Abstract
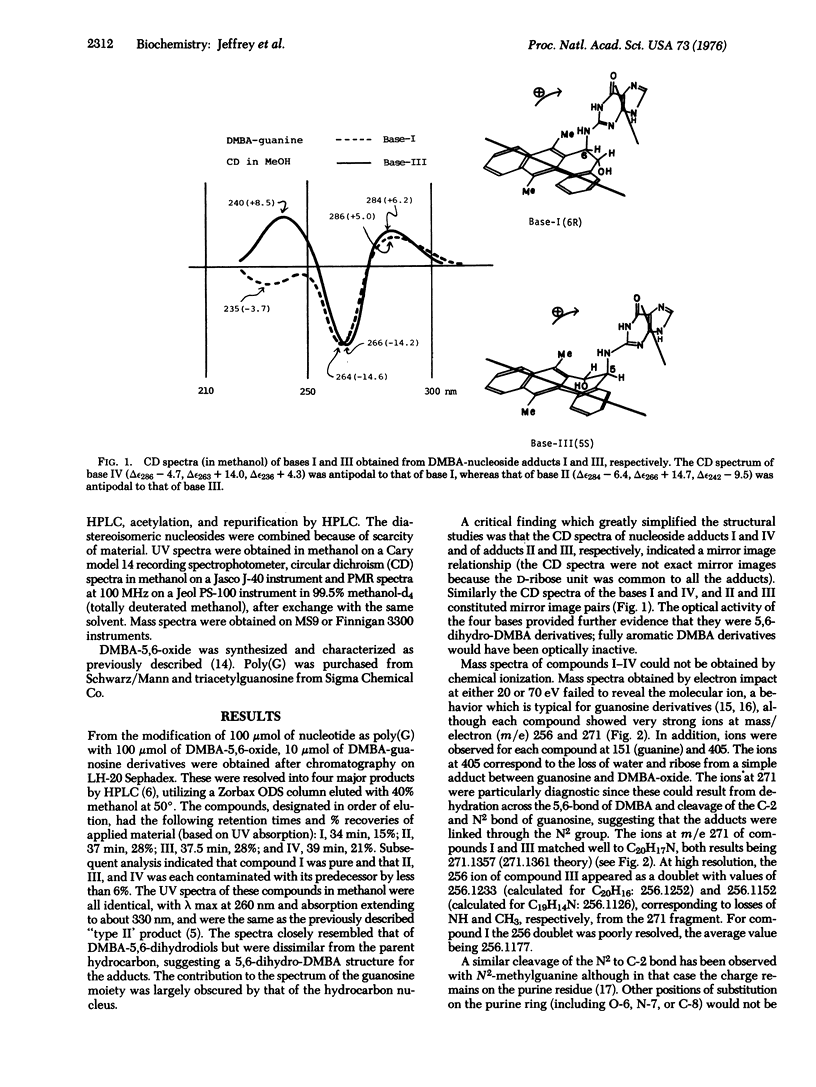
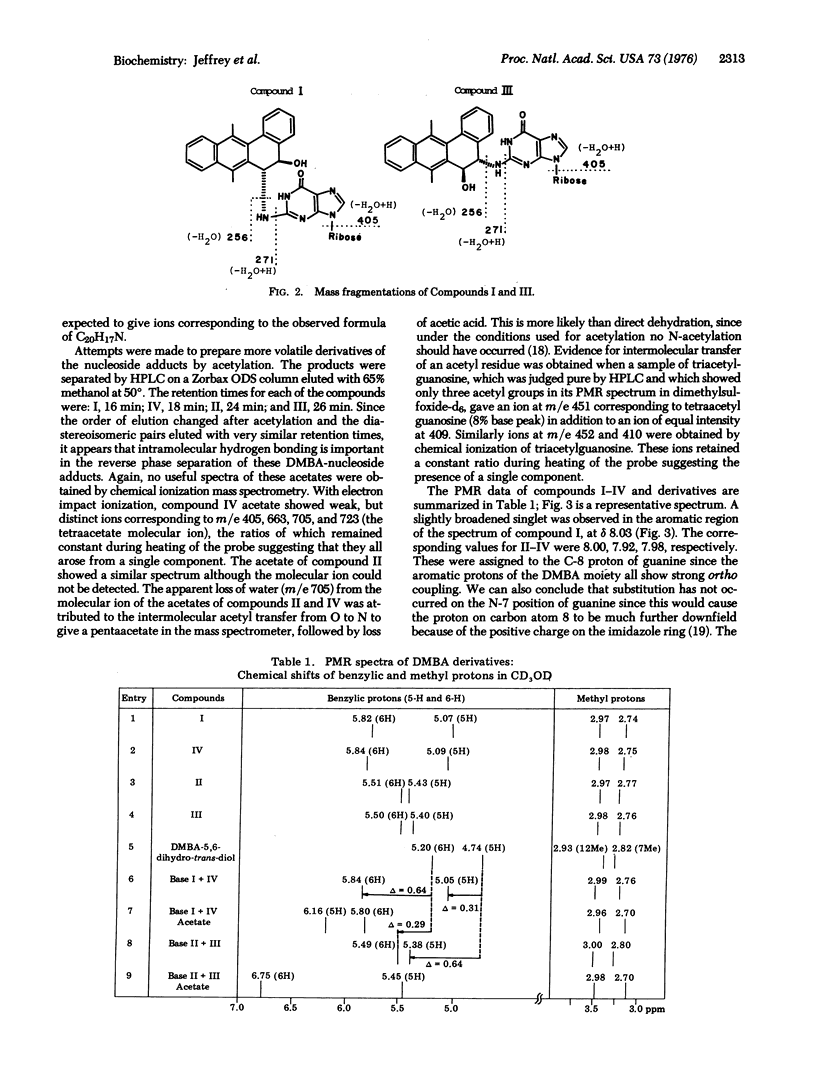
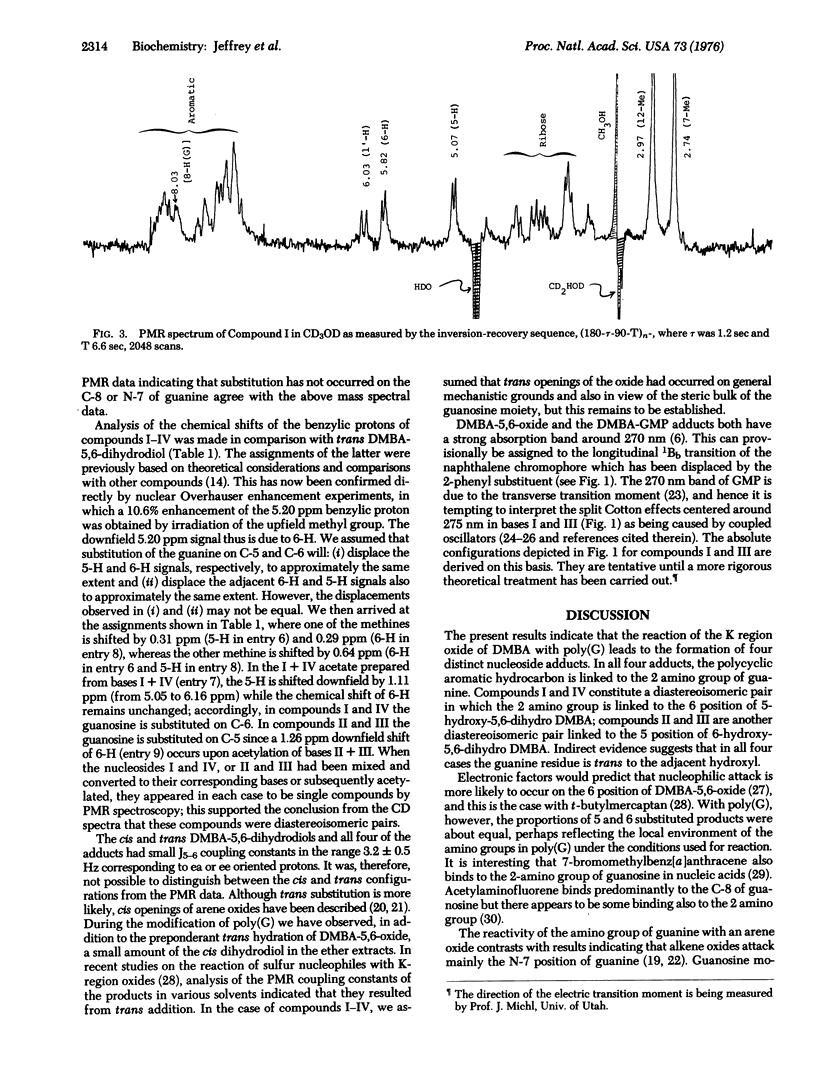
Arene oxides have been proposed as the reactive intermediates in the process of carcinogenesis induced by polycyclic aromatic hydrocarbons. The present study defines the structures of four guanosine adducts formed by the reaction of 7,12-dimethylbenz[a]anthracene-5,6-oxide with polyguanylic acid. The modified polymer was hydrolyzed to nucleotides and the hydrophobic guanosine adducts separated from unmodified guanosine by LH-20 column chromatograhy. The adducts were further resolved into four components (I-IV) by reverse phase high pressure liquid chromatography. Analysis of the ultraviolet, circular dichroism, mass, and proton magnetic resonance spectra of these compounds, or their acetate and free base derivatives, indicates that in all four compounds the aromatic hydrocarbon is present on the 2 amino group of guanine. Compounds I and IV, and II and III constitute diastereoisomeric pairs, respectively. In the I and IV pair, the adducts result from addition at the 6 position of the original dimethylbenz[a]anthracene oxide, whereas in the II and III pair, the addition occurs at the 5 position. Indirect evidence suggests that trans opening of the oxide occurred in all cases but this remains to be established.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobstein S. H., Weinstein I. B., Grunberger D., Weisgras J., Harvey R. G. Products obtained after in vitro reaction of 7,12-dimethylbenz[alpha]anthracene 5,6-oxide with nucleic acids. Biochemistry. 1975 Jul 29;14(15):3451–3458. doi: 10.1021/bi00686a025. [DOI] [PubMed] [Google Scholar]
- Dansette P., Jerina D. M. A facile synthesis of arene oxides at the K regions of polycylic hydrocarbons. J Am Chem Soc. 1974 Feb 20;96(4):1224–1225. doi: 10.1021/ja00811a046. [DOI] [PubMed] [Google Scholar]
- Dipple A., Brookes P., Mackintosh D. S., Rayman M. P. Reaction of 7-bromomethylbenz(a)anthracene with nucleic acids, polynucleotides, and nucleosides. Biochemistry. 1971 Nov;10(23):4323–4330. doi: 10.1021/bi00799a026. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical basis of chemical carcinogenesis by N-2-fluorenylacetamide derivatives and analogs. FEBS Lett. 1973 Aug 15;34(2):295–298. doi: 10.1016/0014-5793(73)80815-4. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P., Huberman E., Marquardt H., Kuroki T., Heidelberger C. In vitro transformation of rodent cells by K-region derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1098–1101. doi: 10.1073/pnas.68.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. G., Goh S. H., Cortez C. "K-region" oxides and related oxidized metabolites of carcinogenic aromatic hydrocarbons. J Am Chem Soc. 1975 Jun 11;97(12):3468–3479. doi: 10.1021/ja00845a032. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Gupta A. S., Leonard N. J. Mass spectra of nucleoside components of tRNA. Anal Biochem. 1969 Aug;30(2):249–270. doi: 10.1016/0003-2697(69)90396-0. [DOI] [PubMed] [Google Scholar]
- Huberman E., Aspiras L., Heidelberger C., Grover P. L., Sims P. Mutagenicity to mammalian cells of epoxides and other derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3195–3199. doi: 10.1073/pnas.68.12.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriek E. Carcinogenesis by aromatic amines. Biochim Biophys Acta. 1974 Sep 9;355(2):177–203. doi: 10.1016/0304-419x(74)90003-1. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Jarman M. Alkylation by propylene oxide of deoxyribonucleic acid, adenine, guanosine and deoxyguanylic acid. Biochem J. 1972 Feb;126(4):893–900. doi: 10.1042/bj1260893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. F., Fink L. M., Weinstein I. B., Grunberger D. Effect of N-2-acetylaminofluorene modification on the conformation of nucleic acids. Cancer Res. 1974 Feb;34(2):319–327. [PubMed] [Google Scholar]
- Marquardt H., Kuroki T., Huberman E., Selkirk J. K., Heidelberger C., Grover P. L., Sims P. Malignant transformation of cells derived from mouse prostate by epoxides and other derivatives of polycyclic hydrocarbons. Cancer Res. 1972 Apr;32(4):716–720. [PubMed] [Google Scholar]
- Marquardt H., Kuroki T., Huberman E., Selkirk J. K., Heidelberger C., Grover P. L., Sims P. Malignant transformation of cells derived from mouse prostate by epoxides and other derivatives of polycyclic hydrocarbons. Cancer Res. 1972 Apr;32(4):716–720. [PubMed] [Google Scholar]
- Marquardt H., Sodergren J. E., Sims P., Grover P. L. Malignant transformation in vitro of mouse fibroblasts by 7,12-dimethylbenz(A)anthracene and 7-hydroxymethylbenz(A)anthracene and by their K-region derivatives. Int J Cancer. 1974 Mar 15;13(3):304–310. doi: 10.1002/ijc.2910130305. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Miller E. C. Chemical carcinogenesis: mechanisms and approaches to its control. J Natl Cancer Inst. 1971 Sep;47(3):V–XIV. [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. NUCLEOTIDE SEQUENCE ANALYSIS OF POLYRIBONUCLEOTIDES BY MEANS OF PERIODATE OXIDATION FOLLOWED BY CLEAVAGE WITH AN AMINE. J Biol Chem. 1964 Sep;239:2927–2934. [PubMed] [Google Scholar]
- Nelson J. H., Grunberger D., Cantor C. R., Weinstein I. B. Modification of ribonucleic acid by chemical carcinogens. IV. Circular dichroism and proton magnetic resonance studies of oligonucleotides modified with N-2-acetylaminofluorene. J Mol Biol. 1971 Dec 14;62(2):331–346. doi: 10.1016/0022-2836(71)90431-1. [DOI] [PubMed] [Google Scholar]
- Sims P., Grover P. L., Swaisland A., Pal K., Hewer A. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature. 1974 Nov 22;252(5481):326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- Stewart R. F., Davidson N. Polarized absorption spectra of purines and pyrimidines. J Chem Phys. 1963 Jul 15;39(2):255–266. doi: 10.1063/1.1734238. [DOI] [PubMed] [Google Scholar]
- Swaisland A. J., Hewer A., Pal K., Keysell G. R., Booth J., Grover P. L., Sims P. Polycyclic hydrocarbon epoxides: the involvement of 8,9-dihydro-8,9-dihydroxybenz (a) anthracene 10,11-oxide in reactions with the DNA of benz (a) anthracene-treated hamster embryo cells. FEBS Lett. 1974 Oct 1;47(1):34–38. doi: 10.1016/0014-5793(74)80420-5. [DOI] [PubMed] [Google Scholar]


