburgh PA), was embedded in a microRNA (miRNA) configuration and placed under the control of the U6 polymerase III promoter. This shRNA cassette was cloned into a third-generation lentiviral SIN vector construct (pRRL-HPRTshRNA) that also contains a Ubiquitin-c (Ubc) promoter-driven GFP fluorescent reporter gene cassette. Lentivirus was produced using a standard third-generation packaging system , and the titer was determined on K562 cells to be approximately 3×108 TU per ml. The parental construct, which consists of the same vector backbone sequence but only contains the reporter gene cassette without any shRNA cassette, was used as a control (RRL-ctr).
Lentiviral vector-mediated inhibition of HPRT expression and protection against 6TG cytotoxicity in a murine cell line
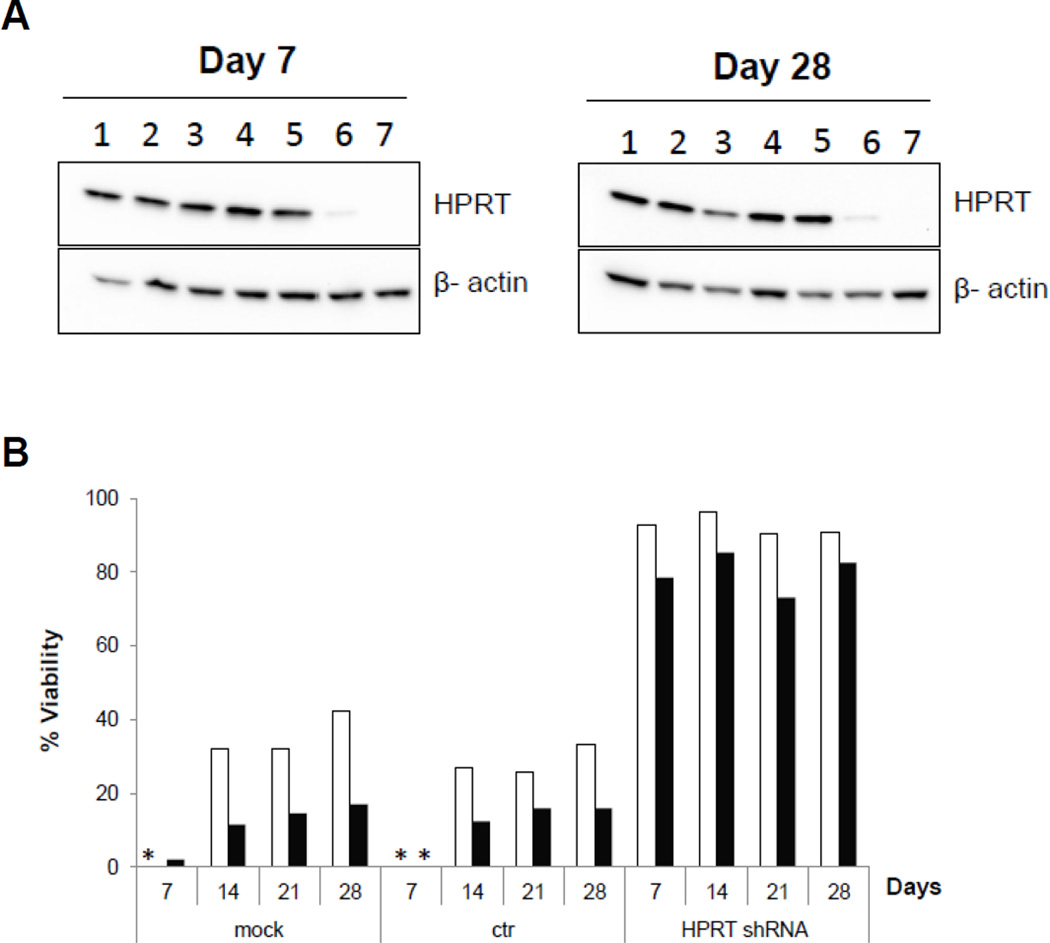
Lentiviral vectors expressing HPRT shRNA (RRL-HPRTshRNA) and no shRNA (RRL-ctr; control vector) were used to transduce murine Neuro-2a cells, which normally express high levels of HPRT, at increasing MOI. In contrast to naïve Neuro-2a cells and those transduced with the control vector, RRL-ctr-transduced cells showed effective knockdown of HPRT protein expression in an MOI-dependent manner, with significant reductions observed at MOI 100 and 400, and knockdown levels were stable for at least 4 weeks (Fig. 1a). Neuro-2a cells transduced with RRL-ctr or RRL-HPRTshRNA at MOI 100 were then cultured up to 4 weeks and incubated with different 6TG concentrations on day 7, 14, 21, or 28 after transduction, respectively. As expected, naïve Neuro-2a cells and those transduced with the control vector were sensitive to the cytotoxic effect of 6TG. In contrast, RRL-HPRTshRNA vector-transduced cells maintained good viability at all 6TG concentrations and time points tested, indicating stable and long-term protection from 6TG cytotoxicity (Fig. 1b).
Fig. 1. Efficient knockdown of HPRT expression can be achieved by lentiviral gene transfer resulting in protection against 6TG.
(A) Neuro-2a cells were transduced with lentiviral vectors expressing HPRT shRNA (RRL-HPRTshRNA) or no shRNA (control construct; RRL-ctr) at different MOI, and whole cell lysates were analyzed after transduction at the indicated time points by Western blot. Lane 1: mock, untransduced; Lane 2: ctr, MOI 10; Lane 3: HPRT shRNA, MOI 10; Lane 4: mock, untransduced; Lane 5: ctr, MOI 100; Lane 6: HPRT shRNA, MOI 100; Lane 7: HPRT shRNA, MOI 400. (B) At weekly intervals, Neuro-2a cells (untransduced (mock), or transduced with RRL-ctr or RRL-HPRTshRNA at MOI 100) were incubated with 0, 3 or 6 µM 6TG for 3 days before cell viability was measured by MTS assay. Cell viability values for cells treated with 3 µM 6TG (white bars) and 6 µM 6TG (black bars) were normalized to each respective set of untreated (0 µM 6TG) cells. (*: below limit of quantitation.)
Lentiviral vector-mediated expression of HPRT-targeted shRNA protects primary mouse BM cells from 6TG cytotoxicity
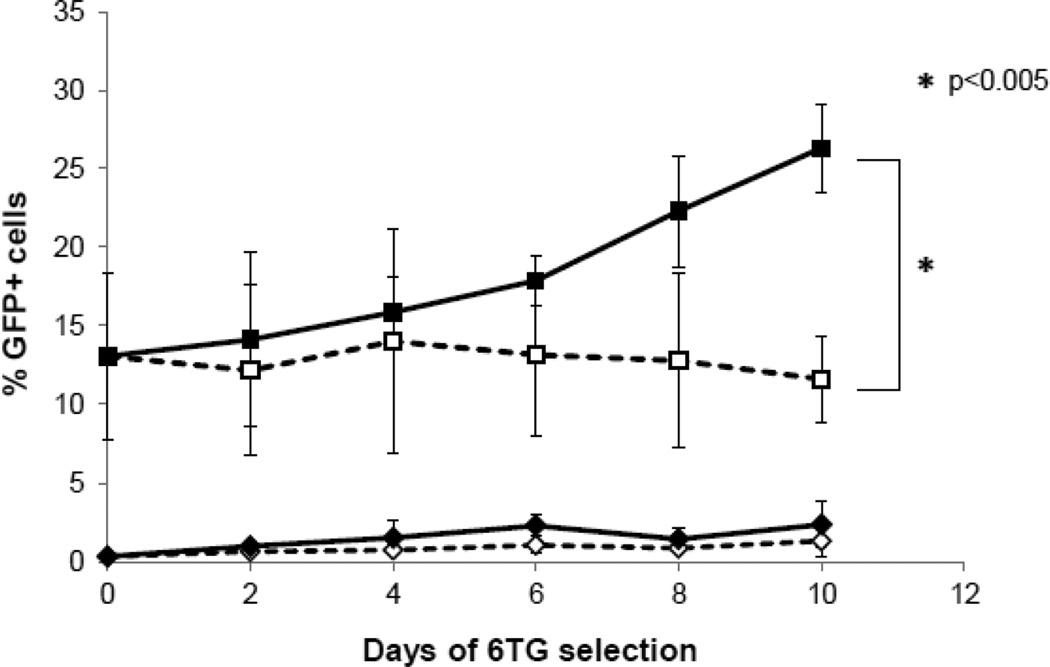
We then examined whether lentiviral vector-mediated knockdown of HPRT could also confer 6TG-resistance to primary murine BM cells in vitro. Lineage-depleted (lin-) BM cells harvested from HPRT-wt mice were transduced with RRL-HPRTshRNA, while non-transduced lin- BM cells were compared as controls. Four days later, both non-transduced and transduced BM cells were cultured in the presence or absence of 6TG for 10 days. As expected, untransduced cells showed no significant level of GFP expression regardless of 6TG treatment status (Fig. 2), although the overall number of viable cells decreased over time with 6TG treatment (data not shown). In contrast, 6TG treatment of RRL-HPRTshRNA-transduced BM cells resulted in a progressive increase in percentage of the GFP-positive population over time, indicating that the transduced cells were protected against 6TG cytotoxicity (Fig. 2). By day 10 of culture, significant differences were observed between untreated and 6TG-treated RRL-HPRTshRNA-transduced BM cells (P<0.005).
Fig. 2. 6TG chemoselection in vitro: mouse lin- BM cells transduced with a lentiviral construct expressing HPRT shRNA are protected from 6TG cytotoxicity.
BM cells were harvested from wild-type mice, lineage depleted and transduced with RRL-HPRTshRNA at MOI 100. Four days later, non-transduced and RRL-HPRTshRNA transduced lin- BM cells were cultured in the presence or absence of 100 nM 6TG for 10 days. GFP marker gene expression was analyzed by flow cytometry at 2 day intervals. Results are expressed as percentage of GFP-positive cells (mean ± SD; n=3). ■: RRL-HPRTshRNA-transduced, 6TG treated; □: RRL-HPRTshRNA-transduced, untreated; ♦: non-transduced, 6TG treated; ◊: non-transduced, untreated.
DISCUSSION
In this study, we have demonstrated that a third-generation lentiviral vector expressing an HPRT-specific miRNA-mimic shRNA could achieve effective and long-term reduction of HPRT expression, associated with enhanced resistance to 6TG cytotoxicity in an established cell line as well as in primary mouse lin- BM cells.
Porter and DeGregori [21] previously reported the use of a second-generation lentiviral vector carrying a shRNA cassette targeting HPRT, which they demonstrated was effective in protecting mouse BM cells against 6TG myelotoxicity in vitro and in vivo. Several studies have shown that vector design and the resulting shRNA expression levels were important for determining whether or not that shRNA will be cytotoxic, and optimization of those levels can yield shRNA that are both effective and minimally cytotoxic [22, 23]. In this context, miRNA-based shRNA designs, which are processed through natural microRNA biogenesis pathways, have been found to be less toxic [24, 25]. In the present study, we designed and tested an optimized lentiviral construct expressing a different HPRT-specific shRNA sequence embedded in an miRNA context, and packaged as a third-generation SIN vector, considered a more advanced and safer gene delivery system for translational application [20].
Transduction with increasing MOIs of this optimized vector resulted in progressive knockdown of HPRT expression, although it should be noted that the MOIs were determined on a standard cell line, and so may not correspond exactly to the same biological titers on other cell types. By day 7, virtually complete silencing of HPRT expression was observed at the highest MOIs, indicating that after knockdown of HPRT mRNA, residual HPRT protein turns over within a few days, and expression did not recover even after 4 weeks due to constitutive expression of HPRT shRNA from the stably integrated lentiviral vector. We also confirmed protection of lentiviral HPRT shRNA-transduced mouse BM cells against 6TG treatment initiated 4 days post-transduction. Notably, in primary lin- mouse BM cells, our optimized lentiviral vector conferred resistance to 6TG concentrations up to 100 nM, i.e., a concentration that is 10- to 20-fold higher than those used previously [21], further supporting the feasibility of using this approach for combined pre-conditioning and chemoselection of genetically modified HSC [13].
In summary, genetic modification by lentiviral gene transfer of HPRT-targeted shRNA may be generally applicable as a method to confer 6TG chemoresistance to primary HSC. This would enable higher engraftment rates and in vivo amplification of genetically modified autologous HSC and their derivative progeny by combined 6TG pre-conditioning and chemoselection. This overall strategy represents a translationally feasible approach to achieve reduced toxicity in BM transplantation for current clinical indications, as well as to enhance the efficiency of ex vivo gene therapy in the future.
ACKNOWLEDGMENTS
The authors would like to thank Nathan Lemp and Pål Sætrom for assistance with designing and cloning the HPRT shRNA lentiviral vector, as well as Karin Gaensler and Donald Kohn for their advice on lentiviral transduction of mouse BM. We also thank the UCLA JCCC/CURE Vector Core, supported by JCCC/P30 CA016042 and CURE/P30 DK041301, for assistance with lentiviral vector production. Virological measures (p24 antigen concentration) were provided by the Center for AIDS Research Virology Core Lab, supported by NIH AI-28697, the UCLA AIDS Institute, and UCLA Council of Bioscience Resources.
Funding disclosure:
This work was supported in part by the National Institute of Allergy and Infectious Diseases-funded UCLA Center for Biological Radioprotectors (U19 AI 067769) (N.K., R.H.S.), UCLA Clinical and Translational Science Institute (NIH Grant# 1UL 1RR033176) Special Seed Research Grant (K.H.), and UCLA In Vivo Cellular and Molecular Imaging Center (ICMIC) (NIH/NCI P50) Career Development Fellowship (K.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katrin Hacke, Email: khacke@mednet.ucla.edu.
Janet A. Treger, Email: jtreger@mednet.ucla.edu.
Brooke T. Bogan, Email: bbogan@ucla.edu.
Robert H. Schiestl, Email: rschiestl@mednet.ucla.edu.
Noriyuki Kasahara, Email: nkasahara@mednet.ucla.edu.
REFERENCES
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.de la Morena MT, Gatti RA. A history of bone marrow transplantation. Hematol Oncol Clin North Am. 2011;25(1):1–15. doi: 10.1016/j.hoc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Hossle JP, Seger RA, Steinhoff D. Gene therapy of hematopoietic stem cells: strategies for improvement. News Physiol Sci. 2002;17:87–92. doi: 10.1152/nips.01343.2001. [DOI] [PubMed] [Google Scholar]
- 4.Williams DA, Hsieh K, DeSilva A, et al. Protection of bone marrow transplant recipients from lethal doses of methotrexate by the generation of methotrexate-resistant bone marrow. J Exp Med. 1987;166(1):210–218. doi: 10.1084/jem.166.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mickisch GH, Aksentijevich I, Schoenlein PV, et al. Transplantation of bone marrow cells from transgenic mice expressing the human MDR1 gene results in long-term protection against the myelosuppressive effect of chemotherapy in mice. Blood. 1992;79(4):1087–1093. [PubMed] [Google Scholar]
- 6.Milsom MD, Williams DA. Live and let die: in vivo selection of gene-modified hematopoietic stem cells via MGMT-mediated chemoprotection. DNA Repair (Amst) 2007;6(8):1210–1221. doi: 10.1016/j.dnarep.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neff T, Beard BC, Kiem HP. Survival of the fittest: in vivo selection and stem cell gene therapy. Blood. 2006;107(5):1751–1760. doi: 10.1182/blood-2005-06-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zielske SP, Reese JS, Lingas KT, et al. In vivo selection of MGMT(P140K) lentivirus-transduced human NOD/SCID repopulating cells without pretransplant irradiation conditioning. J Clin Invest. 2003;112(10):1561–1570. doi: 10.1172/JCI17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larochelle A, Choi U, Shou Y, et al. In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene. J Clin Invest. 2009;119(7):1952–1963. doi: 10.1172/JCI37506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trobridge G, Beard BC, Kiem HP. Hematopoietic stem cell transduction and amplification in large animal models. Hum Gene Ther. 2005;16(12):1355–1366. doi: 10.1089/hum.2005.16.1355. [DOI] [PubMed] [Google Scholar]
- 11.Adair JE, Beard BC, Trobridge GD, et al. Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci Transl Med. 2012;4(133):133ra57. doi: 10.1126/scitranslmed.3003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schambach A, Baum C. Vector design for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. DNA Repair (Amst) 2007;6(8):1187–1196. doi: 10.1016/j.dnarep.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacke K, Szakmary A, Cuddihy AR, et al. Combined preconditioning and in vivo chemoselection with 6-thioguanine alone achieves highly efficient reconstitution of normal hematopoiesis with HPRT-deficient bone marrow. Exp Hematol. 2012;40(1):3–13. doi: 10.1016/j.exphem.2011.09.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107(2):222–239. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haga K, Lemp NA, Logg CR, et al. Permanent, lowered HLA class I expression using lentivirus vectors with shRNA constructs: Averting cytotoxicity by alloreactive T lymphocytes. Transplant Proc. 2006;38(10):3184–3188. doi: 10.1016/j.transproceed.2006.10.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart SA, Dykxhoorn DM, Palliser D, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9(4):493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm D, Kay MA. RNAi and gene therapy: a mutual attraction. Hematology Am Soc Hematol Educ Program. 2007:473–481. doi: 10.1182/asheducation-2007.1.473. [DOI] [PubMed] [Google Scholar]
- 18.Matrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18(3):477–490. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443(3):603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 20.Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72(11):8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter CC, DeGregori J. Interfering RNA-mediated purine analog resistance for in vitro and in vivo cell selection. Blood. 2008;112(12):4466–4474. doi: 10.1182/blood-2008-03-146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimm D, Streetz KL, Jopling CL, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 23.An DS, Qin FX, Auyeung VC, et al. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther. 2006;14(4):494–504. doi: 10.1016/j.ymthe.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudreau RL, Martins I, Davidson BL. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther. 2009;17(1):169–175. doi: 10.1038/mt.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boudreau RL, Monteys AM, Davidson BL. Minimizing variables among hairpin-based RNAi vectors reveals the potency of shRNAs. RNA. 2008;14(9):1834–1844. doi: 10.1261/rna.1062908. [DOI] [PMC free article] [PubMed] [Google Scholar]