Abstract
The brain reward circuit has a central role in reinforcing behaviors that are rewarding and preventing behaviors that lead to punishment. Recent work has shown that the lateral habenula is an important part of the reward circuit by providing ‘negative value’ signals to the dopaminergic and serotonergic systems. Studies also suggest that dysfunction of the lateral habenula is associated with psychiatric disorders including major depression. In this review, we first discuss insights gained from neuronal recordings in monkeys regarding how the lateral habenula processes reward-related information. We next highlight recent optogenetic experiments in rodents addressing normal and abnormal functions of the habenula. Finally, we discuss how deregulation of the lateral habenula may play a role in depressive behaviors.
One of the most conspicuous characteristics of any organism is its ability to react to salient stimuli with an appropriate behavioral response that maximizes survival and avoids threatening or unpleasant events. The brain reward system, thought to play a central role in controlling these behaviors, is a complex circuit containing the forebrain limbic system and its links to the midbrain aminergic (e.g. dopaminergic and serotonergic) centers1–3.
The habenula is a structure that has been preserved through evolution in vertebrates, being found in lamprey, fish, reptiles, and mammals, suggesting that it regulates processes important for survival4. The habenula is located at the most caudal and dorsal part of the thalamus and together with the pineal gland, forms the epithalamus. The stria medullaris provides most of the inputs to the habenula while the fasciculus retroflexus is the main output. The stria medullaris, the habenula and the fasciculus retroflexus form the dorsal diencephalic conduction system5 that, together with the medial forebrain bundle6, transmit most of the cognitive and sensory information from the limbic system to aminergic midbrain areas involved in reward processing and affective control1, 7, 8.
The habenula comprises two well defined nuclei, the medial habenula (MHb, see below) and the lateral habenula (LHb). The LHb receives inputs from the basal ganglia, which participate in many functions including selection of actions, as well as the limbic system, which among other functions controls emotions7, 9. The LHb sends efferents to dopaminergic centers10, serotonergic centers11 and the GABAergic rostromedial tegmental nucleus (RMTg), which inhibits dopaminergic centers12, 13. Aminergic nuclei also send feedback projections to the LHb14, 15. The LHb is thus well positioned to integrate emotion into the selection of actions.
Over the past several decades a number of functions have been ascribed to the LHb: the regulation of sleep, maternal behavior, electromagnetic detection, and navigation7. However, recent findings suggest that the LHb participates in processing reward-related information16, 17. LHb neurons encode negative reward prediction error: they are excited by unexpected non-rewarding or unpleasant events and inhibited by unexpected rewarding events16, 17. The LHb also provides aversive related signals to the dopaminergic centers16, 18. These studies suggest that the LHb plays a central role in goal-directed behaviors. Moreover, findings indicating that the LHb is activated by different type of stressors19 and that habenula lesions induce impaired behavioral responses in stress-related tasks20 also suggest that the LHb might play a central function in behavioral response to stress or in avoidance of punishment.
Deregulation of aminergic centers have been propose to be central in depressive disorders2, 21. Given that the LHb encodes reward-related information, responds to aversive stimuli, and regulates aminergic centers, it has been suggested that deregulation of LHb function could underlie a number of psychiatric disorders, including schizophrenia, drug-induced psychosis, addiction, and depression7, 22, 23; we will focus on depression since recent animal studies have provided insights into possible underlying neural mechanisms.
In this review, we first discuss how the LHb processes reward- and punishment-related information, based mainly on single neuronal recording in the macaque monkey. We next highlight recent optogenetic experiments examining LHb function and refining LHb circuits in rodents. Finally, we discuss how deregulation of the LHb may contribute to depressive behaviors.
Selective effects of LHb on heterogeneous dopamine systems
A prominent ability of animals is to predict rewards and punishments, and change their behavior accordingly. This gives animals a higher chance of survival. But, how behavior should be changed is different between reward and punishment prediction.
When reward is predicted, a change in behavior depends mostly on the recent history of reward, which is often referred to ‘reward prediction error (RPE)’24. When the actual reward is larger than the predicted reward (i.e., positive RPE), the action associated with the reward is facilitated (approach or Go). When the actual reward is smaller (i.e., negative RPE), the action is suppressed (avoid or Nogo).
LHb plays an important role in the RPE-guided behavior: LHb neurons encode RPE by changing their activity phasically in response to the actual or estimated reward (i.e., inhibition with positive RPE, excitation with negative RPE)16. The RPE signals in LHb neurons are transmitted, at least partly, to midbrain dopamine (DA) neurons16, 25. Since the LHb-DA effect is largely mediated by RMTg which consists of GABAergic inhibitory neurons12, 13, 25, DA neurons are excited with positive RPE and inhibited with negative RPE26. Behavioral changes would then be mediated by the striatum which receives strong inputs from DA neurons: approach (or Go) by direct pathway neurons (via D1 receptors); avoidance (or Nogo) by indirect pathway neurons (via D2 receptors)27, 28. The bidirectional effects of RPE on behavior help animals to maximize their rewards29.
LHb neurons are also sensitive to punishment: they are excited by sensory cues predicting punishments17, similarly to their responses to negative RPE. This seems natural because both negative RPE and punishment are worthless or bad for animals. The punishment-predicting excitatory response of LHb neurons is translated into an inhibitory response of DA neurons30, which would lead to avoidance or Nogo.
However, simply suppressing actions while anticipating punishments may not always be good31, 32. In many cases, performing alternative actions would get the animal out of the bad situation. Indeed, DA neuron’s response to punishments is complex: some are excited and others are inhibited30, 33–36. In the rat ventral tegmental area (VTA), DA neurons in its dorsal part are inhibited, whereas DA neurons in its ventral part are excited by foot shocks33. Among putative DA neurons in the substantia nigra pars compacta (SNc) in the macaque monkey, neurons located medially are inhibited, whereas neurons located laterally are excited by a punishment or its predictor30.
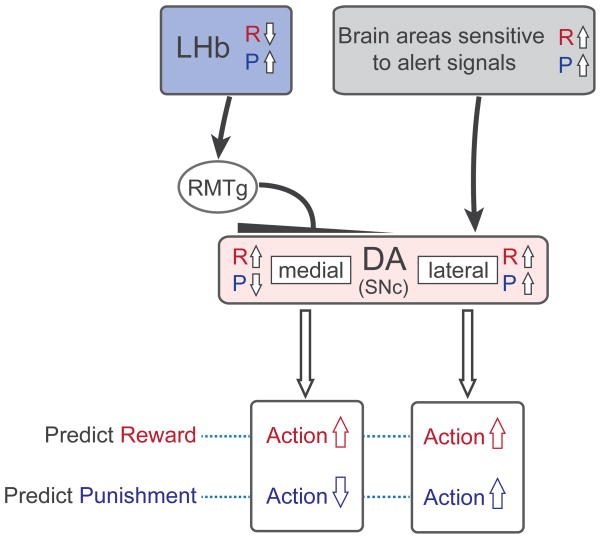
The heterogeneity of DA neurons is partly caused by the heterogeneous inputs from LHb. In the macaque monkey, LHb preferentially inhibits DA neurons located in the medial SNc which consequently are inhibited by punishments and their predictors (Fig. 1). In other words, LHb-DA (medial) circuit suppresses actions leading to punishments37, which is supported experimentally38–41. In contrast, lateral DA neurons are excited by punishments and their predictors (partly because they receive inputs from brain areas that are sensitive to alert signals42 and therefore facilitate actions evoked by the punishment predictions.
Figure 1.
Inhibitory effects originating from the lateral habenula (LHb) create functional heterogeneity among dopamine (DA) neurons. LHb neurons encode negative motivational values (inhibited by reward and excited by punishment) and inhibit DA neurons in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) through the rostromedial tegmental nucleus (RMTg). In the monkey the LHb-induced inhibition is stronger for DA neurons in the medial part of SNc. The medial DA neurons thus signal positive values, and thereby facilitate reward-approaching actions (shown in red) when reward is predicted and suppress actions (blue) when punishment is predicted. In contrast, the lateral DA neurons are excited when either reward or punishment is predicted, and thereby facilitate both reward-approaching actions when reward is predicted (red) and punishment-avoiding actions when punishment is predicted (blue). R and P indicate reward and punishment. The punishment-related excitation of the lateral DA neurons may be caused by inputs from other brain areas sensitive to alert signals. Note that this scheme may not apply to all DA neurons; for example, some DA neurons in the rodent VTA receive excitatory inputs from the LHb (see text for details).
The LHb-DA (medial) circuit and the DA (lateral) circuit are also different in their ways to treat reward uncertainty. When the reward outcome is unpredictable, medial DA neurons and LHb neurons show little response reflecting its objective value, which implies that they promote no action30. Indeed, they treat the resolution of uncertainty (i.e., advanced information) more valuable than certainty (i.e., no information)43, 44. In contrast, lateral DA neurons tend to be excited even when the reward outcome is uncertain30, which implies that they promote actions. These data raise the possibility that LHb-DA (medial) circuit may guide risk-avoiding behavior, whereas the DA (lateral) circuit may support risk-preferring behavior. Possibly in line with these observations, LHb inactivation produces a deficit in choice when the reward outcome is uncertain45.
To summarize, the balance between LHb-DA (medial) circuit and DA (lateral) circuit in an animal (or human) may contribute to the behavioral and emotional trends of the animal (or person). If the LHb-DA (medial) circuit were to become overactive, the animal may become less active, avoiding aversive or risky situations, reluctant to explore, and unwilling to initiate challenging actions. These phenomena may constitute some of the symptoms of major depression and other psychiatric disorders2.
Differential monitoring of reward values by the LHb
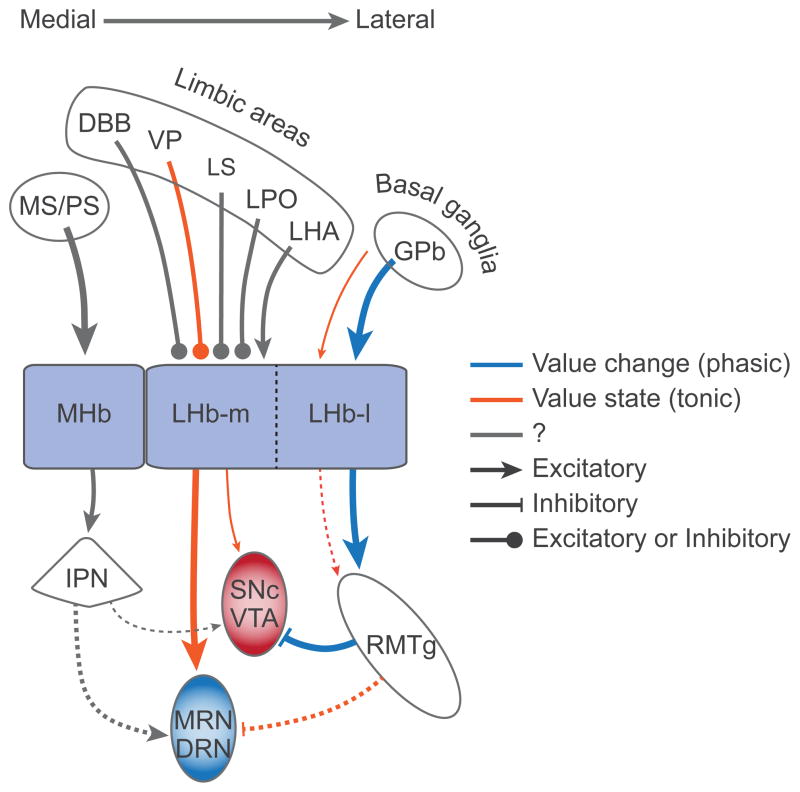
The discussion so far has been focused on the effect of LHb on the DA system. However, it is known that LHb affects other neuromodulator systems7, 46. Particularly relevant to the current theme is serotonin (5HT). The LHb-DA circuit and the LHb-5HT circuit seem segregated mediolaterally (Fig. 2). The lateral part of LHb (LHb-l) receives inputs mainly from the basal ganglia through the border region of the globus pallidus (GPb)47 and sends outputs mainly to the DA system (SNc and VTA) through RMTg48. In contrast, the medial part of LHb (LHb-m) receives inputs from the limbic areas (e.g., diagonal band of Broca)47 and sends outputs mainly to the 5HT system (dorsal and median raphe nuclei, DRN and MRN)11, 48, 49. Manipulations of LHb outputs (electrical stimulation, lesion, inactivation) induce changes in 5HT release in the 5HT nuclei and their target brain areas50, 51, 52 which may lead to changes in behavior and possibly emotion53, 54. In human subjects, a transient depressive relapse, induced by acute tryptophan depletion, leads to a decrease in 5HT neurotransmission associated with co-variation of DRN and habenula activity, particularly in individuals showing the greatest depression55.
Figure 2.
Both lateral habenula (LHb) and medial habenula (MHb) control the dopaminergic (DA) and serotonergic (5HT) systems. The lateral part of LHb (LHb-l) mainly influences the DA system (SNc and VTA) through the rostromedial tegmental nucleus (RMTg), whereas the medial part of LHb (LHb-m) mainly influences the 5HT system (MRN and DRN) directly. The RMTg-VTA/SNc connection is inhibitory; the other connections are mostly excitatory, although they may target inhibitory interneurons. MHb may influence the DA-5HT systems indirectly through the interpeduncular nucleus (IPN), although these connections need to be confirmed. Inputs to DA neurons may signal ‘value change’ by changing their level phasically, whereas inputs to 5HT neurons may signal ‘value state’ by changing their level tonically. It remains to be studied whether neurons in LHb and MHb transmit the phasic and tonic signals differentially to the DA-5HT systems. Dashed lines indicate connections that need to be confirmed and line thickness indicates the presumed strength of connection. DBB, diagonal band of Broca; DRN, dorsal raphe nucleus; GPb, globus pallidus, border region; LHA, lateral hypothalamic area; LPO, lateral preoptic area; LS, lateral septum; MRN, median raphe nucleus; MS, medial septum; PS, posterior septum; SNc, substantia nigra pars compacta; VP, ventral pallidum; VTA, ventral tegmental area.
This LHb-5HT circuit seems important from the psychiatric viewpoint, because changes in 5HT neurotransmission are associated with changes in emotion8. However, little is known about how LHb-5HT circuit might be involved in major depression. A fundamental but unresolved issue here is: what kinds of information are transmitted through this circuit, especially by individual neurons? Recent data provide some hints to this question, as described below.
Neurons in the DRN in the macaque monkey encode reward values, but differently from DA neurons56, 57. While DA neurons show phasic changes in activity every time reward value changes (i.e., RPE), 5HT neurons show tonic changes in activity after reward value is updated. In other words, while DA neurons signal ‘value changes’, 5HT neurons signal ‘value state’. DA neurons alone may not indicate what the current value state is (e.g., Am I comfortable?); 5HT neurons alone may not indicate whether the value is increasing or decreasing (e.g., Am I on the right track?). Combining these two kinds of signals would constitute a better mechanism that monitors and controls the value accurately and efficiently.
Previous studies on LHb neurons emphasized their ‘value change’ coding16. However, this may not be a universal feature of LHb neurons, because the LHb consists of many subnuclei which are characterized by molecular and chemical features58, 59. In particular, its medial division (LHb-m), which projects to the DRN/MRN directly, may contain neurons that encode ‘value state’; preliminary data support this hypothesis (unpublished). Furthermore, brain areas upstream or downstream to LHb also contain neurons encoding ‘value state’. The upstream areas include the border region of the globus pallidus (GPb)60, 61, and ventral pallidum (VP)62, 63. GPb contains two types of neurons – positive and negative value coding types – both of which project to LHb64. While the negative type encodes ‘value change’, the positive type encodes ‘value state’. VP also contains positive and negative type neurons, but both encode ‘value state’65, although it is unknown which type projects to the LHb. A prominent downstream area is RMTg, where neurons show negative value coding and exert GABAergic inhibitions on DA neurons12. Some of them encode ‘value change’ and other encode ‘value state’25.
These results suggest the following hypothesis (Fig. 2): LHb conveys both ‘value change’ and ‘value state’ signals downstream, but the ‘value change’ signal is selectively directed to DA neurons while the ‘value state’ signal is selectively directed to 5HT neurons. This creates a system where ‘value change’ and ‘value state’ can be manipulated separately. The ‘value change’ signal controlled by DA neurons may be related to ‘motivation’ or ‘wanting’66; the ‘value state’ signal controlled by 5HT neurons may be related to ‘mood’ or ‘liking’67.
It is unknown, however, whether these LHb circuits controlling the DA and 5HT systems are essential for controlling motivation and mood. The amygdala, for example, is crucial for emotional control68 and contains neurons encoding ‘value state’. Yet, little is known about whether and how the amygdala circuits and the LHb circuits might interact with each other69.
Optogenetic assessment of the habenula circuits
While studies in monkeys have provided rich information by correlating activity of LHb neurons to subtle behavioral contingencies, more versatile approaches are available for experiments using rodents which allow better dissection of neural circuits to assess the roles of specific pathways in different behavioral contexts, in normal and pathological states.
Recent studies in rodents have used optogenetic approaches70 to examine the functional role of specific inputs and outputs of the LHb in processing reward/aversive information. One study examined the effects of selective activation of inputs to the LHb originating from the entopeduncular nucleus (EP), a basal ganglia nucleus corresponding to the GPb in monkeys. Optogenetic activation of EP terminals in the LHb produced aversion in a passive avoidance test71. As for outputs, optogenetic activation of LHb terminals contacting neurons in the RMTg produced passive, active and conditioned behavioral avoidance41. While LHb activation of RMTg can reduce activity of DA neurons25 (which may explain the generally opposite response properties of LHb and DA neurons in monkeys16, 25), LHb also sends direct excitatory projections to the VTA that activate DA neurons18. Surprisingly, optogenetic activation of this LHb-VTA pathway in mice is also aversive, and injection of a dopamine antagonist in the mPFC blocks the aversion induced by activation of the LHb-VTA pathway18. These results show that LHb activity can lead to reduced DA neuronal activity (by way of the RMTg) or increased DA neuronal activity (by direct pathways), but in both cases the result is to produce aversion. Stamatakis et al. recently identified in mice an inhibitory input to the LHb arising from a unique population of VTA neurons expressing dopaminergic markers72. Optogenetic activation of axon terminals from this VTA input releases GABA (and not dopamine) in the LHb, suppressing postsynaptic firing of LHb neurons. Activation of these terminals in the LHb also reduces firing activity of RMTg neurons, increases the spontaneous firing rate of dopaminergic neurons in the VTA, and promotes reward-related behavioral responses72. By combining tract tracing methods and immunohistochemistry, a previous study showed that LHb terminals, in addition to contacting onto VTA dopaminergic neurons, also contact onto VTA GABAergic neurons10. Optogenetic activation of VTA GABAergic neurons has been shown to disrupt reward consumption73. How direct activation of GABAergic VTA neurons by the LHb affects dopaminergic activity and behavior still needs to be explored. These results demonstrate the intricate relationship between the LHb and the dopaminergic midbrain system and lead to this interesting hypothesis: some LHb neurons might project to this unique dopaminergic population of VTA neurons72 that project back to, and inhibit, the LHb, offering a negative feedback mechanism to LHb neurons.
These new studies indicating that LHb activity can drive aversion support previous studies that linked LHb activity to the processing of different type of stressors19, 74, 75 and support electrophysiological recordings in macaque monkeys that have shown the central role of the LHb in the processing of negative reward-related information. Thus, aversive stimuli activate the LHb, which in turn triggers circuits that drive aversive behaviors.
Lateral habenula in depressive disorders
Monkey and rodent studies have linked the normal function of the LHb to the processing of rewarding/punishing information. In particular, the absence of reward (i.e. disappointment) and the expectation of punishment (i.e. sense of doom) are strong activators of the LHb. What occurs when the LHb functions incorrectly?
Several pieces of evidence have suggested that LHb hyperactivity may play an important role in depression. fMRI studies in depressed humans as well as metabolic labeling in animal models of depression have shown increased LHb activity55, 76, and LHb lesions in rodents have reduced depressive behaviors53, 77. Perhaps most revealing, a clinical case study in which the habenula was inactivated by deep brain stimulation (DBS) resulted in a full remission (albeit with a several week delay period) of major depression in a therapy-refractory patient22. Notably, inadvertent stopping of DBS (which was unknown to the patient) brought back depressive symptoms within days, which were then ameliorated by restarting the DBS.
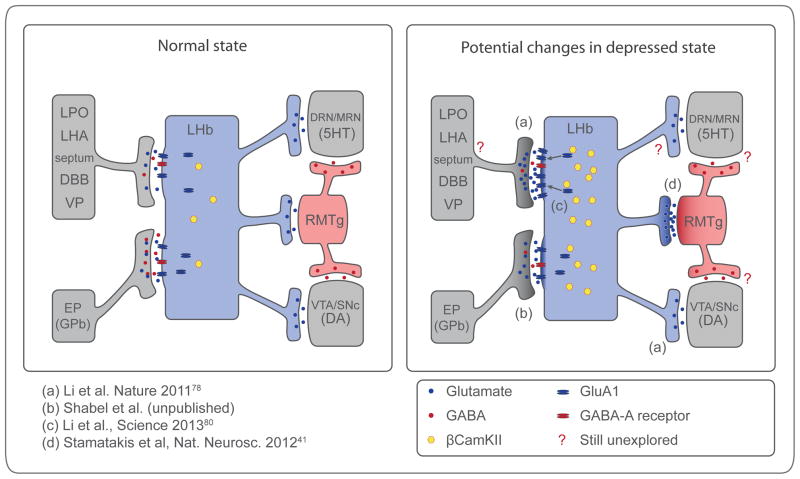
A number of recent studies in rodents suggest that hyperactivity of LHb neurons may contribute to depression (Fig. 3). One study, in models of depression78, detected enhanced excitatory synaptic inputs onto LHb neurons that project to the VTA. These LHb neurons may selectively excite mPFC-projecting dopaminergic VTA neurons that produce aversion in rodents18 and might be involved in cognitive disturbances observed in major depression79. A separate study showed that exposure of mice to protocols that induce behaviors modeling depression increased LHb excitatory drive onto RMTg neurons41, which would be expected to reduce dopaminergic activity25, and potentially play a role in depression2.
Figure 3.
Potential hyperactive synapses in human depression. Schematic showing main inputs and outputs from the lateral habenula (LHb) in normal state and potential cellular and molecular changes in depressed state. Dark shading represents overactive synapses. Depressed state may include increase in excitatory drive onto the LHb (a, b, c) and increased LHb output to RMTg (d) and VTA (a). See text for details. EP, entopeduncular nucleus; GPb, globus pallidus, border region; LPO, lateral preoptic area; LHA, lateral hypothalamic area; DBB, diagonal band of broca; VP, ventral pallidum; DRN/MRN, dorsal and median raphe nucleus respectively; RMTg, rostromedial tegmental neucleus; VTA, ventral tegmental area; SNc, substantia nigra pars compacta.
Recent studies suggested two molecular mechanisms for the depression-related potentiation of depolarizing drive to LHb neurons: 1) a biochemical change inside LHb neurons, and 2) a change in neurotransmitters targeting LHb neurons (Fig. 3). In the first mechanism, CaMKIIβ was identified as a potentially key molecular determinant of depression, through a proteomic screen of LHb tissue in rodent models of depression80. Increased levels of CaMKIIβ were detected in rat models of depression (illustrated as yellow dots in the LHb neuron in Fig. 3). Experimentally increasing CaMKIIβ function in the LHb enhanced synaptic efficacy onto LHb neurons by phosphorylating and driving into synapses GluA1-type glutamate-sensitive receptors. This led to increased spike output of LHb neurons and was sufficient to produce behaviors that model human depression. Conversely, down-regulation of CaMKIIβ levels in the LHb was sufficient to reverse these behaviors. The second mechanism was found in the EP input to LHb neurons (Shabel et al, unpublished results). They found co-release of GABA (which was inhibitory) and glutamate (which was excitatory) from individual EP terminals, and even vesicles. The GABA/glutamate ratio (illustrated as blue and red dots at EP-LHb synapse in Fig. 3) controlled LHb activity. Co-release of GABA was reduced in a model of depression, thus increasing the net depolarizing drive to the LHb. In contrast, chronic treatment with an antidepressant increased the GABA /glutamate ratio.
Taken together, studies examining the normal and abnormal function of the LHb in macaque monkeys and rodents suggest that aberrantly overactive LHb neurons may produce an unpleasant (i.e. aversive) state of constant disappointment or sense of doom, which may contribute to human depression.
Lateral habenula and medial habenula
The habenula consists of two regions, lateral habenula (LHb) and medial habenula (MHb), and these two nuclei together constitute a unique structure that is distinguished from the surrounding thalamic areas, especially in terms of phylogeny4, 81 and ontogeny82. A prominent difference between LHb and MHb is their chemical features. Whereas most neurons in LHb are glutamatergic, neurons in MHb use acetylcholine, substance P, and glutamate as their neurotransmitters83, 84. The cholinergic effect of MHb neurons is mediated by nicotinic receptors85, and is heavily involved in nicotine-related behavioral changes86, 87.
Despite the differences in chemical features, LHb and MHb seem to have shared or related functions. This is hinted by similarities in malfunctions of LHb and MHb. In humans with major depression, both LHb and MHb are smaller than control subjects88. Furthermore, both LHb and MHb are hyperactive in rodent models of major depression76. Experimental manipulations of MHb (like LHb described above) cause changes in behavior, especially under stress or fear. Selective genetic ablation of MHb neurons in mice, which is associated with a decrease in ACh in the interpeduncular nucleus (IPN), induces hyperactive, impulsive, and compulsive behaviors, effort aversion, and memory dysfunctions89. In zebrafish, genetic inactivation of the dorsal and ventral habenula, which correspond to MHb and LHb respectively, changes the pattern of avoidance/freezing responses, somewhat differently, in anticipation of punishment90. In short, both LHb and MHb may control motivation and emotion. This view may be supported by recent studies suggesting that the MHb also has substantial influences on DA and 5HT neurons91, 92, which are largely mediated by the IPN (Fig. 2). Differences in the effects of LHb and MHb manipulations could be explained by their differential effects on DA and 5HT, or on other systems (e.g., norepinephrine system, hippocampus, immune system)46, 93,, 94, 95. It is possible that LHb and MHb have fundamentally different functions that have not yet been revealed.
Strategies for habenula targeting and manipulation
Histochemical and morphological analysis distinguished at least 10 subnuclei in the LHb and 5 subnuclei in the MHb58, 59. Moreover, as mentioned above, the LHb and MHb have distinct inputs and outputs (Figs. 2 and 3). This heterogeneity suggests that different habenular nuclei and subnuclei might be involved in different biological functions. The inability to manipulate specific habenular subnuclei by classic methods, such as electrical stimulation or pharmacological perturbation, likely contributes to the difficulty in clearly determining the biological function of the LHb and MHb.
To improve our understanding of the habenula, it will be important to delineate 1) the precise nature of the circuitry of each of these nuclei/subnuclei; 2) how manipulation of individual nuclei/subnuclei affects behavior during normal and stressful/aversive situations; and 3) what are the changes that can be linked to specific pathological states. In order to answer these questions, it is critical to be able to specifically manipulate these nuclei and subnuclei.
Powerful ways of manipulating neuronal activity now exist. Optogenetic approaches use microbial opsins that, when expressed in neurons and illuminated, can rapidly generate time-locked action potentials or can rapidly block action potentials by hyperpolarizing neurons (see70 for a detailed description of optogenetic tools and technical strategies for optogenetic investigation in rodents). In cases where long lasting activation or inhibition of neuronal activity is preferable, an alternative approach is the use of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)96.
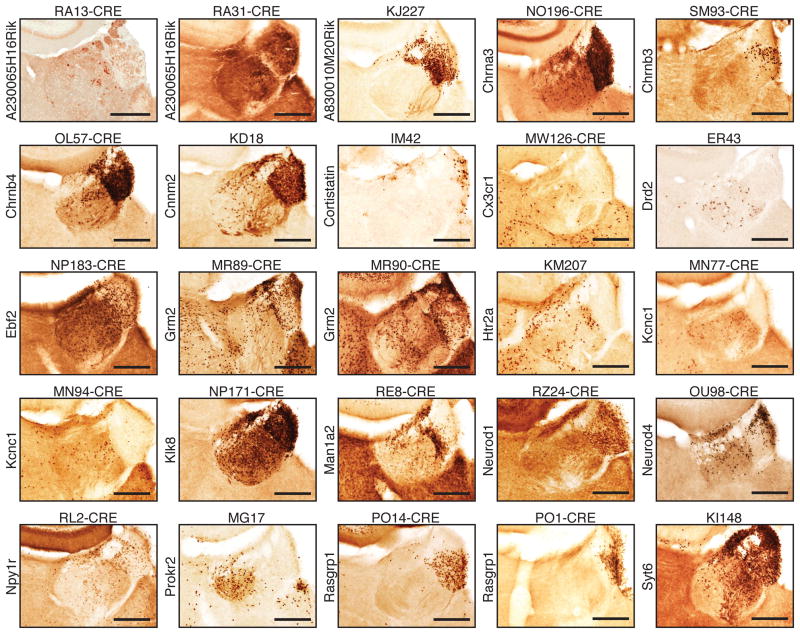
In order to drive expression of opsins or DREADDs in specific subset of neurons, an increasing collection of cre-reporter transgenic lines are now available70, 97. A combinatorial approach, using cre-driver mouse lines and viral delivery of cre-dependent opsins or DREADDs, allows temporally and spatially specific manipulation of neuronal ensembles. Notably, a survey of available cre-driver mouse lines from GENSAT (gensat.org) (Fig. 4) shows that many cre-driver mouse lines may be readily used to target different subnuclei of either the LHb or the MHb. Viral delivery of Cre-dependent opsins or DREADDs into the habenula of these cre-driver mouse lines may greatly advance our understanding of habenular function in normal and pathological states.
Figure 4.
Transgenic cre-driver mouse lines from GENSAT (gensat.org) that permit targeting of different neuronal ensemble in the LHb and MHb. A representative in situ histochemistry image encompassing the habenula is shown for different founder cre lines (founder line name on top of each image) where cre expression is driven under specified gene promoter (gene name on the left of each images). These lines are available from GENSAT (gensat.org). Scale bars are 250 um.
Conclusions
In addition to the habenula, there are many brain areas that are involved in the control of emotion. Why then is the habenula in a pivotal position to guide or misguide emotional behaviors? It may be because the habenula acts as a hub of emotional information: it receives inputs widely and delivers outputs widely through multiple neuromodulator systems and brainstem circuits. Recent studies in monkeys and rodents have provided evidence that the LHb plays important roles in decision making by sending reward-related and aversive signals to the dopaminergic centers. By processing these signals as value state and value change, the LHb is centrally positioned to transform emotional information into proper behavioral responses, particularly during aversive, stressful situation.
Human and non-human studies have both provided evidence that dysfunction of the LHb may play a role in depression. Hyperactivity of LHb neurons could reduce dopamine-related rewards signals, likely through intermediary actions of RMTg; or by directly activating dopamine neurons that project to mPFC. Overactivity of these pathways may affect decision-making (e.g. overprocessing of aversive events, reduced motivation, or altered learning from positive or negative reinforcement).
It is notable that the LHb also sends projections to serotonergic centers. In addition to reward value processing, serotonin has been implicated in the prediction of aversive events and in behavioral inhibition, as well as being centrally involved in depressive disorders. Little is known regarding how exactly the LHb affects these serotonergic centers. The type of signals provided by the LHb to these centers, and their potential deregulation in depressive disorders should be investigated more extensively.
Hyperactivity of the LHb may contribute to depression, and its suppression may reduce depression symptoms. This provides a rationale and potential opportunity for treating depression. It will be important to identify how the LHb becomes hyperactive, and which molecular targets would permit selective reduction of LHb hyperactivity pharmacologically and thereby reduce depressive symptoms. We can hope that future studies elucidating molecular, cellular and circuit properties of the LHb, and how these are linked to behavior, will aid in addressing these issues.
Acknowledgments
C.D.P. received support from the Institutions de Recherche en Santé du Canada and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. O.H. and R.M. received support from the NIH.
References
- 1.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 2.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephenson-Jones M, Floros O, Robertson B, Grillner S. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc Natl Acad Sci U S A. 2012;109:E164–173. doi: 10.1073/pnas.1119348109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neuroscience & Biobehavioral Reviews. 1982:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 6.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci (Regul Ed) 2008:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Lecourtier L, Lecourtier L, Kelly PH, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience & Biobehavioral Reviews. 2007:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. European Journal of Neuroscience. 2009:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. The Journal of comparative neurology. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 12.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. The Journal of comparative neurology. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- 14.Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. The Journal of comparative neurology. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 15.Reisine TD, et al. Evidence for a dopaminergic innervation of the cat lateral habenula: its role in controlling serotonin transmission in the basal ganglia. Brain Res. 1984;308:281–288. doi: 10.1016/0006-8993(84)91067-9. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012 doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox KS, Christoph GR, Double BA, Leonzio RJ. Kainate and electrolytic lesions of the lateral habenula: effect on avoidance responses. Physiol Behav. 1986:413–417. doi: 10.1016/0031-9384(86)90307-0. [DOI] [PubMed] [Google Scholar]
- 21.Dayan P, Huys QJM. Serotonin, inhibition, and negative mood. PLoS Comp Biol. 2008:e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- 24.Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 27.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci. 2006;26:5360–5369. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz W. Behavioral theories and the neurophysiology of reward. Annual review of psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 32.Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nat Rev Neurosci. 2007;8:300–311. doi: 10.1038/nrn2119. [DOI] [PubMed] [Google Scholar]
- 33.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 36.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman A, et al. Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology. 2011;60:381–387. doi: 10.1016/j.neuropharm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilango A, Shumake J, Wetzel W, Scheich H, Ohl FW. Electrical stimulation of lateral habenula during learning: frequency-dependent effects on acquisition but not retrieval of a two-way active avoidance response. PloS one. 2013;8:e65684. doi: 10.1371/journal.pone.0065684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto M, Hikosaka O. Electrical Stimulation of the Primate Lateral Habenula Suppresses Saccadic Eye Movement through a Learning Mechanism. PloS one. 2011;6:e26701. doi: 10.1371/journal.pone.0026701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong S, Hikosaka O. Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.07.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–126. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nat Neurosci. 2011:1209–1216. doi: 10.1038/nn.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stopper CM, Floresco SB. What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci. 2014;17:33–35. doi: 10.1038/nn.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience and biobehavioral reviews. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. The Journal of comparative neurology. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- 48.Goncalves L, Sego C, Metzger M. Differential projections from the lateral habenula to the rostromedial tegmental nucleus and ventral tegmental area in the rat. The Journal of comparative neurology. 2012;520:1278–1300. doi: 10.1002/cne.22787. [DOI] [PubMed] [Google Scholar]
- 49.Kim U. Topographic commissural and descending projections of the habenula in the rat. The Journal of comparative neurology. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- 50.Reisine TD, Soubrie P, Artaud F, Glowinski J. Involvement of lateral habenula-dorsal raphe neurons in the differential regulation of striatal and nigral serotonergic transmission cats. J Neurosci. 1982;2:1062–1071. doi: 10.1523/JNEUROSCI.02-08-01062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalen P, Strecker RE, Rosengren E, Bjorklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- 52.Amat J, et al. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 53.Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behavioural brain research. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 54.Pobbe RL, Zangrossi H., Jr The lateral habenula regulates defensive behaviors through changes in 5-HT-mediated neurotransmission in the dorsal periaqueductal gray matter. Neuroscience letters. 2010;479:87–91. doi: 10.1016/j.neulet.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 55.Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci. 2010;30:6262–6272. doi: 10.1523/JNEUROSCI.0015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geisler S, Andres KH, Veh RW. Morphologic and cytochemical criteria for the identification and delineation of individual subnuclei within the lateral habenular complex of the rat. The Journal of comparative neurology. 2003;458:78–97. doi: 10.1002/cne.10566. [DOI] [PubMed] [Google Scholar]
- 59.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. The Journal of comparative neurology. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 60.Parent A, Gravel S, Boucher R. The origin of forebrain afferents to the habenula in rat, cat and monkey. Brain Res Bull. 1981;6:23–38. doi: 10.1016/s0361-9230(81)80066-4. [DOI] [PubMed] [Google Scholar]
- 61.Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. The Journal of comparative neurology. 2001;439:162–175. doi: 10.1002/cne.1340. [DOI] [PubMed] [Google Scholar]
- 62.Haber SN, Lynd-Balta E, Mitchell SJ. The organization of the descending ventral pallidal projections in the monkey. The Journal of comparative neurology. 1993;329:111–128. doi: 10.1002/cne.903290108. [DOI] [PubMed] [Google Scholar]
- 63.Tripathi A, Prensa L, Mengual E. Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct Funct. 2013;218:1133–1157. doi: 10.1007/s00429-012-0451-0. [DOI] [PubMed] [Google Scholar]
- 64.Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76:826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 67.Seymour B, Daw ND, Roiser JP, Dayan P, Dolan R. Serotonin selectively modulates reward value in human decision-making. J Neurosci. 2012;32:5833–5842. doi: 10.1523/JNEUROSCI.0053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 69.Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the Basal Ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamatakis AM, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA Neurons Disrupts Reward Consumption. Neuron. 2012:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kazi JA, Mori S, Kuchiiwa S, Nakagawa S. Prolonged expression of c-Fos protein in the lateral habenular nucleus of the Japanese monkey (Macaca fuscata) after eye enucleation. Neuro-Signals. 2004;13:130–133. doi: 10.1159/000076566. [DOI] [PubMed] [Google Scholar]
- 75.de Jong TR, Measor KR, Chauke M, Harris BN, Saltzman W. Brief pup exposure induces Fos expression in the lateral habenula and serotonergic caudal dorsal raphe nucleus of paternally experienced male California mice (Peromyscus californicus) Neuroscience. 2010;169:1094–1104. doi: 10.1016/j.neuroscience.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 76.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 77.Amat J, et al. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 78.Li B, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizoguchi K, et al. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li K, et al. βCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Signore IA, et al. Zebrafish and medaka: model organisms for a comparative developmental approach of brain asymmetry. Philosophical transactions of the Royal Society of London. 2009;364:991–1003. doi: 10.1098/rstb.2008.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Contestabile A, et al. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- 84.Qin C, Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161:827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- 85.Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frahm S, et al. Aversion to Nicotine Is Regulated by the Balanced Activity of beta4 and alpha5 Nicotinic Receptor Subunits in the Medial Habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 88.Ranft K, et al. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychological medicine. 2010;40:557–567. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi Y, et al. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front Behav Neurosci. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agetsuma M, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 91.McCallum SE, Cowe MA, Lewis SW, Glick SD. alpha3beta4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology. 2012;63:434–440. doi: 10.1016/j.neuropharm.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu YW, et al. Medial Habenula Output Circuit Mediated by alpha5 Nicotinic Receptor-Expressing GABAergic Neurons in the Interpeduncular Nucleus. J Neurosci. 2013;33:18022–18035. doi: 10.1523/JNEUROSCI.2927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sugama S, Conti B. Interleukin-18 and stress. Brain research reviews. 2008;58:85–95. doi: 10.1016/j.brainresrev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Goutagny R, et al. Interactions between the Lateral Habenula and the Hippocampus: Implication for Spatial Memory Processes. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aizawa H, et al. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:8909–8921. doi: 10.1523/JNEUROSCI.4369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nair SG, Strand NS, Neumaier JF. DREADDing the lateral habenula: A review of methodological approaches for studying lateral habenula function. Brain Res. 2013:93–101. doi: 10.1016/j.brainres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]