Abstract
The role of pigments in generating the colour and maculation of birds' eggs is well characterized, whereas the effects of the eggshell's nanostructure on the visual appearance of eggs are little studied. Here, we examined the nanostructural basis of glossiness of tinamou eggs. Tinamou eggs are well known for their glossy appearance, but the underlying mechanism responsible for this optical effect is unclear. Using experimental manipulations in conjunction with angle-resolved spectrophotometry, scanning electron microscopy, atomic force microscopy and chemical analyses, we show that the glossy appearance of tinamou eggshells is produced by an extremely smooth cuticle, composed of calcium carbonate, calcium phosphate and, potentially, organic compounds such as proteins and pigments. Optical calculations corroborate surface smoothness as the main factor producing gloss. Furthermore, we reveal the presence of weak iridescence on eggs of the great tinamou (Tinamus major), an optical effect never previously documented for bird eggs. These data highlight the need for further exploration into the nanostructural mechanisms for the production of colour and other optical effects of avian eggshells.
Keywords: eggshell, gloss, iridescence, nanostructure, structural colour, tinamou
1. Introduction
Animal coloration is diverse in form. It includes colours that humans can and cannot see [1], colours that are matte or glossy [2] and colours that are perceived differently as the angles of observation and illumination shift (iridescence [3,4]). Colour can be produced through selective absorbance of light at particular wavelengths by pigments, by nanoscale structures that interact with light (structural colour) or by the interaction of pigments and nanoscale structures [5–8]. For example, a basal layer of melanin in Steller's jay (Cyanocitta stelleri) feathers absorbs incoherently scattered light, thereby enhancing the blue coloration that is produced by a quasi-ordered nanostructure of keratin and air [5].
Although nanostructure is typically associated with the production of iridescent colours [7], it can also produce non-iridescent colours (e.g. white on beetle carapaces [9]) and optical effects such as gloss. Iridescence can be produced by diffraction gratings, or when light passes through multiple semi-transparent materials that differ in refractive index, causing light to phase-shift and cancel out particular wavelengths at particular viewing angles [4,10,11]. Gloss, which is loosely defined as the specular or mirror-like component of light reflection, is a common component of animal coloration and is present in invertebrates, vertebrates and plants [2,12–14]. Gloss is often produced by smooth or polished surfaces. Light hitting a smooth surface is mostly reflected in the specular direction, causing the material to appear glossy, whereas light hitting a rough surface is scattered in a range of directions by the surface topography, causing the material to appear matte [15,16]. The refractive index of surface materials can also affect gloss. Materials of higher refractive index reflect more light, and therefore appear glossier [16]. Iridescence and gloss are often not independent because production of both is strongest when light is reflected from a smooth flat surface. As a result, most iridescent materials are glossy [17] and some glossy materials are weakly iridescent [2].
Avian eggs are extremely diverse in visual appearance [18,19]. The avian eggshell is a complex and multifunctional structure that is mainly composed of calcium carbonate [20]. Eggshell coloration can play a role in thermoregulation, crypsis, sexual selection, brood parasitic interactions, embryonic development and protection, and as a result, eggshells are often used as a model system to study the functional and structural evolution of animal coloration [19,21,22]. However, despite over 140 years of study on eggshell coloration [23–25], only two major pigments are thought to be commonly responsible for producing the full spectrum of avian eggshell colours: (i) biliverdin IXα, which absorbs light in the near-ultraviolet and yellow range and produces blue–green colours and (ii) protoporphyrin IX, which absorbs variably between 300 and 700 nm and produces brown colours [24,25]. Although structural coloration is a common mechanism for production of colour in both plants and animals [7,26], and can be produced using materials composed of similar components as avian eggshells (e.g. mother of pearl [27]), a nanostructural basis of coloration in bird eggs is yet to be examined. Moreover, while iridescence is widespread in nature [7], it has not previously been reported in avian eggs.
Tinamous are a basal lineage of birds (order: Tinamiformes) and lay brightly coloured eggs that often exhibit an exceptionally glossy appearance that is similar to a highly polished surface (figure 1). Although the glossiness of tinamou eggs is widely appreciated [28], the mechanism of its production is unclear. Given that gloss is usually produced by smooth surfaces, and may also be influenced by how surface materials reflect light, we hypothesized that the gloss on tinamou eggs is produced by the eggshell cuticle (the outermost layer of the eggshell [29]). A cuticle is present on the eggs of most avian species and is deposited onto the calcareous portion of the eggshell (true eggshell) as a thin non-crystallized layer [29]. When present, its thickness and chemical composition can vary across taxa, and may contain proteins, polysaccharides, lipids, calcium carbonate and calcium phosphates [29–33]. Although the cuticle's roles in embryonic development and antimicrobial defence of eggshells are well studied [32,34–36], its role in modulating the visual appearance of eggshells is poorly understood [37–39]. The chemical composition and structure of the cuticle can differ from the true eggshell underneath [33], and therefore may interact with light differently.
Figure 1.

Photographs of (a) Tinamus major, (b) Eudromia elegans and (c) Nothura maculosa nests. Average length × breadth of eggs (a–c): 58 × 48 mm, 53 × 39 mm and 40 × 29 mm. Photo credits: Karsten Thomsen, Sam Houston and Shirley Sekarajasingham. (Online version in colour.)
Here, we used angle-resolved spectrophotometry, scanning electron microscopy and atomic force microscopy to investigate the nanostructural mechanism(s) of gloss production and the presence of iridescence in tinamou eggs. We experimentally removed the eggshell cuticle to examine its role in producing these effects and used Fourier-transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) to examine its chemical composition.
2. Material and methods
2.1. Sample collection and removal of cuticle
We sourced unincubated eggs of four tinamou species from captive birds: blue eggs of the great tinamou (Tinamus major; n = 3), the Dallas World Aquarium; green eggs of the elegant crested tinamou (Eudromia elegans; n = 3), the Bronx Zoo; brown eggs of the Chilean tinamou (Nothoprocta perdicaria; n = 3) and dark brown eggs of the spotted nothura (Nothura maculosa; n = 1), a private breeder in California. As a comparison for size and colour, we also included a bluish, but matte, egg from an Araucana chicken (Gallus gallus; n = 1) sourced from a private breeder in New York City. Tinamou eggs were sourced in late 2012 and stored frozen in a dark container, whereas the Araucana egg was sourced in 2014. We followed governmental and institutional guidelines in sourcing and using biological materials. Although pigment-based colours can fade over time [40], colours produced by structural mechanisms may be less likely to be affected by such degradation [41]. We fragmented eggshells using soft pressure and washed each fragment using 100% ethanol. We measured gloss and iridescence, and conducted scanning electron microscopy and chemical analysis on eggshells before and after removal of the eggshell cuticle.
To experimentally verify the role of the cuticle and surface topography in producing gloss and iridescence, we disrupted the surface topography and removed the cuticle from eggshell fragments using EDTA, a disodium salt that has previously been used to remove cuticles from chicken eggshells [33,38]. We floated eggshell fragments on top of a solution of 0.37 M EDTA (pH 8.4), with the cuticle side down, for 25 min; the cuticle was then gently brushed away using soft tissue paper. Wiping eggshells with tissue paper produced similar results as using a jet of water to remove the cuticle [33,38]. Removal of the cuticle was verified using scanning electron microscopy (see below).
2.2. Measurement of gloss and iridescence
We measured specular and diffuse spectral reflectance on eggshell fragments between 300 and 700 nm. To minimize geometric variation associated with shell curvature, which can affect measurement of gloss [42], we took measurements from the flattest part of fragments taken from the equatorial region of eggs. We measured specular reflectance between 10° and 50° from coincident normal at 5° increments using a spectrometer equipped with two fibres that rotate independently from one another; one fibre was connected to a light source (AvaLight-XE pulsed xenon light) and the other fibre to a spectrometer (AvaSpec-2048 spectrometer, Avantes Inc., Broomfield, CO, USA); our equipment set-up did not allow us to take specular measurements at angles below 10°. We then rotated the eggshell fragment 90° clockwise and repeated the measurement procedure, thus producing two measurements at each angle for each of five eggshell fragments per egg (for sample sizes, see table 1). In addition, for each egg, we measured specular reflectance between 10° and 50° from coincident normal at 5° increments on a single eggshell fragment that was treated with EDTA to remove the cuticle. Preliminary analysis found evidence of iridescence on the blue eggs of the great tinamou. To further examine the role of the cuticle in production of iridescence, we selected the great tinamou egg with the highest level of iridescence and measured the same five eggshell fragments before and after EDTA treatment. We used an integrating sphere (AvaSphere-50-REFL), which had a black gloss trap to exclude specular reflectance, to measure diffuse reflectance at three locations per fragment. All reflectance measurements were taken as percentages relative to a diffuse white standard (WS-2, Avantes).
Table 1.
Hunter's contrast gloss and surface roughness values for eggs of four tinamou species and the Araucana chicken.
| species | colour | Na | glossb | gloss following EDTAb | roughness Rq (nm)c |
|---|---|---|---|---|---|
| G. gallus | blue | 1 | 1.29 | 1.04 | 168 |
| T. major | blue | 3 | 4.91 (1.54) | 1.39 (0.45) | 41.2 |
| E. elegans | green | 3 | 7.55 (1.26) | 1.37 (0.33) | 26.4 |
| N. perdicaria | dark | 3 | 15.09 (0.15) | 2.22 (1.02) | 13.5 |
| N. maculosa | brown | 1 | 18.12 | 1.78 | 14.2 |
aCorresponds to the number of eggs on which gloss was measured.
bMean contrast gloss values (s.d.) measured as a ratio of specular to diffuse reflectance.
cSurface roughness measured as a root mean square. Smaller values represent a smoother texture.
We measured gloss as the total specular reflectance at 10° incidence divided by the total diffuse reflectance (Hunter's contrast gloss [16]) and identified the presence of iridescence using linear models to test if hue (wavelength at maximum reflectance) changes with viewing angle. We used the peakshape() function of the R package PAVO [43] to extract hue from reflectance curves of eggs at different angles of incidence. This could only be accomplished for the blue eggs of the great tinamou and the Araucana chicken because the higher levels of gloss on the other tinamou eggs flattened reflectance curves (figure 2), preventing accurate measurement of hue. We excluded measurements where high levels of reflectance impeded reliable measurement of hue for eggs of the great tinamou. We constructed general linear models for each egg with hue as the response, angle of incidence as a continuous predictor variable and measurement location as a discrete predictor variable. All statistical tests were implemented in R v. 3.0.1 [44].
Figure 2.
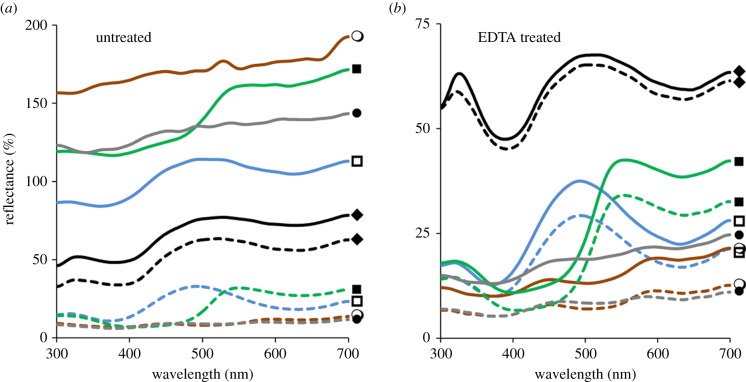
Contrast gloss of eggshells. Average diffuse (dashed lines) and specular (10° from normal incidence; solid lines) reflectance spectra for eggshells of four species of tinamou and an Araucana chicken. Spectra shown are prior to (a) and following (b) removal of the cuticle using EDTA. Black (closed diamond), G. gallus; blue (open square), T. major; green (closed square), E. elegans; grey (closed circle), N. perdicaria; brown (open circle), N. maculosa. All measurements were taken relative to a diffuse white standard (WS-2, Avantes). Glossiness is associated with the ratio between specular (solid lines) and diffuse (dashed lines) reflectance spectra for each egg. Note that the y-axis scales are different for (a,b). (Online version in colour.)
2.3. Examination of eggshell surface topography
We used a JSM-7401F scanning electron microscope (JEOL Japan) to examine the surface features of eggs that may contribute to their visual appearance. For example, surface features, such as cracks and rough texture, can cause scattering of incident light and impede mirror-like reflection that is associated with glossy appearances [15]. To examine the presence of these features, we mounted untreated or EDTA-treated eggshell fragments onto aluminium stubs, allowing visualization of both the eggshell's surface and cross-section, which we then sputter-coated with gold/palladium for 1 min. We viewed samples at a working distance of 7 mm and using an accelerating voltage of 7 kV.
To measure the height profiles of eggshell surfaces, and subsequently their roughness, we used a Nanoscope IIIA Atomic Force Microscope (AFM) and NanoScope software v. 4.43r8 (Bruker Scientific Instruments, USA). We measured a 30 × 30 µm2 area using tapping mode, a scan rate of 0.5 Hz, 512 × 512 pixels and aluminium-coated silicon tips with an estimated radius of 6–10 nm and resonant frequency of 150 kHz (Applied NanoStructures Inc., USA). We used Gwyddion software [45] to measure surface roughness as a root mean square (Rq; greater values indicate a rougher texture). We measured surface roughness for a single egg per species and calculated the association between measured gloss and surface roughness measurements using Spearman's rank correlations (rs).
We used standard optical calculations to determine (i) whether eggshell surfaces are theoretically smooth enough to produce gloss and (ii) the refractive index required to produce measured gloss independently of smoothness. Glossiness is affected by both the surface topography and the refractive index of materials [16]. A surface that is smooth enough to cause gloss fulfils Rayleigh's criterion (equation (2.1)), where h is the standard deviation for the height of surface features from the lowest point on a surface, λ is the wavelength of light and i is the angle of incidence.
| 2.1 |
For h we used the roughness measurements from the AFM (Rq; table 1), and for i we used 10°, which was the angle used to calculate Hunter's contrast gloss (see above). However, materials with a higher refractive index reflect more light [16]. For non-polarized light travelling through air, this is represented mathematically by Fresnel's equation (equation (2.2)), where i is the angle of incidence and n is refractive index.
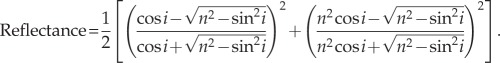 |
2.2 |
We applied this equation to determine whether a change in refractive index alone can realistically explain the differences in reflectance between eggs with and without a cuticle. There is no published information on the crystallinity or refractive index of tinamou eggshells; therefore, for the refractive index for the true eggshell below the cuticle, we used 1.56, which is the mean value of the extraordinary and ordinary refractive indices of calcium carbonate [46]. For the angle of incidence, (i) we used 10°. We calculated reflectance using this refractive index, and then determined the change in refractive index required for the cuticle to produce the observed 7× increase in reflectance (figure 2).
2.4. Chemical analyses
We used XPS and FT-IR to identify the chemical composition of the spotted nothura eggshell cuticle. We used the dark brown egg of the spotted nothura for this analysis because it had the greatest amount of surface gloss. Eggshell fragments were mounted on double-sided carbon tape for analysis. XPS spectra were obtained using a VersaProbe II Scanning XPS Microprobe from Physical Electronics (PHI) and under vacuum conditions of 2 × 10−6 Pa. Automated dual beam charge neutralization was used during the analysis of the samples to provide accurate data. The analyser pass energy was 117.4 eV for the survey spectra and 11.75 eV for the high-resolution C 1s, N 1s and O 1s scans. Each spectrum was collected using a monochromatic (Al Kα) X-ray beam (hν = 1486.7 eV) operating at 100 W over a 200-μm diameter probing area. Survey scans were collected using a pass energy of 80 eV over the binding energy range 1200–0 eV and were used to evaluate the atomic percentage of C, O, Ca, N, Na and P on the surface (top 2–3 nm) using PHI MultiPak software. These elements were quantified using peak areas for the C 1s, O 1s, Ca 2p, N 1s and P 2p regions. The binding energy scale was calibrated against the C 1s signal at 285.0 eV from adventitious hydrocarbons. FT-IR spectra were collected on a Perkin Elmer Spectrum Two FT-IR spectrometer with UATR attachment. Twenty scans at 4 cm−1 resolution were co-added to produce a spectrum. The FT-IR spectra for the tinamou eggshells were compared in relation to spectra collected for reference compounds, including CaCO3, Ca5(PO4)3(OH) and Ca3(PO4)2. In addition, we collected FT-IR spectra for an untreated tinamou eggshell that was powdered using a mortar and pestle.
3. Results
Tinamou eggs were highly glossy prior to, but not following, EDTA treatment (figure 2 and table 1). The specular reflectance of all tinamou eggs was above 100% relative to the commercial diffuse white standard and greater than the chicken egg (figure 2). By contrast, the tinamou eggs' diffuse reflectance was less than 25% relative to the white standard and lower than that of the chicken eggshell (figure 2). The four tinamou species varied in overall eggshell gloss: the dark brown eggs of the spotted nothura were most glossy, followed by the brown egg of the Chilean tinamou, green eggs of the elegant crested tinamou and blue eggs of the great tinamou (figure 2 and table 1). EDTA treatment removed gloss from all tinamou eggs but did not alter the hue of the background coloration (figure 2 and table 1).
In addition to being glossy, blue coloured great tinamou eggs showed weak iridescence (figure 3; electronic supplementary material, table S1). The hue changed from greener (mean ± s.e.: 514 ± 0.90 nm) to bluer (mean ± s.e.: 489 ± 7.65 nm) as the angle of specular incidence increased from 10° to 50° from normal incidence (tmajor1: F1,77 = 8.10, p = 0.006; tmajor2: F1,78 = 873.39, p < 0.001; tmajor3: F1,75 = 53.69, p < 0.001; figure 3). By contrast, hue did not change with specular angle for the chicken egg (F1,78 = 0.21, p = 0.65; figure 3) or for the blue coloured tinamou egg after treatment with EDTA (F1,39 = 1.63, p = 0.21; figure 3).
Figure 3.
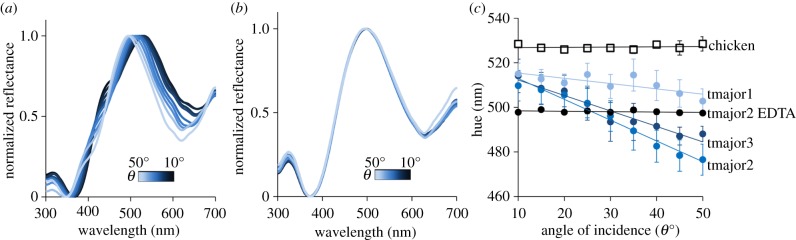
Iridescence of eggshells. Specular reflectance of a T. major eggshell fragment at different angles of incidence before (a) and after (b) treatment with EDTA to remove the cuticle. Shown are specular reflectance curves at 5° angle increments between 10° (dark blue) and 50° (light blue) from coincident normal (θ = 0°). (c) Hue, the wavelength (nm) with maximum reflectance, of the primary blue–green peak as a function of the angle of specular incidence (θ = 0° at coincident normal). Shown are means ± s.e.m. at different angles for three T. major eggs (blue symbols), one T. major egg following treatment with EDTA (black circles) and a G. gallus egg (open squares). (Online version in colour.)
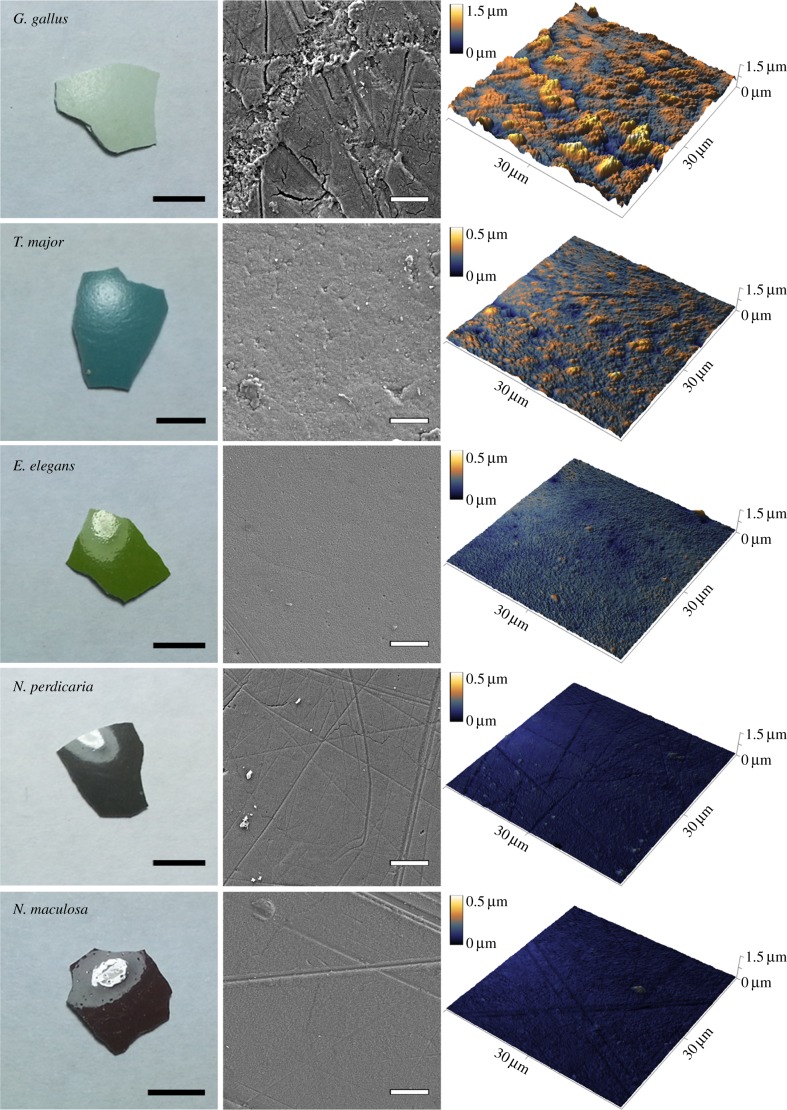
The glossiness of tinamou eggshells was produced by extremely smooth cuticles that coat the eggshells (figure 4). The highly glossy dark brown and brown coloured tinamou eggs had the smoothest surface textures, whereas the green coloured tinamou egg had a slightly rougher surface texture and the intermediately glossy blue coloured tinamou egg had the roughest surface of the four tinamou eggs (figure 4 and table 1). All four tinamou species met the Rayleigh criterion for surface smoothness to produce gloss (equation (2.1)). Threshold h, below which eggshell surfaces are smooth enough to produce gloss, increased from 38.1 to 88.9 nm for wavelengths between 300 and 700 nm; all tinamou eggs had roughness measurements below this threshold, with the exception of the blue coloured great tinamou eggs that were only under the threshold for wavelengths between 325 and 700 nm (electronic supplementary material, figure S1; table 1). Moreover, the level of glossiness of tinamou eggshells was negatively correlated with the amount of surface roughness (rs = −0.93; table 1). By contrast, the blue chicken egg was very rough with large cracks (figure 4 and table 1) and was above the smoothness threshold for production of gloss (electronic supplementary material, figure S1; table 1). Calculations using Fresnel's equation (equation (2.2)) showed that an increase in refractive index from 1.56 to 3.8 would be required to produce a 7× increase in reflectance independently of surface smoothness. The cuticles of the different tinamou species' eggs varied in both thickness and structure across species (electronic supplementary material, figure S2). Treatment using EDTA produced a rough pock-marked exterior surface on all eggshells, with the exception of a small area on a single fragment (figure 5).
Figure 4.
Photographs, SEM images, and AFM images illustrating the association between glossiness and smoothness of eggshell surface texture. Light areas in the eggshell photographs are reflections of a lamp above that illustrate the glossiness of eggshells. Measurements of glossiness are presented in figure 2 and table 1. Scale bars: left column 5 mm; middle column 10 µm. Note that the height colour map scales differ between the chicken and tinamou eggshell AFM images. (Online version in colour.)
Figure 5.
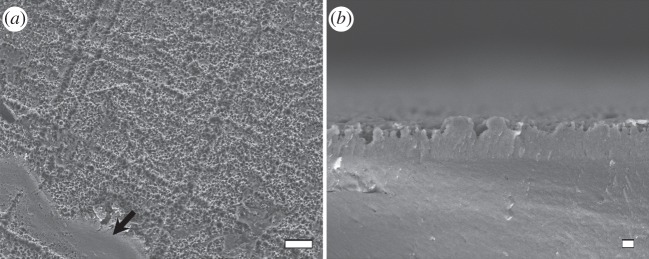
SEM images of N. maculosa eggshell surface in top-view (a) and cross-section (b) following treatment with EDTA to remove the cuticle (see Material and methods for protocol). Images illustrate the rough pock-marked surface associated with reduced gloss. Arrow indicates the residual presence of the cuticle across a 1000 µm2 area of the eggshell illustrating how the cuticle fits on top of the rough pock-marked surface. Scale bars: (a) 10 µm; (b) 1 µm.
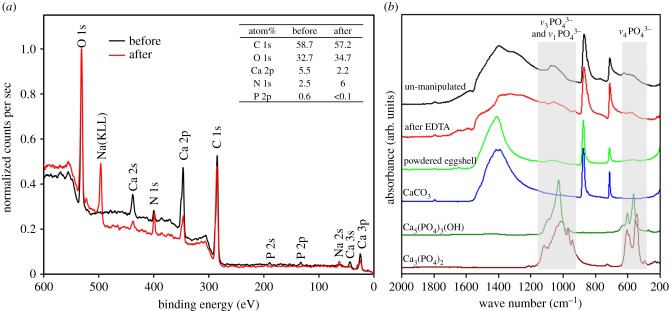
Chemical analyses revealed the presence of calcium carbonate, calcium phosphates and, potentially, organic compounds such as proteins and pigments. XPS detected the presence of calcium, phosphate and nitrogen in the eggshell cuticle (figure 6a). The presence of calcium can be attributed to both calcium carbonate and calcium phosphates (figure 6a). The presence of nitrogen on the surface of untreated eggshells is likely attributed to organic compounds (figure 6a), such as proteins and pigments. There was more than 85% reduction in phosphate composition following EDTA treatment to remove the cuticle (figure 6a). Phosphate can be attributed to the presence of hydroxyapatite [Ca5(PO4)3(OH)] and/or tricalcium phosphate [Ca3(PO4)2]; however, FT-IR was unable to differentiate the presence of hydroxyapatite or tricalcium phosphate in the cuticle (figure 6b). Hydroxyapatite is a component of chicken eggshell cuticles [31], and therefore is expected to be the dominant phosphate-containing compound here.
Figure 6.
(a) XPS spectra for a N. perdicaria egg before and after treatment with EDTA to remove the cuticle. Table shows atom percentages for different chemical elements present on the surface of the eggshell. N and Na following treatment with EDTA may be attributed to the residual presence of EDTA. (b) FT-IR spectra for a N. perdicaria egg before and after treatment with EDTA to remove the cuticle, and after being ground down into a powder. Spectra for calcite [CaCO3], hydroxyapatite [Ca5(PO4)3(OH)] and tricalcium phosphate [Ca3(PO4)2] are also illustrated. The absorption between 1200 and 950 cm−1 is characteristic for P–O stretching modes (ν3 and ν1) of PO43– and the absorption between 650 and 550 cm−1 is due to the triply degenerate O–P–O bending mode (ν4) of PO43−. The FT-IR spectrum of the powdered tinamou eggshell was dominated by signals due to calcite, confirming that the calcium phosphate species were predominantly localized on the surface. (Online version in colour.)
4. Discussion
Tinamous have some of the most colourful and glossy eggs of all birds (figure 1), and here we show that an extremely smooth eggshell cuticle produces their mirror-like sheen. Furthermore, we reveal the presence of iridescence on the blue eggs of the great tinamou, an optical effect that has not been previously reported for avian eggs. The eggshell cuticle only modifies the underlying background coloration of tinamou eggs because their colour is retained following its removal. These results establish a nanostructural basis for production of gloss on birds' eggs and highlight the cuticle's role in modulating the eggshell's visual appearance. The presence of iridescence in particular opens the door for further investigation into nanostructural mechanisms of colour production in eggshells.
Smooth surfaces are well known to produce glossy appearances ([13,15,47]; although see [2]), so it is not surprising that they account for production of gloss on tinamou eggshells. The surface of tinamou eggs is smoother than that of the chicken egg and in most cases fulfils Rayleigh's criterion for production of gloss. Moreover, the differences in glossiness between eggs of the different tinamou species were associated with differences in surface smoothness. Experimental removal of the cuticle caused roughening of the eggshell surface and eliminated gloss, again strongly supporting the role of surface smoothness in production of gloss. Although increasing the refractive index of surface materials may also increase glossiness, our calculations show that the refractive index of the tinamou eggshell cuticle would need to be higher than that of diamond (RI > 2.4) to produce the observed gloss independently of surface smoothness. Therefore, the refractive index likely plays a minor role in producing gloss relative to smoothness.
The glossy appearance of tinamou eggs has been noted for many years by scientists and collectors alike [18]; however, the presence of iridescence, to our knowledge, has not been reported for tinamou or any other birds' eggs. This may be in part because the iridescence on great tinamou eggs is not clearly visible to the human eye. Although the weak iridescence detected here is theoretically detectable by both the avian and the human eyes (514–489 nm change in hue), it may be masked by high levels of gloss. Alternatively, the tinamou eggs may not be iridescent under natural light conditions. For example, some bird feathers that are iridescent under directional light, such as that produced by spectrophotometers, are not iridescent under omni-directional light as a result of the isotropic nature of the feathers' quasi-ordered or amorphous nanostructures [48–50]. Although some aspect of the cuticle may produce iridescence through thin-film interference, the cuticle of great tinamou eggshells as a whole is too thick to act as a thin film (more than 700 nm). If iridescence is produced by thin-film interference, it should theoretically be visible on glossy surfaces under natural light conditions (e.g. soap bubbles [7]). The mechanisms producing iridescence of great tinamou eggs require further investigation.
The gloss and associated iridescence of tinamou eggs appear to be produced independently of the background colour. Indeed, removal of the eggshell cuticle caused loss of both iridescence and gloss, but not background coloration. By contrast, removal of materials that produce iridescence in other materials also results in loss of colour [7]. Although a structural mechanism may play a role, background colour of tinamou eggshells is most likely produced by pigments as in other avian eggs [24,25,51], although this requires further investigation.
The smooth cuticle of tinamou eggshells is composed of calcite, calcium phosphate and, potentially, organic compounds such as proteins, lipids, polysaccharides and pigments [29–33]. However, the relative contributions of the different components in producing a smooth cuticle are unclear from our findings. The inorganic component of avian eggshells is largely composed of calcite, but calcium phosphates have also been reported in eggshells of a number of species [31,35,52–54]. In brush turkey eggs (Alectura lathami), phosphate is associated with approximately 300 nm spheres that produce a rough surface [35], whereas in chicken eggs, phosphate is associated with needle-like hydroxyapatite crystals that form spherical patterns in the cuticle [31]. Calcium phosphate is thus associated with both rough and smooth surfaces, suggesting that it may contribute to surface modification in entirely opposing ways. This may be potentially associated with the relative quantity of phosphate. For example, phosphate is present in greater quantity in the cuticle of brush turkey eggs than in the cuticle of tinamou eggs (D.F-L. 2014, unpublished data).
The function of gloss or iridescence on avian eggshells is unclear. Gloss and iridescence may increase conspicuousness of tinamou eggs; however, this would be in contradiction to the hypothesis that egg coloration functions in crypsis, which is considered to be a major driver of avian egg colour evolution [19]. The mating system of tinamous and many of the closely related ratites is unusual: multiple females lay their eggs into the same nest, usually on the ground, which are then solely incubated by a male [55]. Bright egg colours may signal the presence of nests to other females, which in turn could be beneficial if nests with larger clutches are more successful [56,57]. Indeed, tinamou females are known to lay eggs in artificial clutches where existing eggs are the only cue of nest location [57]. However, a phylogenetic study found no evidence that tinamou eggs from communal nesting species are more conspicuous than eggs from non-communal species [28]. On the other hand, gloss and colour of tinamou eggs, which fade through the incubation period, may provide females with a cue to assess the age of nests and enable them to avoid laying eggs in nests where incubation has begun [28]. Bright eggs may also ‘blackmail’ males into comparatively high incubation attendance to conceal conspicuous eggs, thereby shortening their incubation time and reducing the risk of predation [58]. Any selective disadvantage of increased conspicuousness may be offset by high incubation attendance, and therefore limited exposure of the eggs to visually oriented predators when incubated by males with cryptic plumage. Indeed, male tinamous have extraordinarily high incubation attendance rates compared with other birds [59]. However, the role of visual predation on great tinamou eggs is likely minimal because most predation occurs at night after incubation has started [57].
Alternative to a signalling function, gloss and iridescence may be a by-product of mechanisms that protect the developing embryo. For example, a smooth eggshell surface may prevent water from clogging pores and impeding gas exchange by minimizing resistance for sliding water droplets [60]. A highly reflective eggshell surface may also help prevent damage to the embryo from solar radiation [61]. Our results open the door for further investigation into the mechanisms, functions, and evolution of non-pigmentary contributors to avian eggshell appearance.
Supplementary Material
Acknowledgements
We thank C. Eliason, B. Hsiung, R. Maia, J. Peteya and four anonymous referees for their comments on the manuscript, and Z. Nikolov for help with the XPS analysis. For several egg samples, we thank C. Julian, G. Bergera, and the Ornithology team at the Bronx Zoo of the Wildlife Conservation Society.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.d8810.
Funding statement
M.E.H., T.G., M.D.S. and G.I.N.W. acknowledge financial support from the Human Frontier Science Program. M.D.S. acknowledges financial support from the Air Force Office of Scientific Research FA9550–13–1–0222 and National Science Foundation DEB. When participating in this work D.H. was co-financed by the European Social Fund and the state budget of the Czech Republic (project no. CZ.1.07/2.3.00/30.0041).
References
- 1.Hunt S, Bennett AT, Cuthill IC, Griffiths R. 1998. Blue tits are ultraviolet tits. Proc. R. Soc. Lond. B 265, 451–455. ( 10.1098/rspb.1998.0316) [DOI] [Google Scholar]
- 2.Maia R, D'Alba L, Shawkey MD. 2011. What makes a feather shine? A nanostructural basis for glossy black colours in feathers. Proc. R. Soc. B 278, 1973–1980. ( 10.1098/rspb.2010.1637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox H, Vevers G. 1960. The nature of animal colours. London, UK: Sidgwick and Jackson Limited. [Google Scholar]
- 4.Newton I. 1704. Opticks. London, UK: William Innys. [Google Scholar]
- 5.Shawkey MD, Hill GE. 2006. Significance of a basal melanin layer to production of non-iridescent structural plumage color: evidence from an amelanotic Steller's jay (Cyanocitta stelleri). J. Exp. Biol. 209, 1245–1250. ( 10.1242/jeb.02115) [DOI] [PubMed] [Google Scholar]
- 6.Bagnara JT, Fernandez PJ, Fujii R. 2007. On the blue coloration of vertebrates. Pigment Cell Res. 20, 14–26. ( 10.1111/j.1600-0749.2006.00360.x) [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita S. 2008. Structural colors in the realm of nature. Singapore: World Scientific. [Google Scholar]
- 8.D'Alba L, Kieffer L, Shawkey MD. 2012. Relative contributions of pigments and biophotonic nanostructures to natural color production: a case study in budgerigar (Melopsittacus undulatus) feathers. J. Exp. Biol. 215, 1272–1277. ( 10.1242/jeb.064907) [DOI] [PubMed] [Google Scholar]
- 9.Vukusic P, Hallam B, Noyes J. 2007. Brilliant whiteness in ultrathin beetle scales. Science 315, 348 ( 10.1126/science.1134666) [DOI] [PubMed] [Google Scholar]
- 10.Vašíček A. 1960. Optics of thin films. Amsterdam, The Netherlands: North-Holland Pub. Co. [Google Scholar]
- 11.Liu Y, Shigley J, Hurwit K. 1999. Iridescent color of a shell of the mollusk Pinctada margaritifera caused by diffraction. Opt. Express 4, 177–182. ( 10.1364/OE.4.000177) [DOI] [PubMed] [Google Scholar]
- 12.Hegedüs R, Szél G, Horváth G. 2006. Imaging polarimetry of the circularly polarizing cuticle of scarab beetles (Coleoptera: Rutelidae, Cetoniidae). Vis. Res. 46, 2786–2797. ( 10.1016/j.visres.2006.02.007) [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen PV, Dyck J. 2000. Silkiness in brown mink pelts characterized with optical methods. J. Anim. Sci. 78, 1697–1709. [DOI] [PubMed] [Google Scholar]
- 14.Vignolini S, Thomas MM, Kolle M, Wenzel T, Rowland A, Rudall PJ, Baumberg JJ, Glover BJ, Steiner U. 2012. Directional scattering from the glossy flower of Ranunculus: how the buttercup lights up your chin. J. R. Soc. Interface 9, 1295–1301. ( 10.1098/rsif.2011.0759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willmouth FM. 1986. Transparency, translucency and gloss. In Optical properties of polymers (ed. Meeten GH.), pp. 265–334. London, UK: Elsevier Applied Science Publishers. [Google Scholar]
- 16.Hunter RS. 1937. Methods of determining gloss. J. R. Natl Bur. Stand. 18, 19–39. ( 10.6028/jres.018.006) [DOI] [Google Scholar]
- 17.Toomey MB, Butler MW, Meadows MG, Taylor LA, Fokidis HB, McGraw KJ. 2010. A novel method for quantifying the glossiness of animals. Behav. Ecol. Sociobiol. 64, 1047–1055. ( 10.1007/s00265-010-0926-z) [DOI] [Google Scholar]
- 18.Hauber M. 2014. The book of eggs. Chicago, IL: University of Chicago Press. [Google Scholar]
- 19.Kilner R. 2006. The evolution of egg colour and patterning in birds. Biol. Rev. 81, 383–406. ( 10.1017/S1464793106007044) [DOI] [PubMed] [Google Scholar]
- 20.Fernandez MS, Araya M, Arias JL. 1997. Eggshells are shaped by a precise spatio-temporal arrangement of sequentially deposited macromolecules. Matrix Biol. 16, 13–20. ( 10.1016/S0945-053X(97)90112-8) [DOI] [PubMed] [Google Scholar]
- 21.Cassey P, Thomas GH, Portugal SJ, Maurer G, Hauber ME, Grin T, Lovell PG, Mikšík I. 2012. Why are birds’ eggs colourful? Eggshell pigments co-vary with life-history and nesting ecology among British breeding non-passerine birds. Biol. J. Linn. Soc. 106, 657–672. ( 10.1111/j.1095-8312.2012.01877.x) [DOI] [Google Scholar]
- 22.Cassey P, Portugal SJ, Maurer G, Ewen JG, Boulton RL, Hauber ME, Blackburn TM. 2010. Variability in avian eggshell colour: a comparative study of museum eggshells. PLoS ONE 5, e12054 ( 10.1371/journal.pone.0012054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorby HC. 1875. On the colouring-matters of the shells of birds’ eggs. Proc. Zool. Soc. Lond. 23, 351–365. [Google Scholar]
- 24.Kennedy G, Vevers H. 1976. A survey of avian eggshell pigments. Comp. Biochem. Physiol. B Comp. Biochem. 55, 117–123. ( 10.1016/0305-0491(76)90183-8) [DOI] [PubMed] [Google Scholar]
- 25.Gorchein A, Lim C, Cassey P. 2009. Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 23, 602–606. ( 10.1002/bmc.1158) [DOI] [PubMed] [Google Scholar]
- 26.Vukusic P, Sambles JR. 2003. Photonic structures in biology. Nature 424, 852–855. ( 10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 27.Grégoire C. 1957. Topography of the organic components in mother-of-pearl. J. Biophys. Biochem. Cytol. 3, 797–808. ( 10.1083/jcb.3.5.797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanley D, Stoddard MC, Cassey P, Brennan PLR. 2013. Eggshell conspicuousness in ground nesting birds: do conspicuous eggshells signal nest location to conspecifics? Avian Biol. Res. 6, 147–156. ( 10.3184/175815513X13617279883973) [DOI] [Google Scholar]
- 29.Mikhailov KE, Ornithologists’ Club B. 1997. Avian eggshells: an atlas of scanning electron micrographs. Newbury, British Ornithologists’ Club Occasional Publications, No. 3. The Nature Conservancy Bureau Limited.
- 30.Rose-Martel M, Du J, Hincke MT. 2012. Proteomic analysis provides new insight into the chicken eggshell cuticle. J. Proteomics 75, 2697–2706. ( 10.1016/j.jprot.2012.03.019) [DOI] [PubMed] [Google Scholar]
- 31.Dennis JE, Xiao S-Q, Agarwal M, Fink DJ, Heuer AH, Caplan AI. 1996. Microstructure of matrix and mineral components of eggshells from white leghorn chickens (Gallus gallus). J. Morphol. 228, 287–306. () [DOI] [PubMed] [Google Scholar]
- 32.Wellman-Labadie O, Picman J, Hincke MT. 2008. Antimicrobial activity of the Anseriform outer eggshell and cuticle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 149, 640–649. ( 10.1016/j.cbpb.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 33.Baker J, Balch D. 1962. A study of the organic material of hen's-egg shell. Biochem. J. 82, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeming D. 1987. Effect of cuticle removal on the water vapour conductance of egg shells of several species of domestic bird. Br. Poultr. Sci. 28, 231–237. ( 10.1080/00071668708416957) [DOI] [Google Scholar]
- 35.D'Alba L, Jones DN, Badawy HT, Eliason CM, Shawkey MD. 2014. Antimicrobial properties of a nanostructured eggshell from a compost-nesting bird. J. Exp. Biol. 217, 1116–1121. ( 10.1242/jeb.098343) [DOI] [PubMed] [Google Scholar]
- 36.Peebles E, Brake J, Gildersleeve R. 1987. Effects of eggshell cuticle removal and incubation humidity on embryonic development and hatchability of broilers. Poultr. Sci. 66, 834–840. ( 10.3382/ps.0660834) [DOI] [PubMed] [Google Scholar]
- 37.Richards P, Deeming DC. 2001. Correlation between shell colour and ultrastructure in pheasant eggs. Br. Poultr. Sci. 42, 338–343. ( 10.1080/00071660120055304) [DOI] [PubMed] [Google Scholar]
- 38.Samiullah S, Roberts J. 2013. Protoporphyrin IX in shell and cuticle of brown shelled eggs. In 24th Annual Australian Poultry Science Symp., Sydney, Australia, 17–20 February, pp. 260–263. Poultry Research Foundation. [Google Scholar]
- 39.Riehl C, Jara L. 2009. Natural history and reproductive biology of the communally breeding greater ani (Crotophaga major) at Gatún Lake, Panama. Wilson J. Ornithol. 121, 679–687. ( 10.1676/09-017.1) [DOI] [Google Scholar]
- 40.Cassey P, Maurer G, Duval C, Ewen JG, Hauber ME. 2010. Impact of time since collection on avian eggshell color: a comparison of museum and fresh egg specimens. Behav. Ecol. Sociobiol. 64, 1711–1720. ( 10.1007/s00265-010-1027-8) [DOI] [Google Scholar]
- 41.McNamara ME. 2013. The taphonomy of colour in fossil insects and feathers. Palaeontology 56, 557–575. ( 10.1111/pala.12044) [DOI] [Google Scholar]
- 42.Maurer G, Cassey P. 2011. Evaluation of a glossmeter for studying the surface appearance of avian eggs. J. Ornithol. 152, 209–212. ( 10.1007/s10336-010-0600-2) [DOI] [Google Scholar]
- 43.Maia R, Eliason CM, Bitton P-P, Doucet SM, Shawkey MD. 2013. pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol. Evol. 4, 906–913. [Google Scholar]
- 44.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 45.Nečas D, Klapetek P. 2012. Gwyddion: an open-source software for SPM data analysis. Centr. Eur. J. Phys. 10, 181–188. ( 10.2478/s11534-011-0096-2) [DOI] [Google Scholar]
- 46.Bragg W. 1924. The refractive indices of calcite and aragonite. Proc. R. Soc. Lond. A 105, 370–386. ( 10.1098/rspa.1924.0026) [DOI] [Google Scholar]
- 47.Stamm RF, Garcia ML, Fuchs JJ. 1977. The optical properties of human hair I. Fundamental considerations and goniophotometer curves. J. Soc. Cosmet. Chem. 28, 571. [Google Scholar]
- 48.Stavenga DG, Tinbergen J, Leertouwer HL, Wilts BD. 2011. Kingfisher feathers—colouration by pigments, spongy nanostructures and thin films. J. Exp. Biol. 214, 3960–3967. ( 10.1242/jeb.062620) [DOI] [PubMed] [Google Scholar]
- 49.Noh H, Liew SF, Saranathan V, Mochrie SG, Prum RO, Dufresne ER, Cao H. 2010. How noniridescent colors are generated by quasi-ordered structures of bird feathers. Adv. Mater. 22, 2871–2880. ( 10.1002/adma.200903699) [DOI] [PubMed] [Google Scholar]
- 50.Osorio D, Ham A. 2002. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 205, 2017–2027. [DOI] [PubMed] [Google Scholar]
- 51.Igic B, et al. 2010. Detecting pigments from colourful eggshells of extinct birds. Chemoecology 20, 43–48. ( 10.1007/s00049-009-0038-2) [DOI] [Google Scholar]
- 52.Board R, Perrott H, Love G, Scott V. 1984. The phosphate-rich cover on the eggshells of grebes (Aves: Podicipitiformes). J. Zool. 203, 329–343. ( 10.1111/j.1469-7998.1984.tb02336.x) [DOI] [Google Scholar]
- 53.Board RG, Perrott HR, Love G, Seymour RS. 1982. A novel pore system in the eggshells of the mallee fowl, Leipoa ocellata. J. Exp. Zool. 220, 131–134. ( 10.1002/jez.1402200118) [DOI] [Google Scholar]
- 54.Tullett S, Board R, Love G, Perrott H, Scott V. 1976. Vaterite deposition during eggshell formation in the cormorant, gannet and shag, and in ‘shell-less’ eggs of the domestic fowl. Acta Zool. 57, 79–87. ( 10.1111/j.1463-6395.1976.tb00213.x) [DOI] [Google Scholar]
- 55.Handford P, Mares MA. 1985. The mating systems of ratites and tinamous: an evolutionary perspective. Biol. J. Linn. Soc. 25, 77–104. ( 10.1111/j.1095-8312.1985.tb00387.x) [DOI] [Google Scholar]
- 56.Fernández GJ, Reboreda JC. 1998. Effects of clutch size and timing of breeding on reproductive success of greater rheas. Auk 115, 340–348. ( 10.2307/4089192) [DOI] [Google Scholar]
- 57.Brennan PLR. 2010. Clutch predation in great tinamous Tinamus major and implications for the evolution of egg color. J. Avian Biol. 41, 419–426. ( 10.1111/j.1600-048X.2010.04999.x) [DOI] [Google Scholar]
- 58.Hanley D, Doucet SM, Dearborn DC. 2010. A blackmail hypothesis for the evolution of conspicuous egg coloration in birds. Auk 127, 453–459. ( 10.1525/auk.2009.09090) [DOI] [Google Scholar]
- 59.Brennan PL. 2009. Incubation in great tinamou (Tinamus major). Wilson J. Ornithol. 121, 506–511. ( 10.1676/08-073.1) [DOI] [Google Scholar]
- 60.Bikerman J. 1950. Sliding of drops from surfaces of different roughnesses. J. Colloid Sci. 5, 349–359. ( 10.1016/0095-8522(50)90059-6) [DOI] [Google Scholar]
- 61.Maurer G, Portugal SJ, Hauber ME, Mikšík I, Russell DG, Cassey P. 2014. First light for avian embryos: eggshell thickness and pigmentation mediate variation in development and UV exposure in wild bird eggs. Funct. Ecol. ( 10.1111/1365-2435.12314) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.d8810.