Abstract
Neighborhood walkability has been associated with increased physical activity, but only a few studies have explored the association between walkability and health outcomes related to physical activity, such as type 2 diabetes. The aim of this study was to investigate the association between objectively assessed neighborhood walkability and the 4-year incidence of type 2 diabetes in a sample of 512,061 Swedish adults aged 18 years and older. Neighborhoods were defined by 408 administratively defined geographical areas in the city of Stockholm. We found a negative association between walkability and type 2 diabetes (OR=1.33, 95% CI=1.13–1.55) that remained significant after adjusting for neighborhood deprivation. This association, however, no longer remained statistically significant after adjusting for individual socio-demographic factors. These results were also confirmed using a co-sibling design. Future studies are encouraged to further explore the potential effect of a broader array of the neighborhood built environment on health outcomes related to physical activity.
Keywords: Diabetes mellitus, epidemiology, geographic information systems, neighborhood deprivation, walkability
Introduction
Research on environmental correlates of physical activity has gained increasing interest during the last decade. Neighborhood walkability, a physical environmental characteristic, has been investigated in several studies and it has become one of the most consistent environmental correlates of physical activity in Europe (Sundquist et al., 2011; Van Holle et al., 2012) and worldwide (Owen et al., 2007; Sallis et al., 2009; Bauman et al., 2012).
Previous research on neighborhood walkability has primarily addressed the hypothesis that more walkable environments result in higher levels of physical activity, which in turn may decrease the public health burden of non-communicative diseases such as hypertension, type 2 diabetes, cardiovascular disease and depression (Frank et al., 2006; Owen et al., 2007; Sallis et al., 2009). Most studies have investigated the association between neighborhood walkability and walking and/or total physical activity (Owen et al., 2007; Sallis et al., 2009; Sundquist et al., 2011; Van Dyck et al., 2010a). For example, results from the Swedish Neighborhood and Physical Activity study showed that participants living in highly walkable neighborhoods reported 50 minutes more of walking for transportation per week and had about 21 more minutes of moderate to vigorous physical activity per week (Sundquist et al., 2011). Only a few studies have, however, investigated the association between neighborhood walkability and other types of health-related outcomes and even fewer have used a longitudinal study design. Many of the studies on walkability and health-related outcomes have used body mass index (BMI) as an outcome (Frank et al., 2007; Gebel et al., 2011; Sallis et al., 2009; Van Dyck et al., 2010b), while others were based on mental and physical quality of life (Sallis et al., 2009). The results have often shown that more walkable environments may have a beneficial effect on certain health-related outcomes but not on others, i.e., living in walkable neighborhoods is associated with more physical activity and lower overweight/obesity rates but not with better quality of life.
The worldwide prevalence of type 1 and type 2 diabetes in the adult population is about 6.4% (285 million people) and it is estimated to increase to 7.7% (439 million adults) by 2030 (Shaw et al., 2010). Insufficient levels of physical activity are estimated to cause 27% of the type 2 diabetes burden worldwide (WHO, 2009). Despite the obviously beneficial effects of physical activity in the prevention of type 2 diabetes (Colberg et al., 2010), a majority of individuals at risk of developing type 2 diabetes are not sufficiently physically active (at least 150 minutes of moderate to vigorous physical activity per week) (Morrato et al., 2007). There is also evidence that walking specifically improves 24-h glycemic control in people at risk for type 2 diabetes (DiPietro et al., 2013).
Walkable neighborhoods have therefore been suggested as a potentially preventive factor for type 2 diabetes in the population (Pasala et al., 2010) and self-reported neighborhood resources for physical activity, such as perceived ease of walking and availability of exercise facilities, were shown to be negatively associated with type 2 diabetes in a study of 2,285 adults in the U.S. (Auchincloss et al., 2009). However, while the association between objectively measured neighborhood walkability and physical activity is relatively established, no previous study has investigated the potential association between objectively measured neighborhood walkability and the incidence of type 2 diabetes in the entire adult population of a large city. In addition, previous research has highlighted the importance of objectively assessing walkability; in a recent report that used both objective and subjective assessments of walkability, one-third of individuals in neighborhoods with high objective walkability misperceived it as low (Arvidsson et al., 2012b).
Neighborhood deprivation is an important variable to consider in studies of the potential association between neighborhood characteristics and type 2 diabetes as previous studies have documented associations between neighborhood deprivation and type 2 diabetes, physical activity and diet (Cubbin et al., 2006; Mezuk et al., 2013). Neighborhood walkability was a strong predictor of type 2 diabetes incidence independent of neighborhood deprivation in a large study from Canada. Coexisting poverty and recent immigrant status modified these effects (Booth et al., 2013). Finally, previous research has argued that there is a need to examine whether individual and neighborhood characteristics may modify the association between the built environment, i.e., neighborhood walkability, and health-related behaviors (Lovasi et al., 2009). This is because socioeconomically disadvantaged individuals may be less likely to respond positively to walkable neighborhoods due to lack of financial and other resources.
The first aim of this study was to investigate the association between objectively assessed neighborhood walkability and the incidence of type 2 diabetes, independently of neighborhood - and individual-level potential confounders, in a sample of 512,061 Swedish adults. The second aim was to investigate the potential interactions, i.e., moderating effects of individual socioeconomic characteristics and neighborhood deprivation on this hypothesized association. To gain further insight into the nature of the association between walkability and diabetes, we also used a co-sibling design that allowed us to assess the degree to which the possible association observed in the population might be causal or due to confounding from genetic and/or familial-environmental factors.
Methods
Our study used linked data from multiple Swedish nationwide registries and healthcare data. Linking was achieved via the unique individual 10-digit personal ID number assigned at birth or immigration to all Swedish residents. This ID number was replaced by a serial number, in order to preserve confidentiality. The following sources were used to create our dataset: the Total Population Register, containing annual individual-level sociodemographic data; the Multi-Generation Register, providing information on family relations; the Swedish Hospital Discharge Register, containing all hospitalizations for all Swedish residents; the Swedish Prescribed Drug Register, containing all prescriptions in Sweden picked up by patients; the Swedish Mortality Register, containing causes of death, and; the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA), containing annual information on socio-economic factors on all individuals from 16 years of age. The data were provided to us by Statistics Sweden (the Swedish Government-owned Statistics Bureau) and the National Board of Health and Welfare. All socioeconomic data used in the present study were obtained from national registers maintained by Statistics Sweden, who in turn receives data on the socioeconomic variables from different authorities. For example, the income data comes from the tax authorities and the education data comes from schools and universities. Self-report survey measures are only used for educational level in those immigrants who have not studied in Sweden (Statistics Sweden).
We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409).
Outcome variable
Clinically diagnosed type 2 diabetes was identified in the Swedish Prescribed Drug Register by ATC codes A10A (insulin and analogues to insulin), A10B (blood glucose lowering drugs, excluding insulins) and A10X (other drugs used in diabetes). Individuals were considered to have their first recorded event of type 2 diabetes in our analyses if they had collected a prescribed drug classified from the above ATC codes during the period January 1, 2007 –December 31, 2010. Insulin and antidiabetic agents are almost exclusively prescribed for individuals with a diagnosis of diabetes mellitus, which means that the use of ATC-codes will not include other types of diseases. Swedish doctors follow national guidelines in the diagnosis of type 2 diabetes (Läkemedelsverket), which are based on expert consensus from the WHO. Missing data in the Swedish Prescribed Drug Register varies between 0.02% and 0.6% according to the National Board of Health and Welfare (National Board of Health and Welfare). In addition, the National Diabetes Register in Sweden has used the Swedish Prescribed Drug Register to validate their own data and found that the correspondence between the two registers is high (Nationella Diabetesregistret).
Samples
The city of Stockholm is divided into 408 geographic areas, created for administrative purposes. The mean number of individuals living in these areas is 1,890 (range 1–8,790). The mean size of the areas is 0.53 square kilometers (range 0.01–3.81), which corresponds well with other studies’ definitions of neighborhoods used to estimate walkability indices (Forsyth, 2007).
From the population residing in the 408 geographical areas on December 31, 2005, we identified all individuals born 1988 or earlier (n=634,214). This age restriction ensures that the vast majority of identified incident cases are of type 2 diabetes (Thunander et al., 2008). We excluded individuals who had collected a prescribed drug classified from the above ATC codes from January 1, 2006 – December 31, 2006 in order to increase the probability that our analysis was restricted to incident cases; all drugs in Sweden are prescribed for a period of three months, which means this 12-month wash-out period covered four possible prescriptions. Remaining individuals were followed for the outcome variable described above from 1st Jan 2007 to 31st Dec 2010. We also excluded those individuals hospitalized for cardiovascular diseases (CVDs) during the years 2000–2006 in order to minimize confounding due to CVD. CVD was defined according to the following ICD10 codes: I10-I15, I20-I25, and I60-I69. Other exclusions were individuals who died (n = 28,336) or emigrated (n = 22,998) during the follow-up period. Individuals lacking information on residential location were also excluded (n=3,777). Furthermore, we excluded 67 geographic areas with less than 50 individuals due to unstable statistical estimates. In total, 1,869 eligible individuals were lost due to this restriction. In the final analyses, 512,061 individuals from 341 geographical areas were included. In a second step, by means of the Swedish Multi-Generation Register, we created a sibling dataset where we identified all full sibling pairs in the study database (159,238 pairs). The total number of individuals who did not live in the same neighborhood during the entire follow-up period was 189,898 and these individuals were included in the study. A sensitivity analysis that excluded these individuals showed almost identical results (data not shown in tables).
Neighborhood walkability
The walkability in each geographic area was assessed by calculating a walkability index (Arvidsson et al., 2012a; Sundquist et al., 2011) using Geographic Information Systems (GIS). The index was similar to walkability indices used in the U.S., Australia and Belgium (Owen et al., 2007; Sallis et al., 2009; Van Dyck et al., 2010a) and included three components: (1) residential density, (2) street connectivity, and (3) land use mix. Data on residential density were delivered to us by Statistics Sweden and calculated as the number of residential units per square kilometer (excluding water bodies). Street connectivity was based on data delivered by the City Planning Administration in Stockholm and was calculated as the number of intersections per square kilometer. Highways were not included in the calculations. Cycle paths and footpaths were included if they had an intersection with a street. The land use mix was calculated as the evenness in distribution between five categories of land use: (1) retail/service, (2) entertainment/physical activity, (3) institutional/healthcare, (4) office/workplace, and (5) dwellings. Categories 1 to 4 were based on data delivered by Teleadress, a company founded when the government-owned telecom sector was privatized. The Teleadress database is updated continuously and it includes businesses and services with a registered phone number, as well as those who actively have provided information about their business to the company. Inclusion in their database is free of charge. We performed a small field study to test the validity of the data from Teleadress. The results showed that almost all companies that are present in the Teleadress database have a business at the correct address. In addition, 75% of all businesses that we could find in the neighborhoods included in the small field study were present in the database. The fifth category in the land use mix was based on data obtained from the City Planning Administration in Stockholm. The correlation between the three different components (residential density, street connectivity, and land use mix) in the walkability index varied between 0.27–0.54.
The land use mix was calculated by the Herfindahl-Hirschman Index (HHI) (Rhoades, 1993). The HHI is calculated by summing the squared proportions of each land use category (HHI= p12 + p22 … + p52). A high HHI indicates a low level of land use mix.
The walkability index for each geographic area was calculated as the sum of the z-scores using a formula based on previous research (Frank et al., 2010; Sundquist et al., 2011):
The walkability index scores were then divided into deciles.
Neighborhood deprivation
We used a neighborhood deprivation index (NDI) for each of the geographical areas based on register data for all residents in the neighborhood aged 25–64, i.e., the working-age population, who is assumed to have a stronger socioeconomic impact on the neighborhood than others. The NDI was created as follows: a principal components analysis was used to select deprivation indicators for the entire Swedish population. The following four variables were selected for those aged 25–64: low educational status (<10 years of formal education); low income (income from all sources, including that from interest and dividends, defined as less than 50% of individual median income); unemployment (not employed, excluding full-time students, those completing compulsory military service, and early retirees); and social welfare recipient. Each of the four variables loaded on the first principal component with similar loadings (+0.47 to +0.53) and explained 52% of the variation between these variables. A z score was calculated for each SAMS neighborhood. The z scores, weighted by the coefficients for the eigenvectors, were then summed to create the index (ranging from −3 to 11). Higher scores reflect more deprived neighborhoods. This index has been used in previous research (Winkleby et al., 2007; Crump et al., 2011). Neighborhood-level deprivation and the individual-level socioeconomic variables appeared sufficiently uncorrelated (r = 0.18–0.31) to allow neighborhood effects to be disentangled from individual effects. In addition, the correlation between the continuous NDI and the continuous walkability score was low (−0.20).
Individual information
Our individual-level information was defined in 2006 and included year of birth (a continuous variable); gender (male and female); net annual household income divided into quartiles where one Swedish krona (SEK) equals to about 0.15 USD: high (>442,399 SEK), mid-high (260,200–442,399 SEK), mid-low (161,700-260,199 SEK), and low (<161,700 SEK)) and education, divided into low (9 years or less), middle (10–11 years) and high (12 years or more). Female gender, high income and high education were used as reference categories in the models. We selected individual-level covariates based on our previous research on neighborhood walkability and physical activity (Sundquist et al., 2011) as well as on other, similar studies from the U.S. (Sallis et al., 2009) and Australia (Owen et al., 2007).
Statistical methods
We investigated the association between neighborhood walkability and individual odds of type 2 diabetes. Odds ratios were considered to be a good approximation of relative risks because we had a large sample size, a relatively low incidence rate, risk ratios of moderate size, and a relatively short follow-up period (Davies et al., 1998). Firstly, we performed a multilevel logistic regression with individuals nested within their geographical area at baseline. This technique accounts for the hierarchical structure of the data. We created four consecutive models. In the first model (empty model) we only included the areas as random parameters in the model. In the second model (Model A) we also included the walkability variable. In the third model (Model B) we added neighborhood deprivation. In the final model (Model C) we also included the individuals’ characteristics. We also performed additional analyses where we investigated the association between the different components (residential density, street connectivity and land use mix) of walkability and type 2 diabetes. We followed the same steps for these components as we did for the walkability variable.
We present odds ratios and 95% confidence intervals (CI) for the fixed parameters. For the random part we use the latent variable method in order to convert the variance parameters into the intra class correlation (ICC). This method converts the individual level variance from the probability scale to the logistic scale. It assumes that the propensity for developing clinically identified type 2 diabetes is a continuous latent variable underlying our binary response. Each individual has a propensity to develop type 2 diabetes, but only individuals whose propensity exceeds a certain limit will develop the disease. The unobserved individual variable follows a logistic distribution with individual variance equal to 3.29 (Π2/3). An ICC close to 0% suggest that the areas are not important constructs for understanding the variation in type 2 diabetes, while a higher ICC suggest that the areas are more important for understanding the variation in the outcome. The statistical analyses were performed using MLwiN 2.23.
In the second part we aimed to compare the results from the multilevel logistic regression with the results from a co-sibling design. Using conditional logistic regression (CLR), we performed an analysis on all full sibling pairs that were discordant for type 2 diabetes status and decile of neighborhood walkability (4.056 pairs from 308 neighborhoods). In these models we controlled for the same individual and neighborhood factors as in the multilevel analysis described above. The co-sibling design allows contrasting of the type 2 diabetes odds of siblings living in different neighborhoods with different levels of walkability. This model is adjusted for the familial cluster and, therefore, it accounts for an array of unknown shared genetic and environmental factors. The statistical analyses were performed using SAS 9.3.
Interactions were tested between walkability and the individual-level socio-demographic factors and between walkability and neighborhood deprivation (both multiplicative and additive interactions).
Results
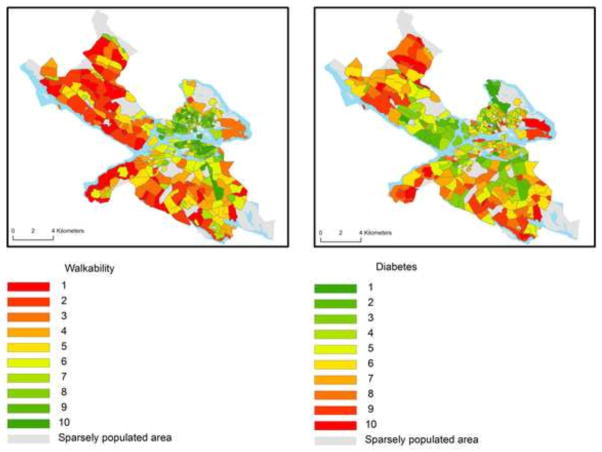
Table 1 shows the distribution of type 2 diabetes incidence by socio-demographic strata. A total of 6,613 of the individuals (1.3%) were identified as incident cases of type 2 diabetes between the years 2007–2010. The incidence proportions of type 2 diabetes were 1.5% in men and 1.1% in women. The mean age among individuals with and without type 2 diabetes was 55 (SD=14.9) and 44 (SD=16.9) years, respectively. The mean NDI was 0.59 (SD=2.11) among individuals with type 2 diabetes and −0.07 (SD=1.7) among individuals without type 2 diabetes. The geographical distribution of neighborhood walkability and the incidence of type 2 diabetes are shown in Figure 1. Neighborhood walkability seemed to be higher in the central areas of Stockholm whereas incidence of DM seemed to be higher in the outskirts of Stockholm.
Table 1.
Descriptive statistics of the 512,061 individuals living in the city of Stockholm in 2006 and included in the analyses. Numbers and percentages (within brackets).
| No diabetes mellitus | Diabetes mellitusa | |
|---|---|---|
| N | 505,448 (98.7) | 6,613 (1.3) |
| Walkability Indexb | 0.51 (2.62) | 0.16 (2.44) |
| Men | 240,325 (98.5) | 3,686 (1.5) |
| Women | 265,123 (98.9) | 2,927 (1.1) |
| Income | ||
| Low (0–161,699 SEKc) | 125,190 (98.5) | 1,922 (1.5) |
| Mid-Low (161,700–260,199 SEK) | 126,574 (98.6) | 1,750 (1.4) |
| Mid-High (260,200–442,399 SEK) | 126,604 (98.7) | 1,670 (1.3) |
| High (442,400–693,106,000 SEK) | 127,080 (99.0) | 1,271 (1.0) |
| Education | ||
| Low (<10 years) | 106,078 (97.9) | 2,288 (2.1) |
| Middle (10–11 years) | 79,520 (98.2) | 1,499 (1.9) |
| High (>11 years) | 319,850 (99.1) | 2,826 (0.9) |
| Age (years) | 44 (16.9)b | 55 (14.9)b |
| NDId | −0.07 (1.7)b | 0.59 (2.11)b |
Individuals that developed DM over the 2007–2010 follow-up period.
Mean (standard deviation)
One Swedish krona (SEK) equals about 0.15 USD
NDI=Neighborhood Deprivation Index
Figure 1.
The geographical distribution of neighborhood walkability and the incidence of diabetes mellitus in the city of Stockholm. Higher numbers represent higher neighborhood walkability and higher incidence of diabetes mellitus.
Table 2 shows the association between neighborhood walkability and odds of incident type 2 diabetes. In the crude model, individuals living in the lower deciles of walkability had significantly higher odds of type 2 diabetes compared to individuals living in the tenth decile, i.e., the decile with the highest walkability (model A). For example, individuals in the first decile of walkability had 30% higher odds (OR=1.30, CI=1.06–1.60) of incident type 2 diabetes compared to individuals in the tenth decile. Adjustment for neighborhood deprivation (model B) resulted in only minor changes of the odds and the overall p-value remained significant. However, further adjustment for age, gender, income and education (model C) resulted in substantial reduction of the estimates and none of the estimates remained statistically significant. The results from the co-sibling design (far right column in Table 2) showed no significant differences in the odds of type 2 diabetes between siblings living in neighborhoods that were discordant in walkability. No significant interactions were found between walkability and the individual-level socio-demographic factors or between walkability and neighborhood deprivation.
Table 2.
Results from the multilevel logistic regression analysis on the 512,061 individuals included in the study with diabetes mellitus as outcome and results from the conditional logistic regression on all sibling pairs. Numbers are odds ratios and 95 % CI.
| Model Aa | Model Ba | Model Ca,d | Sibling Analysis | |
|---|---|---|---|---|
| Walkability decile (walkability value) | ||||
| 1 (−3.44) (lowest) | 1.30 (1.06; 1.60) | 1.33 (1.13; 1.55) | 1.16 (1.00; 1.34) | 0.93 (0.67; 1.30) |
| 2 (−2.57) | 1.25 (0.98; 1.54) | 1.16 (1.00; 1.35) | 1.09 (0.96; 1.25) | 0.75 (0.56; 1.02) |
| 3 (−2.04) | 1.30 (1.02; 1.63) | 1.14 (0.98; 1.26) | 1.11 (0.96; 1.29) | 1.00 (0.74; 1.30) |
| 4 (−1.42) | 1.25 (0.98; 1.55) | 1.08 (0.92; 1.26) | 1.04 (0.90; 1.20) | 0.89 (0.65; 1.22) |
| 5 (−0.83) | 1.51 (1.24; 1.85) | 1.18 (1.03; 1.35) | 1.07 (0.94; 1.20) | 1.01 (0.76; 1.34) |
| 6 (−0.14) | 1.36 (1.09; 1.64) | 1.15 (1.00; 1.34) | 1.12 (0.98; 1.27) | 0.85 (0.64; 1.13) |
| 7 (0.78) | 1.32 (1.04; 1.63) | 1.14 (0.99; 1.33) | 1.08 (0.95; 1.22) | 1.39 (1.05; 1.83) |
| 8 (1.92) | 1.15 (0.93; 1.45) | 1.13 (0.97; 1.31) | 1.13 (0.99; 1.29) | 1.04 (0.79; 1.37) |
| 9 (3.27) | 0.97 (0.78; 1.21) | 1.01 (0.87; 1.16) | 0.97 (0.85; 1.11) | 0.82 (0.62; 1.08) |
| 10 (5.27) (highest) | Reference | Reference | Reference | Reference |
| p-valueb | 0.001 | 0.002 | 0.177 | |
| Neighborhood Deprivation Index | 1.18 (1.15; 1.20) | 1.21 (1.19; 1.23) | ||
| Men vs women | 1.63 (1.55; 1.72) | |||
| Low Income | 1.12 (1.04; 1.21) | |||
| Mid-Low Income | 1.11 (1.03; 1.20) | |||
| Mid-High Income | 1.09 (1.01; 1.17) | |||
| High Income | Reference | |||
| p-valuec | 0.077 | |||
| Low Education | 1.04 (0.97; 1.12) | |||
| Middle Education | 1.44 (1.36; 1.55) | |||
| High Education | Reference | |||
| Birth Year (cen) | 0.96 (0.96; 0.96) |
Model A includes neighborhood walkability, Model B includes + neighborhood deprivation, and Model C includes + neighborhood deprivation, gender, income, education and birth year.
P-values for trends of the overall association between neighborhood walkability and diabetes mellitus in Models A, B and C.
P-value for trend of the overall association between income and diabetes mellitus.
The DIC value for model C equals 66196.82 while a model that includes the random part, the individual characteristics and the neighborhood deprivation index have a DIC value of 66443.22.
The results of the random part of the multilevel logistic regression showed that the ICC was low in all models, i.e., only a small part of the total variation in type 2 diabetes was at the neighborhood level (Table 3).
Table 3.
Results from the random part of the multilevel logistic regression analysis of the 512,061 individuals included in the study with diabetes mellitus as outcome.
| Variance | ICCa | |
|---|---|---|
| Walkability | ||
| Empty Model | 0.147 (0.119; 0.184) | 4.3% |
| Model A | 0.133 (0.104; 0.169) | 3.9% |
| Model B | 0.044 (0.030; 0.064) | 1.3% |
| Model C | 0.016 (0.006; 0.028) | 0.5% |
| Residential density | ||
| Model A | 0.119 (0.093; 0.152) | 3.5% |
| Model B | 0.044 (0.028; 0.060) | 1.3% |
| Model C | 0.014 (0.006; 0.026) | 0.4% |
| Street connectivity | ||
| Model A | 0.141 (0.113; 0.178) | 4.1% |
| Model B | 0.049 (0.031; 0.067) | 1.5% |
| Model C | 0.018 (0.009; 0.029) | 0.5% |
| Land use mix | ||
| Model A | 0.133 (0.105; 0.169) | 3.9% |
| Model B | 0.041 (0.025; 0.057) | 1.2% |
| Model C | 0.013 (0.006; 0.025) | 0.4% |
Intraclass correlation
Model A includes neighborhood walkability, Model B includes + neighborhood deprivation, and Model C includes + neighborhood deprivation, gender, income, education and birth year.
The analyses of the associations between the three components of walkability (residential density, street connectivity and land use mix) and type 2 diabetes were similar to the associations found in the analyses based on the walkability index. That is, there were no significant associations between the separate walkability components and the incidence of type 2 diabetes after adjusting for age, gender, income, education and neighborhood deprivation (Supplementary Table 1).
Discussion
The aim of this study was to investigate the association between objectively assessed neighborhood walkability and the incidence of clinically diagnosed type 2 diabetes in a large sample of adults living in an urban area. The results showed that there was an inverse association between neighborhood walkability and incidence of type 2 diabetes, and this association remained significant after adjusting for neighborhood deprivation. However, the association was no longer statistically significant after accounting for individual sociodemographic factors. There were no moderating effects of individual characteristics and neighborhood deprivation on the association between neighborhood walkability and type 2 diabetes. The co-sibling analysis examining the incidence of type 2 diabetes among siblings living in neighborhoods with different levels of walkability showed no significant differences in the odds of type 2 diabetes between siblings living in neighborhoods that were discordant in walkability. This latter analysis had the additional strength of accounting for unmeasured genetic and family environmental factors that siblings share (e.g., family history of type 2 diabetes and related conditions, early-life dietary habits, participation in sports during childhood and adolescence) that we could not account for in the general population analysis. The values of the ICC calculations indicate that the proportion of the total variance at the neighborhood level is low. Our findings represent a novel contribution as it is, to the best of our knowledge, the first study investigating the association between objectively assessed neighborhood walkability and objectively assessed incidence of type 2 diabetes in the entire adult population in a large city, i.e., the capital of Sweden.
Recent reviews have found neighborhood walkability to be one of the most consistent environmental correlates of physical activity (Bauman et al., 2012; Van Holle et al., 2012), and a previous study of ours found higher levels of walking and moderate to vigorous physical activity among individuals living in high walkability neighborhoods compared to individuals living in low walkability neighborhoods (Sundquist et al., 2011). As physical activity may prevent type 2 diabetes (Colberg et al., 2010; WHO, 2009), we therefore hypothesized that there is a negative association between neighborhood walkability and type 2 diabetes. A study from the U.S. found a negative association between self-reported neighborhood resources for physical activity and incidence of type 2 diabetes after adjusting for individual-level socioeconomic factors, which was in line with our hypothesis. No information of neighborhood socioeconomic factors or neighborhood deprivation was, however, included in that analysis (Auchincloss et al., 2009). Although the present study is not directly comparable to that study, our results no longer remained significant after adjustment for individual-level socioeconomic factors. However, the results remained significant after adjusting for neighborhood deprivation.
Neighborhood deprivation has been associated with type 2 diabetes in previous research (Cox et al., 2007; Cubbin et al., 2006), possibly mediated by factors such as psychological stress from unsafe environments, littering and violent crime and also unhealthy lifestyles (Anderson et al., 1997; Cubbin et al., 2006). In previous studies on walkability and physical activity, the positive associations remained after adjusting for neighborhood socioeconomic status (Owen et al., 2007; Sallis et al., 2009; Sundquist et al., 2011; Van Dyck et al., 2010a). For example, the odds ratio for walking for transport among participants living in high compared to low walkability neighborhoods changed only slightly from 1.92 to 1.77 when neighborhood socioeconomic status and individual socio-demographic factors were included in the model (Sundquist et al., 2011).
Some studies have investigated the associations between neighborhood walkability and other health-related outcomes related to physical activity. For example, a study from the U.S found the odds of being overweight or obese to be 35% higher in participants living in low walkability neighborhoods compared to participants living in high walkability neighborhoods (Sallis et al., 2009). Gebel et al. found associations between low walkability parameters and increases in BMI over a four-year follow-up period in Australia (Gebel et al., 2011). Furthermore, self-reported land use mix was negatively associated with the metabolic syndrome in a study on 1,324 Australian adults. Those results remained significant after adjusting for neighborhood-level socioeconomic status (median household income) (Baldock et al., 2012). Most studies have, however, used rather simple indicators of neighborhood socioeconomic status (e.g., median income in the neighborhood), while the present study used an index based on several socio-economic indicators.
Walkable neighborhoods have been associated with lower levels of motor vehicle ownership and use per capita (Eriksson et al., 2012; Frank et al., 2006), which is health promoting. However, there may be environmental factors associated with neighborhood walkability that may be risk factors for type 2 diabetes, and these risk factors may diminish the potential positive influence of walkability on type 2 diabetes. For example, walkability has been associated with higher levels of traffic-related air pollution (Marshall et al., 2009) due to traffic congestions and a higher concentration of vehicle traffic. Long-term exposure to traffic-related air pollution may increase the risk of type 2 diabetes (Andersen et al., 2012; Kramer et al., 2010), and especially so in physically active individuals (Andersen et al., 2012). Physical activity may increase the penetration of traffic-related particles into the respiratory system, making physically active individuals more susceptible for traffic-related air pollution compared to inactive individuals (Oravisjarvi et al., 2011). Also, long-term exposure to traffic noise may be associated with increased risk of type 2 diabetes (Sorensen et al., 2013). Further understanding of potential risk factors associated with walkable environments and their effect on health would be useful when designing health-promoting neighborhoods.
Strengths of this study include the large sample size, i.e., more than half a million individuals. Using the Swedish personal ID number, replaced by a serial number, made it possible to link population-based health care register data with the geographic area in which the individuals lived. Both the explanatory variables (i.e., neighborhood walkability, neighborhood deprivation and individual socio-demographic factors) and the outcome (type 2 diabetes) were based on objective measures. The use of incident, rather than prevalent, cases of type 2 diabetes is an additional strength. The incidence of type 2 diabetes was assessed using nationwide, highly complete data on collection of anti-diabetic drugs from the Swedish Prescription Drug Register, and is thereby likely to include almost all new cases. More importantly, this register captures all individuals that are medically treated for type 2 diabetes as it is compulsory for all pharmacies in Sweden to report to the register. The geographical areas used in this study were rather small with homogenous types of buildings and their boundaries follow the road network, which makes it more likely that the geographical areas are representative of neighborhoods in social terms.
There are also some limitations in the present study. We had no clinical diagnoses on type of diabetes (type 1 or 2). However, we only included adult individuals aged 18 years or older at study start and after a 12-month washout period, which minimized the proportions of individuals with type 1 diabetes. Another limitation is that we could not detect patients with type 2 diabetes that were not treated with medication. However, few individuals with type 2 diabetes are treated with only lifestyle changes, such as diet, which means that few individuals with type 2 diabetes were missed in our study. Some cases may have been lost, i.e., those who did not collect their prescribed drug. Also, the walkability index in this study was based on only three neighborhood characteristics and an association between walkability and type 2 diabetes incidence might have been found had the index included characteristics of the built environment associated with a wider range of physical activities. Finally, we did not have access to information on many other potential environmental risk factors for type 2 diabetes (e.g., traffic-related noise, air pollution and unhealthy food outlets). Future studies could assess the predictive validity of a healthy food index in order to obtain a more complete picture of the built environment’s role in the development of type 2 diabetes.
Conclusions
The findings of the present study on 512,061 adults showed that there is an association between neighborhood walkability and incidence of type 2 diabetes, after adjusting for neighborhood deprivation. This association, however, no longer remained statistically significant after adjusting for individual socio-demographic factors. Future studies are encouraged to further explore risk factors potentially associated with walkable environments, such as traffic-related noise and air pollution, as well as the potential effects of a broader array of the neighborhood built environment on health outcomes related to physical activity, which may provide important knowledge on how to design neighborhoods that promote good health in several aspects.
Supplementary Material
Highlights.
Objective assessments of walkability and incidence of type 2 diabetes.
A total of 512,061 adults were included in the analyses.
The association between walkability and type 2 diabetes was negative in the crude models.
Adjustment for individual socio-demographic factors diminished this association.
Sibling analyses confirmed the results.
Acknowledgments
This study was supported by grants to Dr. Kristina Sundquist from the Swedish Research Council and ALF Region Skåne as well as the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL116381. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Mezuk is supported by NIH, grants K01-MH093641-01A1 and R21-DK8356430-01A1. The authors want to thank Klas Cederin for his work on Figure 1 in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristina Sundquist, Email: Kristina.Sundquist@med.lu.se.
Ulf Eriksson, Email: kosteruffe@hotmail.com.
Briana Mezuk, Email: bmezuk@vcu.edu.
Henrik Ohlsson, Email: Henrik.Ohlsson@med.lu.se.
References
- Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, Tjonneland A, Overvad K, Sorensen M. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35:92–98. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RT, Sorlie P, Backlund E, Johnson N, Kaplan GA. Mortality effects of community socioeconomic status. Epidemiology. 1997;8:42–47. doi: 10.1097/00001648-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Arvidsson D, Eriksson U, Lonn SL, Sundquist K. Neighborhood Walkability, Income, and Hour-by-Hour Physical Activity Patterns. Med Sci Sports Exerc. 2012a doi: 10.1249/MSS.0b013e31827a1d05. [DOI] [PubMed] [Google Scholar]
- Arvidsson D, Kawakami N, Ohlsson H, Sundquist K. Physical Activity and Concordance between Objective and Perceived Walkability. Med Sci Sports Exerc. 2012b;44:280–287. doi: 10.1249/MSS.0b013e31822a9289. [DOI] [PubMed] [Google Scholar]
- Auchincloss AH, Diez Roux AV, Mujahid MS, Shen M, Bertoni AG, Carnethon MR. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the Multi-Ethnic study of Atherosclerosis. Arch Intern Med. 2009;169:1698–1704. doi: 10.1001/archinternmed.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldock K, Paquet C, Howard N, Coffee N, Hugo G, Taylor A, Adams R, Daniel M. Associations between resident perceptions of the local residential environment and metabolic syndrome. J Environ Public Health. 2012;2012:589409. doi: 10.1155/2012/589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380:258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- Booth GL, Creatore MI, Moineddin R, Gozdyra P, Weyman JT, Matheson FI, Glazier RH. Unwalkable neighborhoods, poverty, and the risk of diabetes among recent immigrants to Canada compared with long-term residents. Diabetes Care. 2013;36:302–308. doi: 10.2337/dc12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg SR, Sigal RJ, Femhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M, Boyle PJ, Davey PG, Feng Z, Morris AD. Locality deprivation and Type 2 diabetes incidence: a local test of relative inequalities. Soc Sci Med. 2007;65:1953–1964. doi: 10.1016/j.socscimed.2007.05.043. [DOI] [PubMed] [Google Scholar]
- Crump C, Sundquist K, Sundquist J, Winkleby MA. Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Ann Epidemiol. 2011;21:231–237. doi: 10.1016/j.annepidem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubbin C, Sundquist K, Ahlen H, Johansson SE, Winkleby MA, Sundquist J. Neighborhood deprivation and cardiovascular disease risk factors: protective and harmful effects. Scand J Public Health. 2006;34:228–237. doi: 10.1080/14034940500327935. [DOI] [PubMed] [Google Scholar]
- Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;28:989–91. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro L, Gribok A, Stevens MS, Hamm LF, Rumpler W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care. 2013;36:3262–3268. doi: 10.2337/dc13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U, Arvidsson D, Gebel K, Ohlsson H, Sundquist K. Walkability parameters, active transportation and objective physical activity: moderating and mediating effects of motor vehicle ownership in a cross-sectional study. Int J Behav Nutr Phys Act. 2012;9:123. doi: 10.1186/1479-5868-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LD, Saelens BE, Powell KE, Chapman JE. Stepping towards causation: Do built environments or neighborhood and travel preferences explain physical activity, driving, and obesity? Soc Sci Med. 2007;65:1898–1914. doi: 10.1016/j.socscimed.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Forsyth A. Twin cities walking study, environment and physical activity: GIS protocols. University of Minnesota and Cornell; 2007. [Google Scholar]
- Frank LD, Sallis JF, Conway TL, Chapman JE, Saelens BE, Bachman W. Many pathways from land use to health - Associations between neighborhood walkability and active transportation, body mass index, and air quality. Journal of the American Planning Association. 2006;72:75–87. [Google Scholar]
- Frank LD, Sallis JF, Saelens BE, Leary L, Cain K, Conway TL, Hess PM. The development of a walkability index: application to the Neighborhood Quality of Life Study. Br J Sports Med. 2010;44:924–933. doi: 10.1136/bjsm.2009.058701. [DOI] [PubMed] [Google Scholar]
- Gebel K, Bauman AE, Sugiyama T, Owen N. Mismatch between perceived and objectively assessed neighborhood walkability attributes: prospective relationships with walking and weight gain. Health Place. 2011;17:519–524. doi: 10.1016/j.healthplace.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kramer U, Herder C, Sugiri D, Strassburger K, Schikowski T, Ranft U, Rathmann W. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118:1273–1279. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läkemedelsverket. Läkemedelsboken. 2014 In Swedish. Available from: http://www.xn--Ikemedelsboken-5hb.se/k1_end_diabetmellitus_2013fm10.html?search=&iso=false&imo=false&nplld=null&id=k1_15.
- Lovasi GS, Neckerman KM, Quinn JW, Weiss CC, Rundle A. Effect of individual or neighborhood disadvantage on the association between neighborhood walkability and body mass index. Am J Public Health. 2009;99:279–284. doi: 10.2105/AJPH.2008.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Brauer M, Frank LD. Healthy neighborhoods: walkability and air pollution. Environ Health Perspect. 2009;117:1752–1759. doi: 10.1289/ehp.0900595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Chaikiat Å, Li X, Sundquist J, Kendler KS, Sundquist K. Depression, neighborhood deprivation and risk of type 2 diabetes. Health Place. 2013;23:63–69. doi: 10.1016/j.healthplace.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- National Board of Health and Welfare. Läkemedelsregistret. In Swedish. Available from: http://www.socialstyrelsen.se/register/halsodataregister/lakemedelsregistret.
- Nationella Diabetesregistret. Årsrapport. 2013 års resultat. In Swedish. Available from: http://www.registercentrum.se/sites/default/files/dokument/nationella_diabetesregistret_arsrapport_2013.pdf.
- Oravisjarvi K, Pietikainen M, Ruuskanen J, Rautio A, Voutilainen A, Keiski RL. Effects of physical activity on the deposition of traffic-related particles into the human lungs in silico. Sci Total Environ. 2011;409:4511–4518. doi: 10.1016/j.scitotenv.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Owen N, Cerin E, Leslie E, duToit L, Coffee N, Frank LD, Bauman AE, Hugo G, Saelens BE, Sallis JF. Neighborhood walkability and the walking behavior of Australian adults. Am J Prev Med. 2007;33:387–395. doi: 10.1016/j.amepre.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Pasala SK, Rao AA, Sridhar GR. Built environment and diabetes. Int J Diabetes Dev Ctries. 2010;30:63–68. doi: 10.4103/0973-3930.62594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades SA. The Herfindahl-Hirschman Index. Fed Res Bull. 1993;79:188. [Google Scholar]
- Sallis JF, Saelens BE, Frank LD, Conway TL, Slymen DJ, Cain KL, Chapman JE, Kerr J. Neighborhood built environment and income: examining multiple health outcomes. Soc Sci Med. 2009;68:1285–1293. doi: 10.1016/j.socscimed.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Sorensen M, Andersen ZJ, Nordsborg RB, Becker T, Tjonneland A, Overvad K, Raaschou-Nielsen O. Long-term exposure to road traffic noise and incident diabetes: a cohort study. Environ Health Perspect. 2013;121:217–222. doi: 10.1289/ehp.1205503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Sweden. Registret över befolkningens utbildning. In Swedish. Available from: http://www.scb.se/sv_/Vara-tjanster/SCBs-data-for-forskning/SCBs-datalager/Registret-over-befolkningens-utbildning-/
- Sundquist K, Eriksson U, Kawakami N, Skog L, Ohlsson H, Arvidsson D. Neighborhood walkability, physical activity, and walking behavior: the Swedish Neighborhood and Physical Activity (SNAP) study. Soc Sci Med. 2011;72:1266–1273. doi: 10.1016/j.socscimed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Thunander M, Petersson C, Jonzon K, Fornander J, Ossiansson B, Torn C, Edvardsson S, Landin-Olsson M. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82:247–255. doi: 10.1016/j.diabres.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Van Dyck D, Cardon G, Deforche B, Sallis JF, Owen N, De Bourdeaudhuij I. Neighborhood SES and walkability are related to physical activity behavior in Belgian adults. Prev Med. 2010a;50(Suppl 1):S74–79. doi: 10.1016/j.ypmed.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Van Dyck D, Cerin E, Cardon G, Deforche B, Sallis JF, Owen N, de Bourdeaudhuij I. Physical activity as a mediator of the associations between neighborhood walkability and adiposity in Belgian adults. Health Place. 2010b;16:952–960. doi: 10.1016/j.healthplace.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Van Holle V, Deforche B, Van Cauwenberg J, Goubert L, Maes L, Van de Weghe N, De Bourdeaudhuij I. Relationship between the physical environment and different domains of physical activity in European adults: a systematic review. BMC Public Health. 2012;12:807. doi: 10.1186/1471-2458-12-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization; Geneva: 2009. [Google Scholar]
- Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. Am J Prev Med. 2007;32:97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.