Abstract
Alzheimer’s disease (AD) is a progressive brain disorder that initially affects medial temporal lobe circuitry and memory functions. Current drug treatments have only modest effects on the symptomatic course of the disease. In contrast, a growing body of evidence suggests that lifelong bilingualism may delay the onset of clinical AD symptoms by several years. The purpose of the present review is to summarize evidence for bilingualism as a reserve variable against AD and discuss potential underlying neurocognitive mechanisms. Evidence is reviewed suggesting that bilingualism may delay clinical AD symptoms by protecting frontostriatal and frontoparietal executive control circuitry rather than medial temporal lobe memory circuitry. Cellular and molecular mechanisms that may contribute to bilingual cognitive reserve effects are discussed, including those that may affect neuronal metabolic functions, dynamic neuronal-glial interactions, vascular factors, myelin structure and neurochemical signaling. Future studies that may test some of these potential mechanisms of bilingual CR effects are proposed.
Keywords: aging, Alzheimer’s, cognitive reserve, bilingualism, neural reserve
1. Introduction
The older adult population is growing rapidly. For example, in the U.S. the number of people aged 65 or older will climb to 72.1 million (one in every five Americans) by 2050 (http://www.aoa.gov/Aging_Statistics/). As our population continues to age, increasing numbers of individuals will be at risk for cognitive decline. Aging is the single greatest risk factor for dementia, with Alzheimer’s disease (AD) being the most common form. AD affects approximately 15% of individuals in the U.S. aged 65 years or older, with the prevalence reaching approximately 50% in people aged 85 years or older (http://www.alz.org). Beyond the obvious decrease in quality of life of affected individuals, caring for older adults with cognitive declines is likely to become a major healthcare issue, especially in current times of uncertainty about the future of health insurance programs such as Medicare. Increasing our understanding about how to promote the maintenance of cognitive health as people grow older has thus become a practical imperative [1].
2. Cognitive Reserve
Cognitive declines in older adults are likely due to multiple forms of age-related neurobiological declines, including atrophy of gray matter brain structures, disruption of white matter connections, reductions in vascular integrity and depletion of neurotransmitter systems [2, 3]. However, considerable heterogeneity exists in the relationship between cerebral declines and cognitive functioning in aging, with variability in task performance tending to increase with age [4]. While neurodegenerative changes result in significant cognitive declines in some older adults, others seem to continue to function like young healthy adults [5-7]. Similar heterogeneity between brain burden and cognitive function exists with respect to age-related dementias such as Alzheimer’s disease (AD). For example, while the majority of individuals who meet criteria for pathological AD also meet clinical AD criteria, a significant number continue to function within the normal range [8-10]. A striking early example of the gap between neuropathology and cognitive functioning came from the Kentucky Nun Study, which found that 32% of older adults with Braak Stage III and IV pathology (where Stage VI indicates the most severe pathology) had normal memory function before death [11].
The theory of cognitive reserve arose as an explanation for the gap between brain pathological burden and cognitive functioning [12]. Cognitive reserve (CR) theory holds that certain variables improve the brain’s ability to cope with damage, mitigating its effects on cognitive functioning [12, 13]. Putative CR variables include education, intelligence, socioeconomic status and aerobic fitness [14-17]. Uncovering other CR variables represents an important step toward maximizing the ability of older adults to live independently. In addition, this line of research has implications for early detection of dementia. Individuals with higher reserve require significantly greater brain declines than their peers before they manifest cognitive declines on standard neuropsychological tests. A more complete understanding of CR variables is required to develop more sensitive tests geared to detect cognitive decline in individuals with high CR.
3. Bilingualism as a Cognitive Reserve Variable
Lifelong bilingualism (hereafter referred to simply as bilingualism) refers to speaking two languages on a regular basis since childhood. Bilingualism has garnered much interest as a potential form of CR in part because it is appears to be a primarily environmental factor for which no special education or intelligence is needed. The initial evidence for bilingualism as a CR variable came from studies showing that lifelong bilinguals tend to develop clinical AD symptoms at an older age than monolinguals [18, 19]. In a seminal study, Bialystok et al. [18] found that bilinguals experienced clinical AD symptoms when they were an average of 4 years older than their monolingual peers. A similar delay to diagnosis of 4.3 years in bilinguals was reported in the Craik et al. [19] study. The older age of symptom onset associated with bilingualism in these studies could not be accounted for by gender, years of education or socioeconomic status.
Chertkow et al. (2010) found an overall delay in diagnosis of AD of approximately 3 years for patients who spoke more than two languages (multilinguals) compared to monolingual patients [20]. The effects were weaker when comparing only native-born Canadian monolinguals and bilinguals, raising the possibility that bilingualism may only confer CR through an interaction with immigration status. However, a recent large-scale study of 648 individuals conducted in India has provided strong evidence for bilingualism as a CR variable independent of immigration effects [21]. Results from this study indicated that native-born bilingual patients developed clinical symptoms consistent with diagnoses of AD or other dementia when they were on average of 4.5 years older than native-born monolingual patients. In addition, even when considering only illiterate individuals, bilingualism was associated with a 6 year delay of dementia symptoms. Finally, results from another recent large-scale study (N = 853) have provided evidence that the positive effects of bilingualism could not be explained by childhood intelligence or verbal fluency [22].
Future research will be required to determine if bilingual protective effects in delaying AD are on par with more well-established CR variables. However, preliminary results are encouraging. Results from the study by Bak et al. [22] indicated effect sizes associated with bilingualism in delaying cognitive declines that were comparable to those reported in the same participants for variation in the gene for apolipoprotein E, physical fitness, and (not) smoking [23]. Overall, bilingualism appears to have met a reasonable set of criteria that should be used to define a CR variable, including demonstration that its effects are independent of other CR variables. The available evidence suggests that speaking more than one language on a regular basis appears to contribute to a delay in the onset of clinical AD symptoms.
4. Effects of Bilingualism on Memory Systems in Aging
Bilingualism could delay the onset of clinical AD symptoms by directly protecting memory circuits affected in early-stage AD or by enhancing other neural systems. Regions of the medial temporal lobe (MTL) form a critical portion of the neural circuitry for declarative memory [24], and are affected in the earliest stages of AD [25, 26] and preclinical stages of amnestic mild cognitive impairment/AD [27, 28]. Other brain structures prominently affected in preclinical stages of AD include midline parietal and basal frontal regions [29, 30]. In addition, the integrity of white matter tracts that connect MTL structures with midline parietal and basal frontal regions such as the cingulum and the fornix are also damaged in early stages of AD [31-33].
There is currently little evidence to suggest that bilingualism protects MTL-based declarative memory systems. For example, bilingualism does not attenuate age-related declines in the ability to overcome memory interference [34]. Similarly, in the large-sample study by Bak et al. (2014), older adult bilinguals performed significantly better than monolinguals on several executive control measures (discussed below) but showed no differences on memory measures. At present there exist two neuroimaging studies with findings directly related to the relative structural integrity of MTL regions in older adult bilinguals and monolingual groups [35, 36].
Before summarizing results from these two studies it is important to describe their recruitment methods, which bear directly on their results. These two studies followed the original approach to the study of CR, in which sub-groups are rigorously matched on a large number of demographic variables and neuropsychological test scores to equate cognitive functioning as closely as possible [37]. As summarized above, CR theory predicts that individuals with higher reserve should require greater structural brain decline than those with lower reserve before they manifest cognitive declines associated with aging or dementia. When sub-groups are rigorously matched for cognitive functioning, the high CR sub-group is therefore predicted to show poorer brain structure, particularly in neural regions that are less relevant contributors to active CR mechanisms.
Results from the structural studies of Schweizer et al. [35] and Gold et al. [36] found that bilinguals maintained similar levels of cognitive functioning as their monolingual peers in the face of poorer structure within MTL-systems. The study by Schweizer et al. (2012) focused on groups of bilingual and monolingual older adults who were diagnosed with mild AD and showed similar levels of cognitive impairment. The two AD patient groups were compared on several estimates of brain volume derived from computed tomography (CT) scans. Results showed that bilinguals had a larger width of the temporal horn ratio, suggesting more atrophy of MTL structures in the bilingual AD patients compared to the monolingual AD patients. Bilingual CR effects were therefore not based upon neuroprotection of MTL-based memory circuits in this study because the bilingual group had more damage to MTL structures than monolinguals.
The study by Gold et al. [36] compared matched sub-groups of cognitively normal older adult bilingual and monolinguals on several measures of white matter (WM) integrity using diffusion tensor imaging. Bilinguals showed lower WM integrity several tracts, particularly in those with MTL-connections. For instance, the bilingual group showed lower WM in the fornix, a tract containing major cholinergic projections from the hippocampus to basal frontal structures. In addition, bilinguals showed lower WM integrity in portions of the inferior longitudinal fasciculus that contain connections between MTL structures and visual association cortex. These findings suggested that bilingual older adults could retain similar cognitive functioning as their monolingual peers despite significantly more damage to MTL memory circuits.
It is important to reiterate that neither the findings from the volumetric of Schweizer et al. [35] nor those from our DTI study [36] suggest that bilingualism causes MTL atrophy. Instead, findings from these studies converge on the view that bilinguals appear to be able to tolerate significant MTL damage without showing the expected cognitive impairments. These findings, along with the available behavioral evidence, suggest that the principal brain circuits underlying bilingual CR effects are likely to be located outside of classic MTL-based memory systems affected in the earliest stages of AD.
5. Effects of Bilingualism on Executive Control Systems in Aging
Executive control (EC) functions decline markedly with aging [38]. Tasks tapping EC functions such as inhibitory control and switching are subserved by frontostriatal and frontoparietal networks [39-41], which undergo significant age-related neurodegenerative changes. For example, aging is associated with marked atrophy in prefrontal cortex, the caudate, putamen [42-46]. Aging also affects the integrity of the WM connections within the frontostriatal and frontoparietal EC circuitries. For example, DTI studies have documented pronounced age-related declines in tracts connecting frontal and striatal structures such as the anterior limb of the internal capsule and tracts connecting frontal and parietal regions such as the superior longitudinal fasciculus [47].
Considerable evidence suggests that age-related declines in EC functions are less steep in older adult bilinguals compared to their monolingual peers [reviewed in 48]. Older adult bilinguals have been found to outperform their monolingual peers even on EC tasks using nonlinguistic perceptual stimuli, suggesting that lifelong bilingualism may strengthen generalpurpose executive control systems [49, 50]. For example, Bialystok et al. (2006) compared older adult monolingual and bilinguals using a modified antisaccade task. Older bilinguals showed proportionally smaller RT suppression costs compared to their monolingual peers, suggesting better maintained inhibitory control functions [51].
Several neuroimaging studies relevant to EC circuitry have now been conducted comparing older adult bilingual and monolingual groups [52, 53], [54] (Grady et al. under review). The first study to suggest that bilingualism attenuates age-related declines in EC systems was conducted by Luk et al. (2011), who compared older adult bilingual and monolingual groups in measures of DTI and resting-state functional connectivity. The bilingual group showed stronger resting-state connectivity between an inferior frontal region and multiple posterior structures in temporal, parietal and occipital cortices. In addition, bilinguals showed better structural WM integrity than the monolinguals in several tracts including the superior longitudinal fasciculus, which connects frontal and parietal portions of the EC network.
Further evidence of resting state functional connectivity differences between bilingual and monolingual older adult groups has recently been reported (Grady et al., under review). Results from this study indicated stronger resting-state connectivity between an inferior frontal region and the overall frontoparietal control (FPC) network in bilinguals than monolinguals. The FPC includes frontal structures such as the DLPFC and VLPFC and the posterior parietal lobes. It is thought to act as a switch to flexibly control the specific EC processes needed to meet task demands [54]. In contrast, the bilingual group did not show higher functional connectivity at rest between MTL or lingual gyrus and their respective mean overall networks. These findings are consistent with a view that bilingualism may enhance EC networks rather than memory networks.
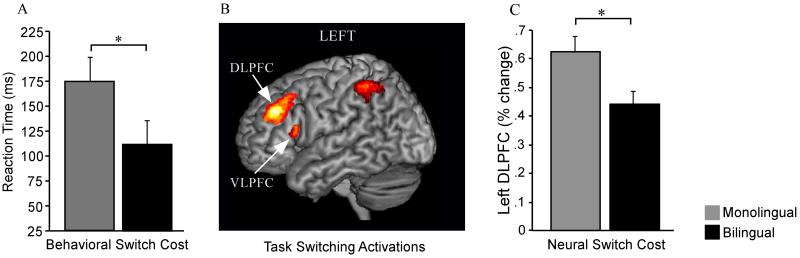
There is also evidence suggesting different strength of frontal cortex activation between older adult bilinguals and monolinguals during EC task performance [53]. In this fMRI study from my lab, the older adult bilingual group was faster than their monolingual peers at switching between tasks. Interestingly, the older bilinguals outperformed their monolingual peers in the context of lower BOLD response in several task-relevant frontal cortex regions (left DLPFC, left VLPFC and ACC) (Figure 1). The pattern of faster performance and lower frontal activation in the older bilingual group suggested that bilingualism may help maintain functional efficiency in frontal regions in aging.
Figure 1.
Behavioral and fMRI task switching differences between older adult bilinguals and monolinguals. Older adult bilinguals showed smaller behavioral switch costs than their monolingual peers (A). Task switching (switch – nonswitch contrast) was associated with the activation of frontostriatal and frontoparietal regions, including left frontal activations seen here in the DLPFC and VLPFC (B). Older adult bilinguals showed lower task switching fMRI activation (i.e. lower neural switch costs) in several frontal regions, including the left DLPFC (C). Adapted from Gold et al. [51] with permission. *P < 0.05.
Finally, there is recent evidence suggesting that bilingualism may enhance and/or protect the macrostructural integrity parietal cortices[55]. This study found greater gray matter volume in the inferior parietal lobules bilaterally in older adult Chinese bilinguals in Hong Kong than their monolingual peers. Interestingly, more prominent volumetric differences were observed in speakers of languages that are linguistically more similar (Cantonese-Mandarin) than linguistically distinct (Cantonese-English). This finding suggested that beneficial effects of bilingualism on the aging brain may increase as inhibitory control demands increase (i.e. from having to suppress highly competing linguistic codes not under active use). [55].
6. Protecting EC Pathways through Experience
The combined evidence from behavioral and neuroimaging studies suggest that bilingualism may offset AD by strengthening EC pathways. In considering how the experience of bilingualism may strengthen EC circuits, it is important to note that bilingual control of two languages involves frontostriatal and frontoparietal structures sharing close correspondence with structures involved in EC processes more generally such as the DLPFC, VLPFC, insula, ACC, basal ganglia, thalamus and posterior parietal cortex [56]. The continuous need for bilinguals to determine when language switches are appropriate and inhibit the language not currently under use may serve to implicitly strengthen the functioning of EC neurocognitive circuits in bilinguals [48, 56, 57].
The precise neural mechanisms underlying CR variables such bilingualism are unknown due to the coarseness of most available neuroimaging methods. However, the animal literature is replete with findings relevant to mechanistic underpinnings of environmental enrichment that likely to have major parallels with neural substrates of human CR. Early enrichment studies showed that housing conditions which enhance sensory, motor, cognitive or social stimulation have beneficial effects on brain structure and function [58, 59]. The relevance of environmental enrichment studies to human CR variables (such as bilingualism) became more apparent with subsequent studies showing that animals placed in enriched environments developed greater ability to adapt to highly conflicting or demanding situations [60-62]. Another pattern to emerge from the enrichment literature of relevance to understanding the brain bases of human CR studies is that specific forms of enrichment (e.g., motor or spatial navigation) tend to produce beneficial effects within specific brain systems underlying those functions [63].
Consideration of the frontostriatal and frontoparietal neural pathways relevant to bilingualism suggests clues about cellular mechanisms that may underlie its CR effects. Many mechanisms of environmental enrichment have been documented and may play a role in human CR including neurogenesis, angiogenesis and synaptogenesis [60, 64, 65]. However, in adult mammals, neurogenesis is thought to be mainly restricted to the hippocampal dentate gyrus [66]. Neurogenesis is thus unlikely to represent an active, ongoing CR mechanism in older adult bilinguals. However, neurogenesis could be relevant as a developmental mechanism that may contribute to brain reserve in later life by endowing older adult bilinguals with an increased number of neurons/synapses in relevant circuits.
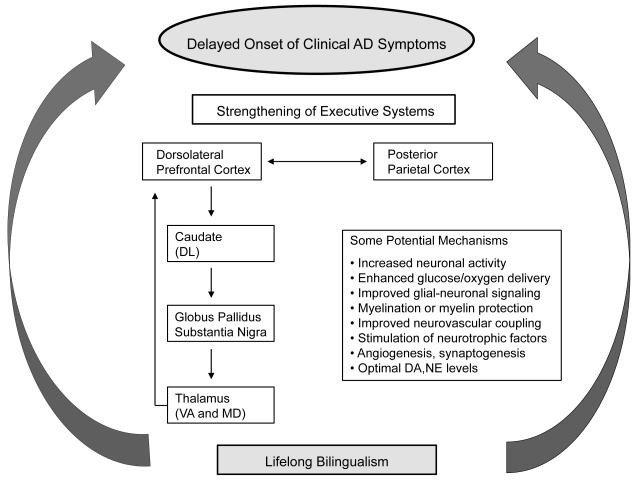
My working hypothesis is that increased activity within frontoparietal and frontostriatal networks associated with the bilingual experience may protect against age-related declines in cellular and synaptic functions within these EC circuits (Figure 2). In the schematic, the DLPFC represents a key hub in both frontoparietal and frontostriatal EC networks. As part of a fronto-striatal-thalamic pathway, the DLPFC has connection to the dorsolateral portion of the caudate nucleus [67, 68]. Neurons in the dorsolateral portion of the caudate nucleus project to the globus pallidus and substantia nigra as part of a direct pathway. Basal ganglia neurons project to the ventral anterior and mediodorsal nuclei of the thalamus via the thalamic fasiculus [68]. The mediodorsal nucleus of the thalamus projects back to neurons within the DLPFC via the anterior limb of the internal capsule [69] completing this so called ‘cognitive portion’ of the fronto-striatal-thalamic circuit.
Figure 2.
A schematic representation of potential bilingual CR mechanisms. Bilingualism may delay the onset of clinical AD symptoms through positive effects on the functioning of frontostriatal and frontoparietal brain systems involved in executive control functions. The DLPFC represents a key hub in both frontoparietal and frontostriatal networks relevant to executive functions. DA, dopamine; NE, norepinephrine. See text for discussion of potential mechanisms that may contribute to bilingual CR effects.
It is hypothesized that increased neuronal activity within EC circuits, and related increases in delivery of oxygen and glucose, may result in a synergistic cascade of beneficial effects in the bilingual brain. One such potential mechanism could relate to strengthening of dynamic neuronal-glial interactions. For example, neuronal activity appears to promote myelination by oligodendroglia [70, 71]. Interestingly, increased myelination associated with neuronal activity may in turn benefit neuronal metabolism given that myelin provides neurons with lactate, an energy-generating metabolite of glucose [72, 73]. Increased neuronal metabolism could also promote angiogenesis, which would appear to be a relevant mechanistic contributor to bilingual CR effects in that age-related small vessel disease appears to predominantly disrupt frontostriatal circuits [74] that appear to be protected by bilingualism.
Strengthening of neuronal-glial interactions would also be expected to benefit neurovascular coupling, which could contribute to some of the fMRI activation differences observed between older adult bilinguals and their peers (summarized above). Cortical astrocytes form an anatomical link between gluatamergic synapses and capillaries via their specialized processes[75]. During neural activity, glutamate activates astrocytic receptors, initiating processes leading to local vasodilation and increased blood flow to active brain regions[75]. In addition to their importance in neuro-vascular coupling, astrocytes may contribute to the BOLD response itself, as they actively respond to visual stimuli with increases in calcium concentration and have many of the receptive-field characteristics of neurons[76].
A contribution of synaptic mechanisms to bilingual CR effects also seems likely given that the BOLD signal is more closely related to local field potentials generated by subthreshold synaptic activity than neuronal spike rate[77]. Beneficial effects of CR variables at the synaptic level could relate to protection of terminal arbours, presynaptic boutons or the density/affinity of neurotransmitter receptors within these circuits [63]. Synaptic plasticity has been welldocumented within fronto-striatal-thalamic circuitry relevant to EC and bilingualism. The link between enrichment and alterations in neuronal branching and synaptogenesis has been most extensively demonstrated in cortex [78, 79]. However, there is evidence of experience-dependent synaptic plasticity in the striatum involving medium spiny neurons [80-82]. In addition, there is evidence for experience dependent synaptogenesis in the thalamus based on markers of excitatory postsynaptic spines [83].
Experience-dependent alterations in receptor affinity may in part operate through modulation of brain-derived neurotrophic factor (BDNF), which is altered by environmental enrichment [84, 85] and promotes synaptic plasticity [86, 87]. Another nerve growth factor that may be modulated by the bilingual experience is glial-derived neurotrophic factor (GDNF), which has been found to promote survival of dopaminergic neurons within the frontostriatal circuitry [88]. Finally, maintenance of optimal synaptic activity in aging may also be in part based on modulation in signaling between astrocytes and neurons in-and-around the synaptic cleft, which is altered with environmental enrichment [89].
Ultimately, protection of synapses associated with CR would be expected to offset age-related declines in neurochemical signaling within relevant brain circuits. Multiple neurotransmitter systems are likely to be implicated in CR and three in particular may be especially relevant to bilingual CR effects within frontostriatal and frontoparietal circuits. Maintenance of dopamine (DA) signaling is likely to play a significant role in bilingual CR effects given its ubiquity in frontostriatal systems and established role of DA projections from the midbrain in modulating frontally-based EC functions [39]. For example, positron emission tomography (PET) imaging in humans has revealed a correlation between performance on EC tasks and both DA receptor availability [90] and dynamic DA release [91]. Other human studies suggest that there may be optimal dopamine levels for EC functions such as attentional capacity [92] and inhibitory control [93].
Bilingualism may also have protective effects on glutamatergic synapses within fronto-striatal-thalamic systems given that glutamate is the main neurotransmitter for thalamo-cortical signaling [94]. Neuroanatomical and neuromodulatory studies have shown that the thalamus plays a critical role in integrating communication between the basal ganglia and cortex [95, 96]. A third neurochemical system that may be modulated by the bilingual experience is norepinephrine (NE), which is part of the brain’s general arousal/alertness system. NE originates from cells in the locus ceruleus of the pons, and contains widespread neocortical projections, but with particularly dense projections to parietal structures involved in attention processes [97]. In addition, pharmacological studies have shown that NE reuptake inhibitors improve some EC functions affected positively by the bilingual experience such as sustained attention and inhibitory control [98, 99].
7. Conclusions
In conclusion, bilingualism appears to delay the onset of clinical symptoms associated with AD. Neuroimaging studies are in their infancy but suggest that bilingual cognitive reserve effects appear to operate through protection of executive control circuits rather than direct protection of memory circuits initially affected in AD. The cellular and molecular mechanisms underlying functional neuroimaging advantages in older adult bilinguals remain to be elucidated. It is hypothesized that the continued use of EC circuits may protect a range of neuronal, glial and synaptic functions within frontostriatal and frontoparietal networks.
Future biomarker studies comparing older adult monolingual and bilingual groups should help elucidate some of the potential mechanisms underlying bilingual CR effects discussed in this review. For example, older adult monolingual and bilingual groups could be compared for levels of specific neurotrophins found in saliva or blood. PET radioligand studies may also contribute to an increased understanding of bilingual CR mechanisms. For instance, it would be relevant to determine if bilingualism modulates the relationship between amyloid deposition and neuropsychological performance. It may be possible to perform this kind of analysis based upon on previously collected data, as multicenter studies such as ADNI have undoubtedly scanned lifelong bilingual participants. Finally, it would be informative to determine if older adult monolingual and bilingual groups differ in DA binding or dynamic DA release in fronto-striatal-thalamic circuits.
Highlights.
Bilingualism may delay the onset of Alzheimer’s disease (AD) symptoms
Evidence of bilingualism as a cognitive reserve variable against AD is reviewed
Bilingualism may protect or enhance the brain’s executive control systems
Potential mechanisms underlying bilingual reserve effects are proposed
Acknowledgements
I thank Dr. Fergus Craik for helpful comments on a previous version of this manuscript. I also gratefully acknowledge my valued colleagues at the Sanders-Brown Center on Aging and the Magnetic Resonance Imaging and Spectroscopy Center for their contributions to several studies described in this review. This work was supported in part by a grant from the National Institutes of Health Institute of Aging (R01-AG033036). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- [2].Raz N, Kennedy KM. A systems approach to age-related change: Neuroanatomical changes, their modifiers, and cognitive correlates. In: Jagust W, Desposito M, editors. Imaging the Aging Brain. Oxford University Press; New York, NY: 2009. pp. 43–70. [Google Scholar]
- [3].Kemper TL. Neuroanatomical and neuropathological changes during aging and in dementia. In: Albert ML, Knoepfel EJE, editors. Clinical Neurology of Aging. Oxford University Press; New York: 1994. pp. 3–67. [Google Scholar]
- [4].Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychol Aging. 1999;14:365–79. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- [5].Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J Cogn Neurosci. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- [6].Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brainactivity in high-performing older adults. Neuroimage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- [7].Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: gauging the evidence for a common cause. Psychol Aging. 2009;24:1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- [8].Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441–54. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- [9].Mortimer JA. Brain reserve and the clinical expression of Alzheimer’s disease. Geriatrics. 1997;52(Suppl 2):S50–3. [PubMed] [Google Scholar]
- [10].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–77. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- [12].Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- [13].Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10:578–89. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- [15].Christensen H. What cognitive changes can be expected with normal ageing? The Australian and New Zealand journal of psychiatry. 2001;35:768–75. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- [16].Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2012;1822:467–73. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- [18].Bialystok E, Craik FI, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45:459–64. doi: 10.1016/j.neuropsychologia.2006.10.009. [DOI] [PubMed] [Google Scholar]
- [19].Craik FI, Bialystok E, Freedman M. Delaying the onset of Alzheimer disease: bilingualism as a form of cognitive reserve. Neurology. 2010;75:1726–9. doi: 10.1212/WNL.0b013e3181fc2a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chertkow H, Whitehead V, Phillips N, Wolfson C, Atherton J, Bergman H. Multilingualism (but not always bilingualism) delays the onset of Alzheimer disease: evidence from a bilingual community. Alzheimer Dis Assoc Disord. 2010;24:118–25. doi: 10.1097/WAD.0b013e3181ca1221. [DOI] [PubMed] [Google Scholar]
- [21].Alladi S, Bak TH, Duggirala V, Surampudi B, Shailaja M, Shukla AK, et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology. 2013;81:1938–44. doi: 10.1212/01.wnl.0000436620.33155.a4. [DOI] [PubMed] [Google Scholar]
- [22].Bak TH, Nissan JJ, Allerhand MM, Deary IJ. Does bilingualism influence cognitive aging? Ann Neurol. 2014;75:959–63. doi: 10.1002/ana.24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort profile: the Lothian Birth Cohorts of 1921 and 1936. International journal of epidemiology. 2012;41:1576–84. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- [24].Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- [25].Jack CR, Jr., Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Convit A, De Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, et al. Specific hippocampal volume reductions in individuals at risk for Alzheimer’s disease. Neurobiol Aging. 1997;18:131–8. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- [27].Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging. 2010;31:1099–106. doi: 10.1016/j.neurobiolaging.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dickerson BC, Wolk DA. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta. 2012;1822:370–9. doi: 10.1016/j.bbadis.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- [33].Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer’s disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822:416–22. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fernandes MA, Craik F, Bialystok E, Kreuger S. Effects of bilingualism, aging, and semantic relatedness on memory under divided attention. Can J Exp Psychol. 2007;61:128–41. doi: 10.1037/cjep2007014. [DOI] [PubMed] [Google Scholar]
- [35].Schweizer TA, Ware J, Fischer CE, Craik FI, Bialystok E. Bilingualism as a contributor to cognitive reserve: evidence from brain atrophy in Alzheimer’s disease. Cortex. 2012;48:991–6. doi: 10.1016/j.cortex.2011.04.009. [DOI] [PubMed] [Google Scholar]
- [36].Gold BT, Johnson NF, Powell DK. Lifelong bilingualism contributes to cognitive reserve against white matter integrity declines in aging. Neuropsychologia. 2013;51:2841–6. doi: 10.1016/j.neuropsychologia.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32:371–5. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- [38].Schaie KW. Intellectual Development in Adulthood: The Seattle Longitudinal Study. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- [39].Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp. 2012;33:130–42. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- [42].Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–73. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- [43].Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–87. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- [44].Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- [46].Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- [47].Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bialystok E, Craik FI, Luk G. Bilingualism: consequences for mind and brain. Trends Cogn Sci. 2012;16:240–50. doi: 10.1016/j.tics.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Costa A, Hernandez M, Sebastian-Galles N. Bilingualism aids conflict resolution: evidence from the ANT task. Cognition. 2008;106:59–86. doi: 10.1016/j.cognition.2006.12.013. [DOI] [PubMed] [Google Scholar]
- [50].Bialystok E, Craik FIM. Cognitive and linguisitc processing in the bilingual mind. Current Directions in Psychological Science. 2010;19:19–23. [Google Scholar]
- [51].Bialystok E, Craik FI, Ryan J. Executive control in a modified antisaccade task: Effects of aging and bilingualism. J Exp Psychol Learn Mem Cogn. 2006;32:1341–54. doi: 10.1037/0278-7393.32.6.1341. [DOI] [PubMed] [Google Scholar]
- [52].Luk G, Bialystok E, Craik FI, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31:16808–13. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J Neurosci. 2013;33:387–96. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Abutalebi J, Canini M, Della Rosa PA, Sheung LP, Green DW, Weekes BS. The neuroprotective effects of bilingualism upon the inferior parietal lobule: A Structural Neuroimaging Study in Aging Chinese Bilinguals. Journal of Neurolinguistics. 2014 [Google Scholar]
- [56].Green DW, Abutalebi J. Language control in bilinguals: The adaptive control hypothesis. J Cogn Psychol (Hove) 2013;25:515–30. doi: 10.1080/20445911.2013.796377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Green DW. Mental control of the bilingual lexico-semantic system. Bilingualism: Language and Cognition. 1998;1:67–81. [Google Scholar]
- [58].Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, et al. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J Comp Neurol. 1966;128:117–26. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- [59].Krech D, Rosenzweig MR, Bennett EL. Effects of environmental complexity and training on brain chemistry. Journal of comparative and physiological psychology. 1960;53:509–19. doi: 10.1037/h0045402. [DOI] [PubMed] [Google Scholar]
- [60].Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002) Progress in Neurobiology. 2004;72:167–82. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [61].Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, et al. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [62].Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–44. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- [63].Petrosini L, De Bartolo P, Foti F, Gelfo F, Cutuli D, Leggio MG, et al. On whether the environmental enrichment may provide cognitive and brain reserves. Brain research reviews. 2009;61:221–39. doi: 10.1016/j.brainresrev.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [64].Ekstrand J, Hellsten J, Tingstrom A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci Lett. 2008;442:203–7. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- [65].Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- [66].Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- [68].Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- [69].Preuss TM, Goldman-Rakic PS. Crossed corticothalamic and thalamocortical connections of macaque prefrontal cortex. J Comp Neurol. 1987;257:269–81. doi: 10.1002/cne.902570211. [DOI] [PubMed] [Google Scholar]
- [70].Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–66. [PubMed] [Google Scholar]
- [72].Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31:538–48. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–8. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schmidtke K, Hull M. Cerebral small vessel disease: how does it progress? J Neurol Sci. 2005;229-230:13–20. doi: 10.1016/j.jns.2004.11.048. [DOI] [PubMed] [Google Scholar]
- [75].Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–7. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- [76].Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–43. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- [77].Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat Rev Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- [78].Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–8. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- [79].Gelfo F, De Bartolo P, Giovine A, Petrosini L, Leggio MG. Layer and regional effects of environmental enrichment on the pyramidal neuron morphology of the rat. Neurobiol Learn Mem. 2009;91:353–65. doi: 10.1016/j.nlm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [80].Comery TA, Shah R, Greenough WT. Differential rearing alters spine density on mediumsized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiol Learn Mem. 1995;63:217–9. doi: 10.1006/nlme.1995.1025. [DOI] [PubMed] [Google Scholar]
- [81].Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain research Developmental brain research. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- [82].Spires TL, Grote HE, Garry S, Cordery PM, Van Dellen A, Blakemore C, et al. Dendritic spine pathology and deficits in experience-dependent dendritic plasticity in R6/1 Huntington’s disease transgenic mice. Eur J Neurosci. 2004;19:2799–807. doi: 10.1111/j.0953-816X.2004.03374.x. [DOI] [PubMed] [Google Scholar]
- [83].Nithianantharajah J, Levis H, Murphy M. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol Learn Mem. 2004;81:200–10. doi: 10.1016/j.nlm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- [84].Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- [85].Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–6. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–9. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- [87].Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–62. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- [88].Brooks DJ, Piccini P. Imaging in Parkinson’s disease: the role of monoamines in behavior. Biol Psychiatry. 2006;59:908–18. doi: 10.1016/j.biopsych.2005.12.017. [DOI] [PubMed] [Google Scholar]
- [89].Nilsson M, Pekny M. Enriched environment and astrocytes in central nervous system regeneration. Journal of rehabilitation medicine. 2007;39:345–52. doi: 10.2340/16501977-0084. [DOI] [PubMed] [Google Scholar]
- [90].Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–9. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- [91].Badgaiyan RD, Wack D. Evidence of dopaminergic processing of executive inhibition. PLoS One. 2011;6:e28075. doi: 10.1371/journal.pone.0028075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Finke K, Dodds CM, Bublak P, Regenthal R, Baumann F, Manly T, et al. Effects of modafinil and methylphenidate on visual attention capacity: a TVA-based study. Psychopharmacology (Berl) 2010;210:317–29. doi: 10.1007/s00213-010-1823-x. [DOI] [PubMed] [Google Scholar]
- [93].Nandam LS, Hester R, Wagner J, Cummins TD, Garner K, Dean AJ, et al. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry. 2011;69:902–4. doi: 10.1016/j.biopsych.2010.11.014. [DOI] [PubMed] [Google Scholar]
- [94].Sherman SM. The function of metabotropic glutamate receptors in thalamus and cortex. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2014;20:136–49. doi: 10.1177/1073858413478490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2001;7:315–24. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- [96].Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–75. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- [97].Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol. 1986;243:117–38. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- [98].Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–84. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [99].Grefkes C, Wang LE, Eickhoff SB, Fink GR. Noradrenergic modulation of cortical networks engaged in visuomotor processing. Cereb Cortex. 2010;20:783–97. doi: 10.1093/cercor/bhp144. [DOI] [PubMed] [Google Scholar]