Abstract
The avian magnetic compass, probably based on radical pair processes, works only in a narrow functional window around the local field strength, with cryptochrome 1a as most likely receptor molecule. Radio-frequency fields in the MHz range have been shown to disrupt the birds' orientation, yet the nature of this interference is still unclear. In an immuno-histological study, we tested whether the radio-frequency fields interfere with the photoreduction of cryptochrome, but this does not seem to be the case. In behavioural studies, birds were not able to adjust to radio-frequency fields like they are able to adjust to static fields outside the normal functional range: neither a 2-h pre-exposure in a 7.0 MHz field, 480 nT, nor a 7-h pre-exposure in a 1.315 MHz field, 15 nT, allowed the birds to regain their orientation ability. This inability to adjust to radio-frequency fields suggests that these fields interfere directly with the primary processes of magnetoreception and therefore disable the avian compass as long as they are present. They do not have lasting adverse after-effects, however, as birds immediately after exposure to a radio-frequency field were able to orient in the local geomagnetic field.
Keywords: magnetic compass, functional window, magnetoreception, radio-frequency fields, cryptochrome 1a
1. Introduction
The magnetic compass of birds works in a way that is very different from our technical compass: it is an inclination compass that ignores polarity and instead relies on the course of the field lines and their inclination; it is finely tuned to the intensity of the local magnetic field in a narrow, but flexible functional window, and it requires short-wavelengths of light (for review, see [1]). These properties of the avian magnetic compass remained enigmatic until the Radical Pair Model [2,3] provided a possible explanation. The model, described in detail by Ritz et al. [3], proposes that the avian magnetic compass is based on spin-chemical processes in specialized photopigments. Upon photon absorption, the receptor molecules generate spin-correlated radical pairs. The magnetic field alters the dynamics of the transition between spin states and thereby modifies singlet and triplet yield, with the magnitude of the response depending on the alignment of the radical pair with respect to the direction of the magnetic field. This reaction is not sensitive to the polarity of the magnetic field. If birds were able to compare the triplet yield in the various spatial directions, this could convey information on the direction of the magnetic field. The eye was suggested as site of magnetoreception [3,4] and cryptochrome, a blue light receptor (for review, see e.g. [5]), as the receptor molecule. A form of this photopigment, cryptochrome 1a (Cry1a), was indeed found in the retina of birds, where it is located at the discs in the outer segment of the UV/violet cones. These cones are more or less evenly distributed all across the retina [6], so that Cry1a fulfils the conditions of the Radical Pair Model, and could thus result in the centrally symmetric activation pattern on the retina proposed by Ritz et al. [3].
When the Radical Pair Model was put forward, applying radio-frequency magnetic fields in the MHz range was suggested as a diagnostic tool [7,8]. The respective experiments supported the model: adding such fields to the geomagnetic field disrupted magnetic compass orientation in several species of birds [9–14]. Most of these tests involved single frequencies, with very sensitive responses reported at the local Larmor frequency, the precession frequency of a free electron [10], but also broadband fields proved disruptive [9,15]. Broadband electromagnetic noise of anthropogenic origin has also been reported to disrupt orientation [15].
The nature of this interference remained unresolved, however. Adding radio-frequency fields could either somehow disrupt the photoreduction of cryptochrome, it could drive the avian compass outside of its functional window by changing the operation point [7,16] or it could provide a source of noise interfering with generating the radical pairs or with the singlet/triplet ratio indicating magnetic directions. In all these cases, radio-frequency fields of sufficient strength could prevent a bird from obtaining meaningful directional information.
The two first mentioned possibilities can be directly tested. An interference with the photo-activation of cryptochrome would become visible in an immuno-histological study exposing birds to radio-frequency fields and then marking the activated Cry1a with immuno-staining [17]. If the compass mechanism is driven outside its functional range, birds would be expected to overcome the disruptive effects if they were exposed to a radio-frequency field prior to testing [16], just as exposure to weaker or stronger static fields allows birds to adjust the functional window of their magnetic compass to these intensities and regain their orientation ability [1,18,19]. We also checked for immediate after-effects of exposure to radio-frequency fields.
Here, we report the results of such experiments with domestic chickens, Gallus gallus, and European robins, Erithacus rubecula (Turdidae), two species that have been demonstrated to have a magnetic compass that is disrupted by radio-frequency fields [9–12] and to have Cry1a in the UV/violet cones in their retinae [6].
2. Material and methods
The experiments were performed in Frankfurt am Main, Germany (50°08′ N, 8°40′ E), where the local geomagnetic field is 47 µT, with 66° inclination.
2.1. Immuno-histological study
This part of the study was performed with young domestic chickens, about three-weeks old, that is, of an age where their magnetic compass is already developed and they can be conditioned to prefer magnetic directions [20].
Before exposure, the chickens had access to daylight. As the behavioural experiments with robins in radio-frequency fields had been performed under 565 nm green light (half band width 550 nm, 583 nm) produced by light emitting diodes [8–10], the chickens were exposed to identical lights for 30 min. For the control birds, this exposure took place in the local magnetic field, for the experimental bird, a radio-frequency field of 1.315 MHz (local Larmor frequency) of 480 nT added vertically, i.e. 24° with respect to the magnetic vector. This field was produced by a coil antenna consisting of a single coaxial cable, with 2 cm of the screening removed opposite the feed. It was measured using a spectrum analyser (HP89410A) and a magnetic field probe (Rohde & Schwarz, model number 7405901, 6 cm probe). That is, this field was produced and controlled in the very same way as described in detail in [9–11]. As a negative control, we also exposed a chicken for 30 min to 645 nm red light, which does not activate cryptochrome.
Immediately after the end of the exposure, the chickens were sacrificed, and their eyes were prepared under the conditions of the exposure, i.e. under green light, under green light in the radio-frequency field and under red light, respectively, with fixation and further processing of the retinae exactly following the protocol described in detail in [6,17]. The whole mounts of the retina were treated with the same Cry1a antiserum used in those studies, i.e. with the antiserum that had been demonstrated to label only the light-activated form of Cry1a [17]. For control, we also labelled violet opsin. The retinae were evaluated with a confocal laser scanning microscope (Zeiss LSM 510 META).
2.2. Behavioural experiments
The test birds were European robins, nocturnal migrants probably of Scandinavian origin. Caught as juvenile transmigrants in September in the Botanical Garden in Frankfurt, they were tested during the autumn migration season in late September and October, kept over the winter and tested again during an advanced spring migration in January and February. The procedures followed the standard protocol for orientation experiments with migrants (e.g. [9–11,18]).
The test rooms and the exposure rooms were wooden buildings where the local geomagnetic field is largely undisturbed. For exposure before testing and for the tests themselves, radio-frequency fields were added vertically. In autumn, we used a 7.0 MHz field of 480 nT, in the following spring experiments, a 1.315 MHz field of 15 nT. These fields were produced as described above [9,10], with the wooden frame surrounding a set of four housing or test cages. The oscillating fields were the same as those used in previous studies (for details, see [9–11]); they were measured every day with the equipment mentioned above before the birds were moved in. In spring, we also ran a test series where we tested the birds in the same 1.315 MHz, 15 nT field without pre-exposure [9]. Tests in the local geomagnetic field served as control.
The individual birds to be tested that night were transferred to a second set of housing cages, where they were exposed to the respective radio-frequency fields, for 2 h in autumn and for 7 h in spring, before they were placed into the test cages, where their activity was recorded for 75 min. The housing cages and all other material inside the coil antennas were made of plastic, thus avoiding any metallic material which may disturb the radio-frequency fields. The pre-exposure cages were lit by ‘white’ light; testing took place under 565 nm green light as in the previous studies [9–11], that is, in conditions under which robins show excellent orientation in their seasonally appropriate migratory direction using their inclination compass (e.g. [21]). To look for possible after-effects of the radio-frequency treatments, robins were exposed in an additional series for 3 h to a 1.315 MHz, 480 nT field prior to testing, but then immediately afterwards tested in the local geomagnetic field.
Testing followed standard procedures: the birds were tested individually once per day in funnel-shaped PVC cages lined with coated paper where they left scratches as they moved. Each bird was tested three times in each condition. From the distribution of the scratches, counted by a person blind to the testing condition, the heading of each test was calculated. The three headings per bird were added to produce a vector with the heading αb and the length rb for each bird. From the 16, respectively 12 mean headings αb, we calculated second-order grand mean vectors which were tested for significant directional preference using the Rayleigh test [22]. The data obtained with oscillating fields added were compared with control data obtained in the geomagnetic field for differences in scatter by the Mann–Whitney U-test applied to the deviations from the mean. From the birds' vectors lengths rb, medians were calculated; they reflect the intra-individual variance.
3. Results
3.1. Light activation of cryptochrome 1a
Figure 1 gives the results of the immuno-histological study. In the retina of the controls as well as in that of the experimental birds treated with radio-frequency fields, activated Cry1a is labelled—there is no obvious difference caused by the treatment. For control labelling of the violet opsin and a negative control labelling after exposure to red light, see the electronic supplementary material.
Figure 1.
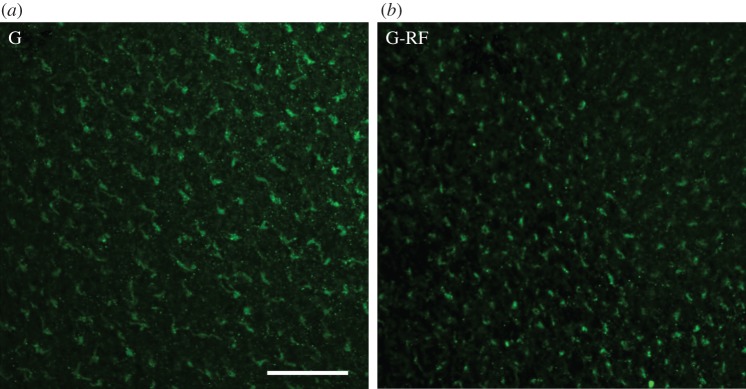
Whole mounts of the retina of chickens, with green immuno-fluorescence labelling of the light-activated Cry1a. (a) G, control exposed to 565 nm green light in the local geomagnetic field (this sample was previously published in [17] as part of fig. 2)); (b) G-RF, experimental exposed to green light in a radio-frequency field of 1.315 MHz, 470 nT, added vertically to the local geomagnetic field. The scale bar represents 50 µm. (For labelling of the negative control exposed to red light and for control staining with an antiserum against SWS1-opsin, see the electronic supplementary material, figure S1.)
This suggests that the radio-frequency field did not disrupt the photoreduction of the Cry1a chromophore from the semiquinone FADH• to the fully reduced form FADH− [23] and the associated conformational change that allows our antiserum to bind [17]—this important step induced by green light appears to have occurred in the normal way.
3.2. Orientation after pre-exposure in radio-frequency fields
Table 1 summarizes the numerical data of the behavioural experiments and indicates significant differences between experimental birds and controls; the data of the individual birds are given in the electronic supplementary material, tables S1–S3.
Table 1.
Orientation behaviour of European robins (based on three recordings each). N, number of birds tested; median rb, median vector length of the individual birds, reflecting the intra-individual variance; αN, rN, grand mean vector based on the mean headings of the 16 or 12 birds, with asterisks indicating significance by the Rayleigh test (direction in parentheses if not significant). ΔC, difference to the respective controls, with asterisks indicating significance of these differences by the Mann–Whitney U-test.
| magnetic conditions | duration of pre-exposure | N | median rb | αN | rN | ΔC, sig.? |
|---|---|---|---|---|---|---|
| autumn | ||||||
| control: local geomagnetic field | — | 16 | 0.87 | 187° | 0.88*** | Cautumn |
| RF field added: 7.0 MHz, 470 nT | 2 h in the same RF field | 16 | 0.50 | (85°) | 0.15n.s. | −102° *** |
| spring | ||||||
| control: local geomagnetic fielda) | — | 12 | 0.81 | 10° | 0.87*** | C1spring |
| RF field added: 1.3 MHz, 15 nT | 7 h in the same RF field | 12 | 0.40 | (297°) | 0.14n.s. | −73° *** |
| RF field added: 1.3 MHz, 15 nTa) | none | 12 | 0.43 | (157°) | 0.13n.s. | +147° ** |
| control: local geomagnetic field | — | 12 | 0.82 | 10° | 0.95*** | C2spring |
| local geomagnetic field | 3 h in 1.315 MHz, 480 nT | 12 | 0.44 | 15° | 0.59* | +5° * |
aThese samples are included in [10].
*p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant.
Since in a previous study [18], 1 h pre-exposure had proved sufficient to allow birds to orient in a static field of twice the intensity of the local geomagnetic field, we began in autumn by pre-exposing the birds for 2 h to a radio-frequency field of 7.0 MHz, 480 nT added to the local geomagnetic field, and subsequently tested them in the same combination of fields. In control tests in the local geomagnetic field, birds were significantly oriented in their seasonally appropriate southerly migratory direction; they were disoriented, however, with the radio-frequency field added (figure 2) just like they had been disoriented in such a field without pre-exposure [10].
Figure 2.
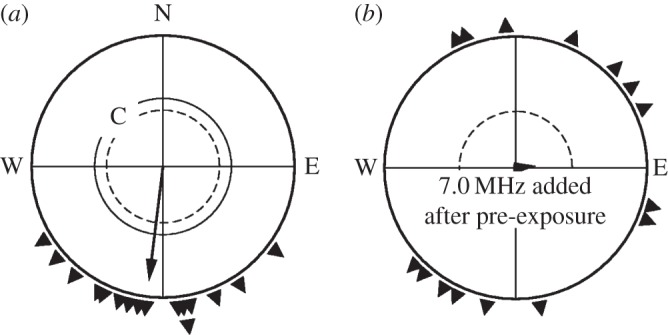
Orientation of European robins in autumn (a) in the local geomagnetic field (C, control) and (b) with a radio-frequency field of 7.0 MHz, 470 nT added vertically, after being pre-exposed to this field for 2 h. The symbols at the periphery of the circle mark the mean headings of individual birds based on three recordings each; the arrows represent the grand mean vectors. The two inner circles are the 5% (dotted) and the 1% significance border of the Rayleigh test [22].
In a second approach in spring, we extended the pre-exposure time to 7 h and used 1.315 MHz, a frequency shown to disrupt orientation at a low intensity of only 15 nT without pre-exposure [10]. The robins' orientation, together with control data and, for comparison, data in the respective oscillating field without pre-exposure, are given in figure 3. In the local geomagnetic field, the birds preferred their northerly migratory direction, but even after a longer pre-exposure to the much weaker radio-frequency field, the birds were still disoriented, just like they were without pre-exposure. Obviously, orientation in radio-frequency fields in the range of at least 1–7 MHz is generally impossible for birds.
Figure 3.
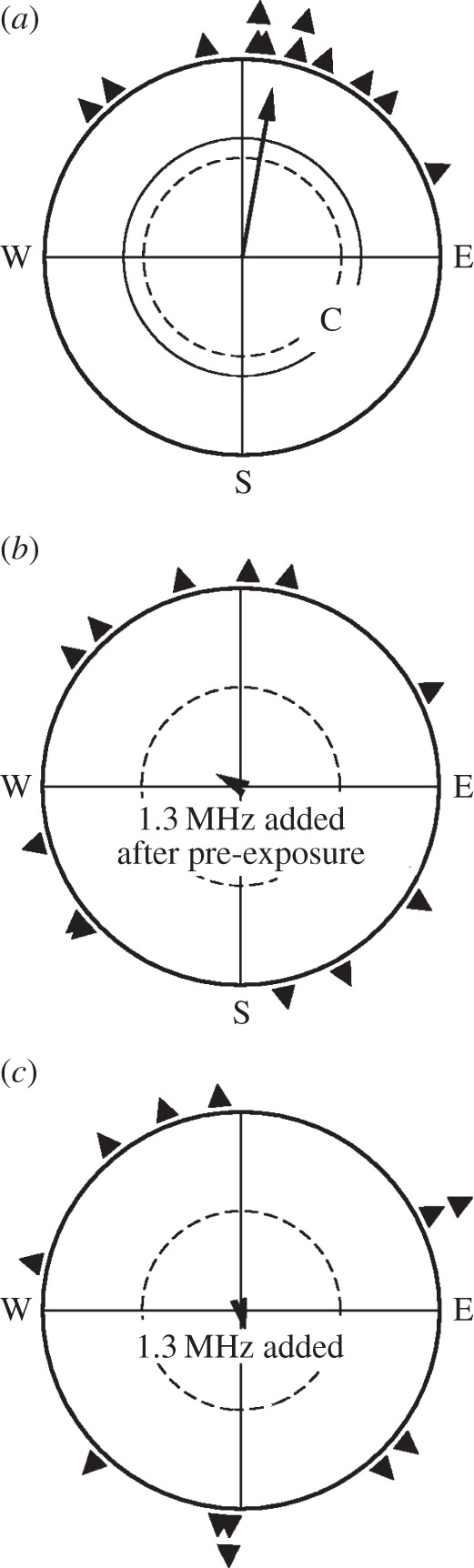
Orientation of European robins in spring (a) in the local geomagnetic field (C, control), (b) with a radio-frequency field of 1.315 MHz, 15 nT added vertically after being pre-exposed to this field for 7 h and (c) with this radio-frequency field added without pre-exposure. Symbols as in figure 2.
In an additional test series in spring, robins were exposed to a 1.315 MHz, 480 nT field for 3 h and immediately afterwards tested in the local geomagnetic field. Their data, together with their orientation in the local field without pre-exposure, are given in figure 4. Although the birds showed more scatter after staying in the radio-frequency field, they are clearly oriented in their northerly migratory direction. This indicates that radio-frequency fields do not have any severe after-effects on the orientation; they appear to disrupt with magnetoreception only while they are present, without causing lasting damage to the reception mechanisms.
Figure 4.
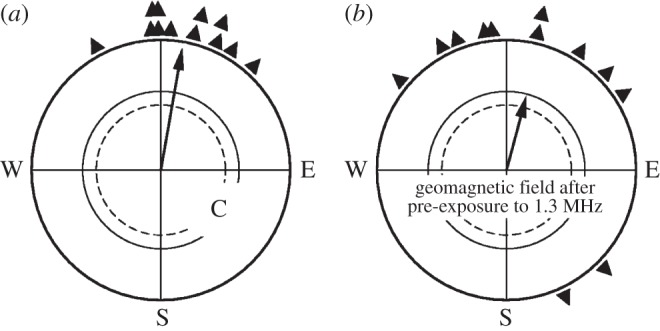
Orientation of European robins in spring in the local geomagnetic field (a) C, control and (b) after a 3-h exposure in a radio-frequency field of 1.315 MHz, 480 nT. Symbols as in figure 2.
4. Discussion
Our findings make some types of possible interferences of radio-frequency fields with magnetoreception appear less likely.
The light activation of the probable receptor molecule Cry1a appears not to be hindered in the presence of a radio-frequency field that disrupts magnetic orientation [10]—the fully reduced form that seems to be the one labelled by the antiserum [17] was found to be present. It must remain open, however, whether the treatment possibly interferes with the re-oxidation part of the flavin redox cycle in Cry1a [23]; effects on the radical pair generated there, its coherence time and its further reactions cannot be excluded.
In contrast to static fields outside the normal functional window, pre-exposure to oscillating fields did not improve the robins' orientation performance. Birds seem unable to adjust their magnetoreception system to radio-frequency fields. This indicates that the effect of radio frequencies is not changing the operating point and driving it out of the functional window, because in this case, one would expect the birds to be able to cope with it. Changes in magnetic intensity are assumed to alter the activation pattern on the retina produced by the different singlet/triplet ratio in the various spatial directions [3]. While an altered pattern would be confusing at first, it retains its central symmetry to the magnetic vector, and birds can learn to interpret it with time, thus regaining their ability to orient by the magnetic field.
The relatively fast adjustment to static fields outside the functional window—about 1 h to a field twice the local geomagnetic field—implies that the adjustment is not based on a modification at the receptor level, but rather on an altered interpretation of the input at higher centres: the adaption to different intensities appears to be a neurological process.
Radio-frequency fields, in contrast, seem to have a very different effect. This effect appears to be a specific interference with the magnetoreception process: radio-frequency fields disrupt orientation only if applied at an angle to the magnetic vector; the same fields applied parallel had no effect [9,11]. This indicates that the disruption is not caused by the radio-frequency fields per se, but that their orientation with respect to the magnetic vector is crucial. It rules out possible artefacts, which should be independent of the alignment of the radio-frequency field with the static field. The radio-frequency fields appear to interfere directly with the processes leading to the sensing of magnetic directions. This raises the crucial question how and where these fields disrupt the reception mechanism.
The change produced by the radio-frequency fields must be considerably large, essentially altering the activation pattern on the retina. One possibility is that the radio-frequency fields could decrease the difference in the singlet/triplet yield in the various directions to an extent that it is no longer detectable by the birds [16]. Any further interpretation is hampered by the fact that the precise mechanisms of magnetoreception are not yet known and that the radical pair model leaves some questions open [24]. To produce a large change in the activation pattern, the spin-coherence lifetime must be rather long—exceeding 100 µs—to allow the weak oscillating field to have a significant effect [16,24,25], and it is unclear whether the spin correlation time of cryptochrome is indeed sufficiently long [16,26]. The same basic difficulty occurs in the proposal of Stoneham et al. [27] that cryptochrome magnetic signalling occurs via the formation of a long-lived charge-separated state which modulates the photocycle of rhodopsin by the electric field arising from its dipole moment. Although the radical pair can have a spin-coherence time of approximately 1 µs in this model, the charge-separated state must have very slow spin-decoherence to account for the effect of the radio-frequency field. There is currently no variant of the radical pair mechanism that can satisfactorily explain the disorientating effect of radio frequencies.
Radio-frequency fields appear to affect magnetoreception only as long as they are present—their disruptive effect appears to be gone when they are no longer applied, without lasting after-effects. It has always been common practice in our laboratory (e.g. [9,10,21] and many others) to test the birds in several test conditions in a pseudo-random sequence, and we never observed any carry-over effect from tests in radio-frequency fields to tests in the next evening. Our present data show that this seems to apply even when the tests in the geomagnetic field immediately follow the exposure to radio frequencies: the birds were significantly oriented. They showed a certain increase in scatter, but it is not clear whether this is a mild immediate after-effect of the radio-frequency field; it could also be a stress response to being moved to another cage and treated not in the usual way. Radio-frequency fields thus do not seem to cause lasting damage in the magnetoreception system.
Supplementary Material
Supplementary Material
Acknowledgements
We sincerely thank P. Hore, Oxford University, for many helpful discussions and advices, and S. Denzau, N. Moldan, T. Pavkovic and K. Stapput for their valuable help with conducting the experiments.
Ethics statement
The experiments were performed in accordance with the rules and regulations of animal welfare in Germany.
Funding statement
Our work was supported by the Human Frontier Science Program (grants to R.W. and T.R.) and by Deutsche Forschungsgemeinschaft (grants to R.W. and W.W.).
References
- 1.Wiltschko R, Wiltschko W. 2014. Sensing magnetic directions in birds: radical pair processes involving cryptochrome. Biosensors 4, 221–243. ( 10.3390/bios4030221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulten K. 1982. Magnetic field effects in chemistry and biology. Adv. Solid State Phys. 22, 61–83. ( 10.1007/BFb0107935) [DOI] [Google Scholar]
- 3.Ritz T, Adem S, Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulten K, Windemuth A. 1986. Model for a physiological magnetic compass. In Biophysical effects of steady magnetic fields (eds Maret G, Kiepenheuer J, Bocarra N.), pp. 99–106. Berlin, Germany: Springer. [Google Scholar]
- 5.Chaves I, et al. 2011. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364. ( 10.1146/annurev-arplant-042110-103759) [DOI] [PubMed] [Google Scholar]
- 6.Nießner C, Denzau S, Gross JC, Peichl L, Bischof H-J, Fleissner G, Wiltschko W, Wiltschko R. 2011. Avian ultraviolet/violet cones identified as probable magnetoreceptors. PLoS ONE 6, 20091 ( 10.1371/journal.pone.0020091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritz T. 2001. Disrupting magnetic compass orientation with radio frequency oscillating fields. In Orientation and navigation—birds, humans and other animals. Oxford, UK: Royal Institute of Navigation, paper No. 4. [Google Scholar]
- 8.Henbest KB, Kukura K, Rodgers TC, Hore PJ, Timmel CR. 2004. Radiofrequency magnetic field effects on a radical recombination reaction: a diagnostic test for the radical pair mechanism. J. Am. Chem. Soc. 126, 8102–8103. ( 10.1021/ja048220q) [DOI] [PubMed] [Google Scholar]
- 9.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. 2004. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180. ( 10.1038/nature02534) [DOI] [PubMed] [Google Scholar]
- 10.Ritz T, Wiltschko R, Hore PJ, Rodgers TC, Stapput K, Thalau P, Timmel CR, Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457. ( 10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. 2005. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften 92, 86–90. ( 10.1007/s00114-004-0595-8) [DOI] [PubMed] [Google Scholar]
- 12.Wiltschko W, Freire R, Munro U, Ritz T, Rogers L, Thalau P, Wiltschko R. 2007. The magnetic compass of domestic chickens, Gallus gallus. J. Exp. Biol. 210, 2300–2310. ( 10.1242/jeb.004853) [DOI] [PubMed] [Google Scholar]
- 13.Keary N, Ruploh T, Voss J, Thalau P, Wiltschko R, Wiltschko W, Bischof HJ. 2009. Oscillating magnetic field disrupts magnetic orientation in zebra finches, Taeniopygia guttata. Front. Zool. 6, 25 ( 10.1186/1742-9994-6-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavokin K, Chernetsov N, Pakomov A, Bojarinova J, Kobylkov D, Namozov B. 2014. Magnetic orientation in garden warblers (Sylvia borin) under 1.4 MHz radiofrequency field. J. R. Soc. Interface 11, 20140451 ( 10.1098/rsif.2014.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engels S, et al. 2014. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory birds. Nature 509, 353–356. ( 10.1038/nature13290) [DOI] [PubMed] [Google Scholar]
- 16.Ritz T. 2011. Quantum effects in biology: bird navigation. Proc. Chem. 3, 262–275. ( 10.1016/j.proche.2011.08.034) [DOI] [Google Scholar]
- 17.Nießner C, Denzau S, Stapput K, Ahmad M, Peichl L, Wiltschko W, Wiltschko R. 2013. Magnetoreception: activated cryptochrome 1a concurs with magnetic orientation in birds. J. R. Soc. Interface 10, 20130638 ( 10.1098/rsif.2013.0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiltschko W, Stapput K, Thalau P, Wiltschko R. 2006. Avian magnetic compass: fast adjustment to intensities outside the normal functional window. Naturwissenschaften 93, 300–304. ( 10.1007/s00114-006-0102-5) [DOI] [PubMed] [Google Scholar]
- 19.Winklhofer M, Dylda E, Thalau P, Wiltschko W, Wiltschko R. 2013. Avian magnetic compass can be tuned to anomalously low magnetic intensities. Proc. R. Soc. B 280, 20130853 ( 10.1098/rspb.2013.0853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denzau S, Nießner C, Rogers LJ, Wiltschko W. 2013. Ontogenetic development of magnetic compass orientation in domestic chickens (Gallus gallus). J. Exp. Biol. 216, 3143–3147. ( 10.1242/jeb.088815) [DOI] [PubMed] [Google Scholar]
- 21.Wiltschko W, Gesson M, Wiltschko R. 2001. Magnetic compass orientation of European robins under 565 nm green light. Naturwissenschaften 88, 387–390. ( 10.1007/s001140100248) [DOI] [PubMed] [Google Scholar]
- 22.Batschelet E. 1981. Circular statistics in biology. London, UK: Academic Press. [Google Scholar]
- 23.Müller P, Ahmad M. 2011. Light-activated cryptochrome reacts with molecular oxygen to form a flavin-superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 286, 21 033–21 040. ( 10.1074/jbc.M111.228940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavokin KV. 2009. The puzzle of magnetic resonance effect on the magnetic compass of migratory birds. Bioelectromagnetics 30, 402–410. ( 10.1002/bem.20485) [DOI] [PubMed] [Google Scholar]
- 25.Gauger EM, Rieper E, Morton JJL, Benjamin SC, Vedral V. 2011. Sustained quantum coherence and entanglement in avian compass. Phys. Rev. Lett. 106, 040503. [DOI] [PubMed] [Google Scholar]
- 26.Liedvogel M, Maeda K, Henbest K, Schleicher E, Simon T, Timmel CR, Hore PJ, Mouritsen H. 2007. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical pairs. PLoS ONE 2, e1106 ( 10.1371/journal.pone.0001106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoneham AM, Gauger E, Porfyrakis K, Benjamin SC, Lovett B. 2012. A new type of radical-pair-based model for magnetoreception. Biophys. J. 102, 961–968. ( 10.1016/j.bpj.2012.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


