Abstract
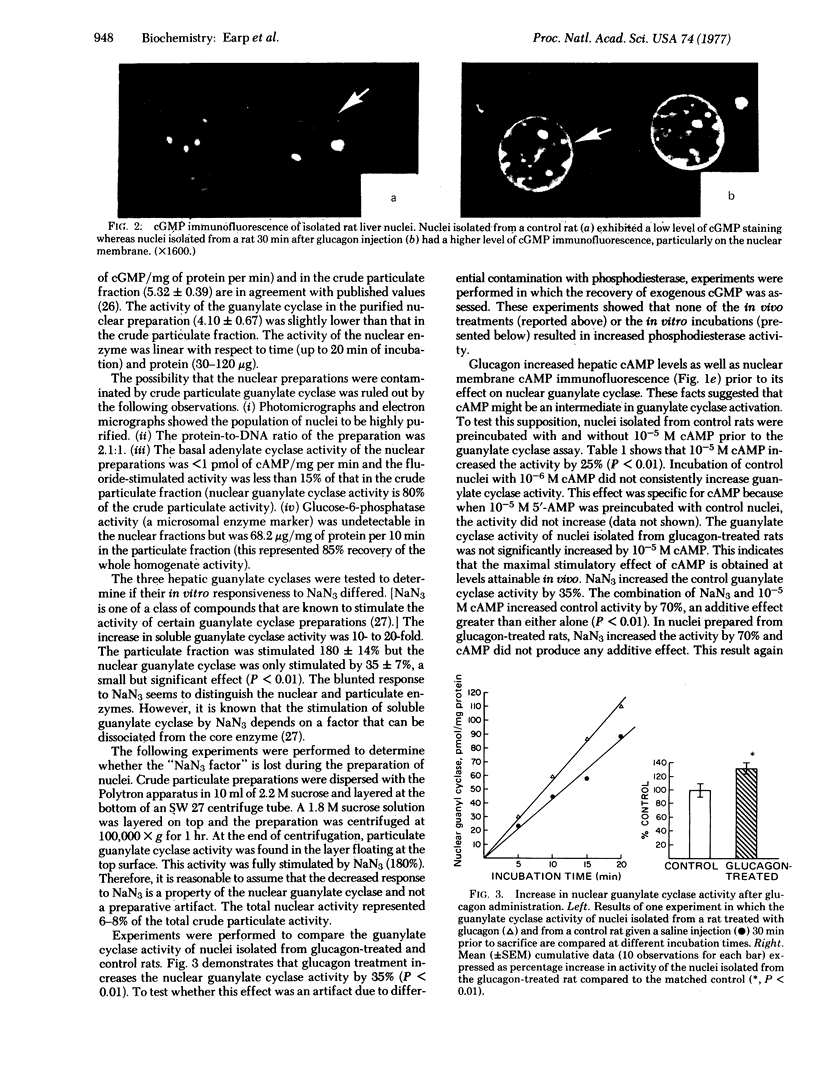
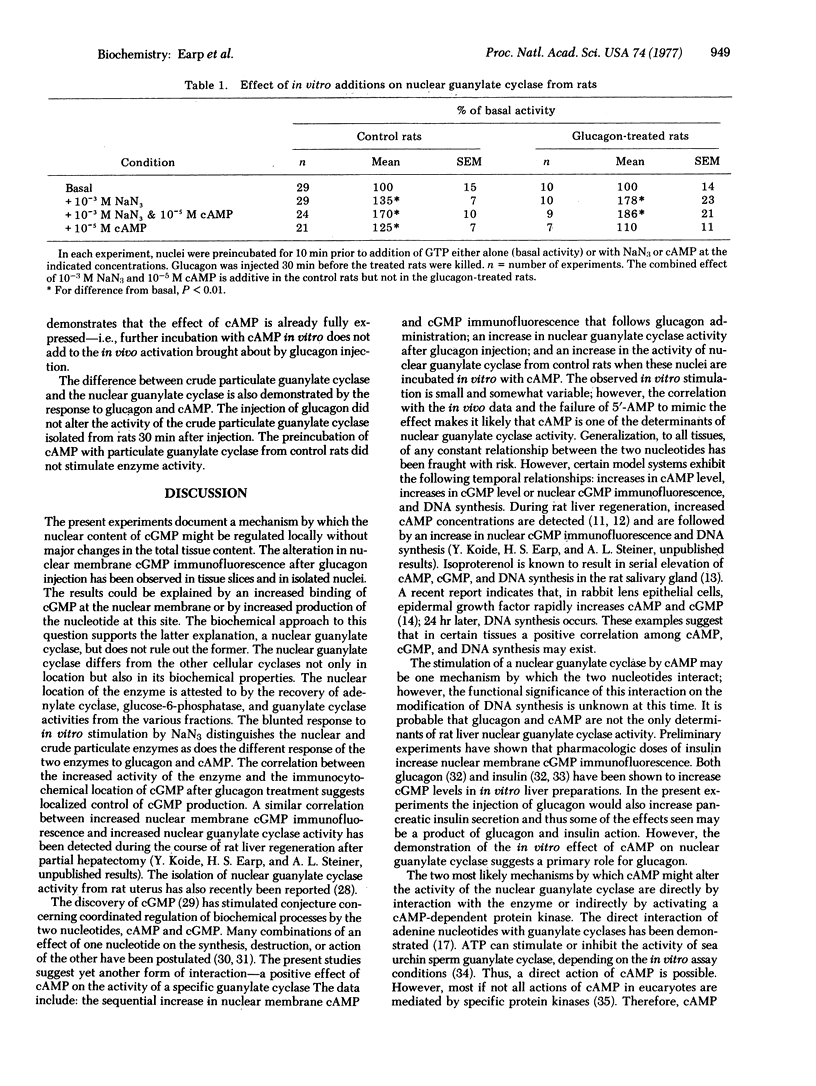
In immunohistochemical studies of rat liver tissue slices and purified nuclei, adenosine 3':5'-cyclic monophosphate (cAMP) and guanosine 3':5'-cyclic monophosphate (cGMP) immunofluorescence on the nuclear membrane are sequentially increased after glucagon administration. An explanation for the increased cGMP immunofluorescence was sought in experiments in which guanylate cyclase [GTP pyrophosphate-lyase (cyclizing), EC 4.6.1.2]activity of hepatic subcellular fractions was determined. The results showed that a nuclear guanylate cyclase exists which can be distinguished from the soluble and crude particulate guanylate cyclases. The activity of the nuclear enzyme was increased by 35% in nuclei isolated from rats 30 min after glucagon injection, the time at which maximal nuclear membrane cGMP immunofluorescence is observed. Because glucagon altered both cAMP location and levels prior to the observed changes in nuclear cGMP metabolism, the hypothesis that cAMP acted as the second messenger was tested. In vitro incubation of nuclei isolated from control rats with 10(-5) M cAMP produced a 25% increase in nuclear guanylate cyclase activity. With nuclei isolated from glucagon-treated rats, no significant increase in enzyme activity was observed; this indicates that maximal stimulation of nuclear guanylate cyclase by cAMP occurred at levels that are obtained in vivo after glucagon administration. These findings suggest that hepatic nuclear cGMP content may be regulated by a specific organelle guanylate cyclase and that cAMP may be one of the determinants of this enzyme's activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHMAN D. F., LIPTON R., MELICOW M. M., PRICE T. D. Isolation of adenosine 3', 5'-monophosphate and guanosine 3', 5'-monophosphate from rat urine. Biochem Biophys Res Commun. 1963 May 22;11:330–334. doi: 10.1016/0006-291x(63)90566-7. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Stimulation of adenosine 3',5'-monophosphate hydrolysis by guanosine 3',5'-monophosphate. J Biol Chem. 1971 Jun 25;246(12):3841–3846. [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Chrisman T. D., Garbers D. L., Parks M. A., Hardman J. G. Characterization of particulate and soluble guanylate cyclases from rat lung. J Biol Chem. 1975 Jan 25;250(2):374–381. [PubMed] [Google Scholar]
- DeRubertis F. R., Zenser T. V., Adler W. H., Hudson T. Role of cyclic adenosine 3',5'-monophosphate in lymphocyte mitogenesis. J Immunol. 1974 Jul;113(1):151–161. [PubMed] [Google Scholar]
- Durham J. P., Baserga R., Butcher F. R. The effect of isoproterenol and its analogs upon adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate levels in mouse parotid gland in vivo. Relationship to the stimulation of DNA synthesis. Biochim Biophys Acta. 1974 Nov 4;372(1):196–217. doi: 10.1016/0304-4165(74)90087-7. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illiano G., Tell G. P., Siegel M. E., Cuatrecasas P. Guanosine 3':5'-cyclic monophosphate and the action of insulin and acetylcholine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2443–2447. doi: 10.1073/pnas.70.8.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Hadden J. W. Phosphorylation of lymphocyte nuclear acidic proteins: regulation by cyclic nucleotides. Science. 1975 Mar 28;187(4182):1198–1200. doi: 10.1126/science.163491. [DOI] [PubMed] [Google Scholar]
- Kimura H., Murad F. Evidence for two different forms of guanylate cyclase in rat heart. J Biol Chem. 1974 Nov 10;249(21):6910–6916. [PubMed] [Google Scholar]
- Kimura H., Murad F. Localization of particulate guanylate cyclase in plasma membranes and microsomes of rat liver. J Biol Chem. 1975 Jun 25;250(12):4810–4817. [PubMed] [Google Scholar]
- Kimura H., Murad F. Two forms of guanylate cyclase in mammalian tissues and possible mechanisms for their regulation. Metabolism. 1975 Mar;24(3):439–445. doi: 10.1016/0026-0495(75)90123-7. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macmanus J. P., Franks D. J., Youdale T., Braceland B. M. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Multer M. M., Boone R. F. Enhanced effects of prostaglandin E1 and dibutyryl cyclic AMP upon human lymphocytes in the presence of cortisol. J Clin Invest. 1973 Sep;52(9):2129–2137. doi: 10.1172/JCI107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Z., Lovelace E., Gallo M., Pastan I. Cyclic guanosine monophosphate and cellular growth. Science. 1975 Dec 19;190(4220):1213–1215. doi: 10.1126/science.173021. [DOI] [PubMed] [Google Scholar]
- Mittal C. K., Kimura H., Murad F. Requirement for a macromolecular factor for sodium azide activation of guanulate cyclase. J Cyclic Nucleotide Res. 1975;1(6):261–269. [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Pointer R. H., Butcher F. R., Fain J. N. Studies on the role of cyclic guanosine 3':5'-monophosphate and extracellular Ca2+ in the regulation of glycogenolysis in rat liver cells. J Biol Chem. 1976 May 25;251(10):2987–2992. [PubMed] [Google Scholar]
- Ryan W. L., Heidrick M. L. Inhibition of cell growth in vitro by adenosine 3',5'-monophosphate. Science. 1968 Dec 27;162(3861):1484–1485. doi: 10.1126/science.162.3861.1484. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- Short J., Tsukada K., Rudert W. A., Lieberman I. Cyclic adenosine 3':5'-monophosphate and the induction of deoxyribonucleic acid synthesis in liver. J Biol Chem. 1975 May 25;250(10):3602–3606. [PubMed] [Google Scholar]
- Siegel M. I., Puca G. A., Cuatrecasas P. Guanylate cyclase. Existence of different forms and their regulation by nucleotides in calf uterus. Biochim Biophys Acta. 1976 Jun 7;438(1):310–323. doi: 10.1016/0005-2744(76)90247-3. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Ong S. H., Wedner H. J. Cyclic nucleotide immunocytochemistry. Adv Cyclic Nucleotide Res. 1976;7:115–155. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Wedner H. F., Hoffer B. J., Battenberg E. B., Steiner A. L., Parker C. W., Bloom F. E. A method for detecting intracellular cyclic adenosine monophosphate by immunofluorescence. J Histochem Cytochem. 1972 Apr;20(4):293–295. doi: 10.1177/20.4.293. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]






