Abstract
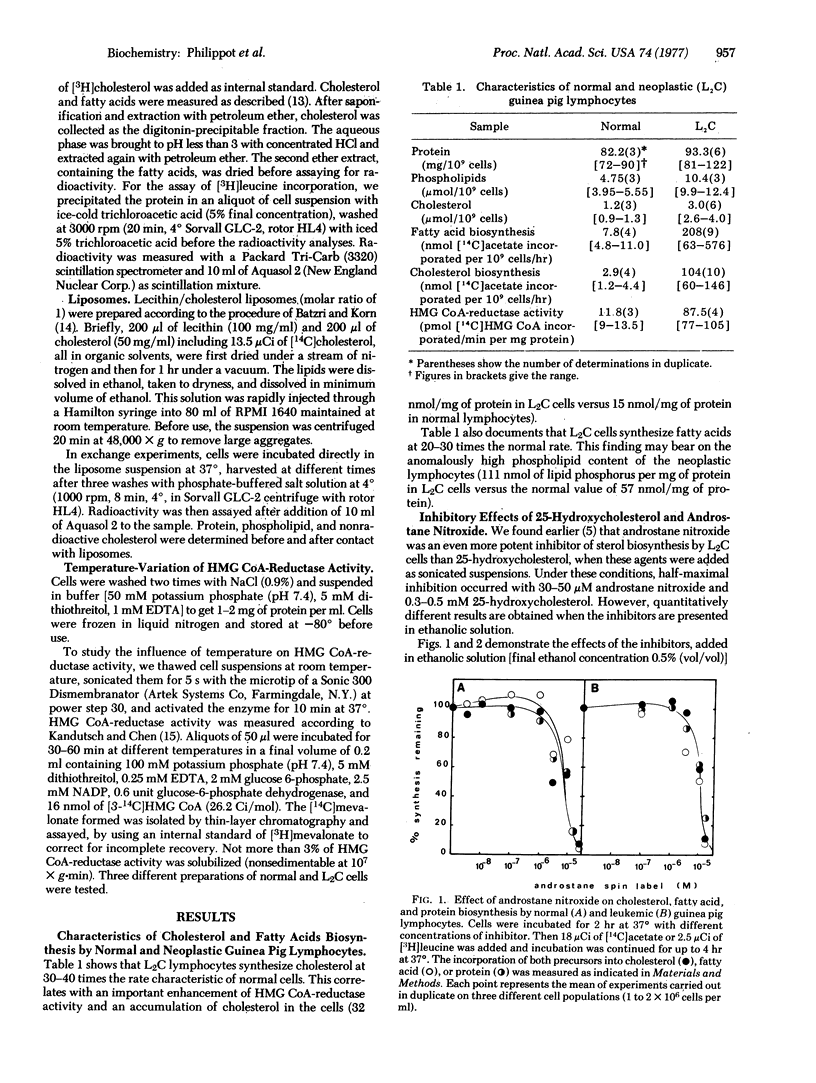
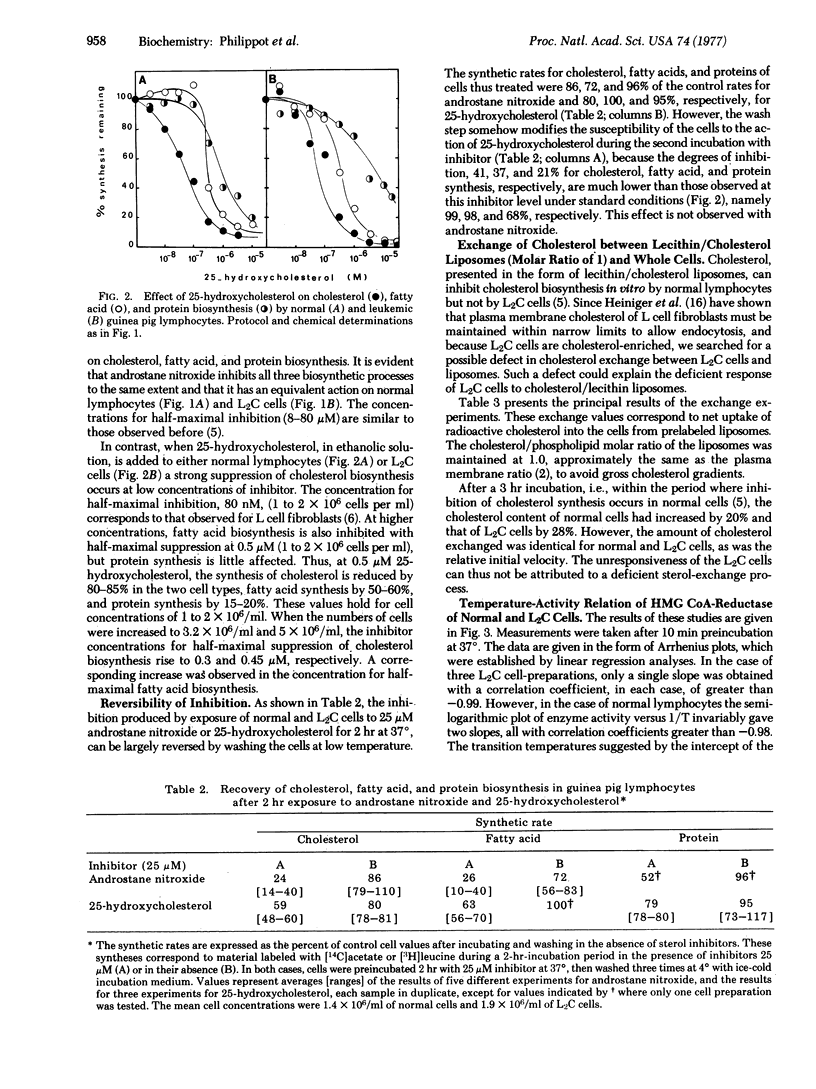
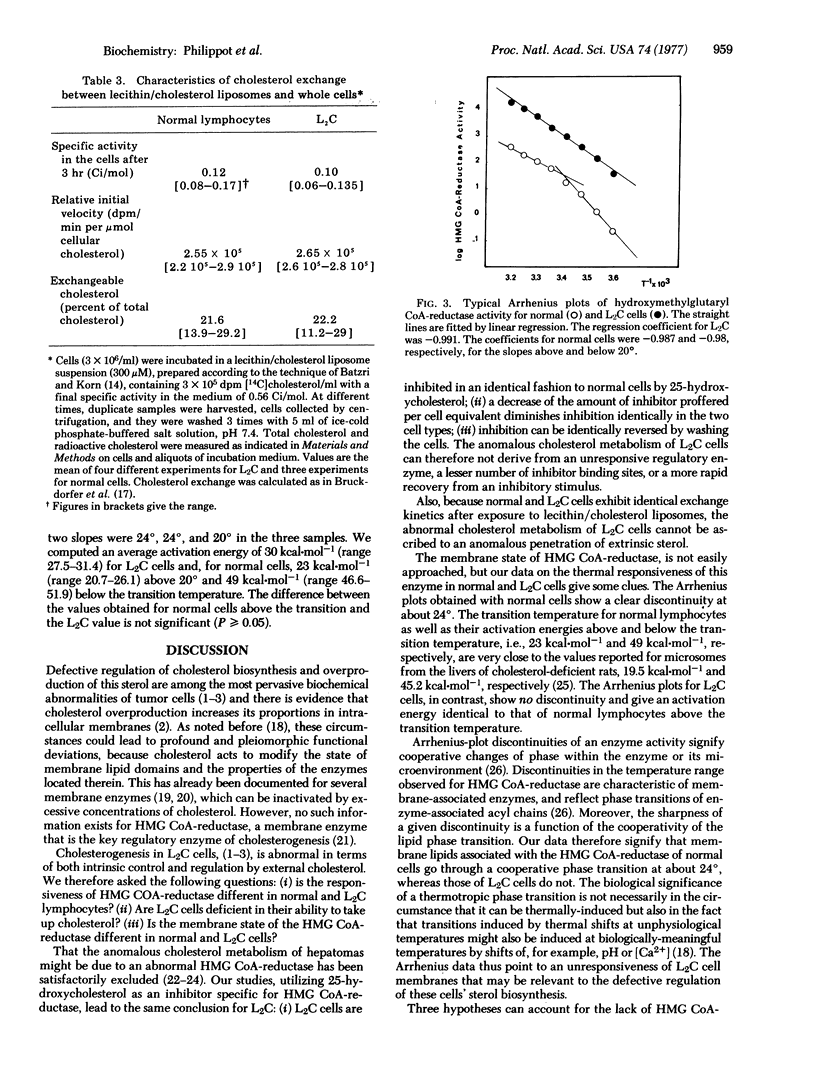
The cholesterol production of guinea pig leukemic (L2C) lymphocytes preceeds at greater than 30 times the rate found in normal cells. Fatty acid biosynthesis is also enhanced in L2C cells. Exposure of L2C cells to cholesterol/lecithin liposomes does not depress their sterol biosynthesis, in contrast to the behavior of normal lymphocytes [Philippot, J.R., Cooper, A.G. & Wallach, D. F. H. (1975) Biochim. Biophys. Acta 406, 161-166]. However, 25-hydroxycholesterol, an inhibitor of hydroxymethylglutaryl-CoA reductase (NADPH) [mevalonate: NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34], the rate limiting enzyme in cholesterogenesis, and 25-hydroxycholecalciferol, a biologically potent form of vitamin D3, block sterol biosynthesis of both normal and L2C lymphocytes [Philippot, j.r., cooper, A.G. & Wallach, D.F.H. (1976) Biochem. Biophys. Res. Commun. 72, 1035-1041]. Moreover, both cell types exchange cholesterol equivalently with cholesterol/lecithin liposomes. The only difference in sterol biosynthesis observed between the two cell types is in the temperature response of the enzyme. Arrhenius plots of this enzyme activity exhibit a prominent discontinuity at about 24 degrees in the case of normal cells, but none in the case of L2C. The activation energies for L2C cells and normal cells, above the normal cell transition temperature, were not significantly different. All of the data suggest that the regulatory defect in L2C lymphocytes arises from a deficiency in these cells' internal membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Siperstein M. D. Properties of 3-hydroxy-3-methylglutaryl coenzyme A reductase solubilized from rat liver and hepatoma. J Biol Chem. 1974 Oct 25;249(20):6585–6589. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Siperstein M. D. Regulation of cholesterol synthesis in normal and malignant tissue. Fed Proc. 1973 Dec;32(12):2168–2173. [PubMed] [Google Scholar]
- Bruckdorfer K. R., Green C. The exchange of unesterified cholesterol between human low-density lipoproteins and rat erythrocyte 'ghosts'. Biochem J. 1967 Jul;104(1):270–277. doi: 10.1042/bj1040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONGDON C. C., LORENZ E. Leukemia in guinea-pigs. Am J Pathol. 1954 Mar-Apr;30(2):337–359. [PMC free article] [PubMed] [Google Scholar]
- Gross L., Dreyfuss Y. The role of the skin in active specific immunization against leukemia in guinea pigs. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3550–3554. doi: 10.1073/pnas.71.9.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiniger H. J., Kandutsch A. A., Chen H. W. Depletion of L-cell sterol depresses endocytosis. Nature. 1976 Oct 7;263(5577):515–517. doi: 10.1038/263515a0. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by 7alpha-hydroxycholesterol, 7beta-hydroxycholesterol, and 7-ketocholesterol. J Biol Chem. 1973 Dec 25;248(24):8408–8417. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem. 1974 Oct 10;249(19):6057–6061. [PubMed] [Google Scholar]
- Kandutsch A. A., Hancock R. L. Regulation of the rate of sterol synthesis and the level of beta-hydroxy-beta-methylglutaryl coenzyme A reductase activity in mouse liver and hepatomas. Cancer Res. 1971 Oct;31(10):1396–1401. [PubMed] [Google Scholar]
- Kandutsch A. A., Saucier S. E. Prevention of cyclic and triton-induced increases in hydroxymethylglutaryl coenzyme A reductase and sterol synthesis by puromycin. J Biol Chem. 1969 May 10;244(9):2299–2305. [PubMed] [Google Scholar]
- Kimelberg H. K. Alterations in phospholipid-dependent (Na+ +K+)-ATPase activity due to lipid fluidity. Effects of cholesterol and Mg2+. Biochim Biophys Acta. 1975 Nov 17;413(1):143–156. doi: 10.1016/0005-2736(75)90065-6. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K. Protein-liposome interactions and their relevance to the structure and function of cell membranes. Mol Cell Biochem. 1976 Feb 25;10(3):171–190. doi: 10.1007/BF01731688. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Philippot J. R., Cooper A. G., Wallach D. F. 25-Hydroxycholecalciferol and 1, 25-dihydroxycholecalciferol are potent inhibitors of cholesterol biosynthesis by normal and leukemic (L2C) guinea pig lymphocytes. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1035–1041. doi: 10.1016/s0006-291x(76)80236-7. [DOI] [PubMed] [Google Scholar]
- Philippot J. R., Cooper A. G., Wallach D. F. Anitroxide-sterol derivative potently modifies cholesterol biosynthesis by normal and neoplastic guinea pig lymphocytes. Biochim Biophys Acta. 1975 Sep 16;406(1):161–166. doi: 10.1016/0005-2736(75)90051-6. [DOI] [PubMed] [Google Scholar]
- Raison J. K. The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. J Bioenerg. 1973 Jan;4(1):285–309. doi: 10.1007/BF01516063. [DOI] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Sabine J. R., James M. J. The intracellular mechanism responsible for dietary feedback control of cholesterol synthesis. Life Sci. 1976 Jun 1;18(11):1185–1192. doi: 10.1016/0024-3205(76)90191-0. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Ellman L., Davie J. M., Green I. L2C Guinea pig lymphatic leukemia: a "B" cell leukemia. Blood. 1972 Jan;39(1):1–12. [PubMed] [Google Scholar]
- Solomonson L. P., Liepkalns V. A., Spector A. A. Changes in (Na+ + K+)-ATPase activity of Ehrlich ascites tumor cells produced by alteration of membrane fatty acid composition. Biochemistry. 1976 Feb 24;15(4):892–897. doi: 10.1021/bi00649a026. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Houslay M. D., Metcalfe J. C., Birdsall N. J. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature. 1975 Jun 26;255(5511):684–687. doi: 10.1038/255684a0. [DOI] [PubMed] [Google Scholar]


