Abstract
Current tools for measuring medication adherence have significant limitations, especially among pediatric populations. We conducted a prospective observational study to assess the use of antiretroviral (ARV) drug levels in hair for evaluating antiretroviral therapy (ART) adherence among HIV-infected children in rural Uganda. Three-day caregiver recall, 30-day visual analog scale (VAS), Medication Event Monitoring System (MEMS), and unannounced pill counts and liquid formulation weights (UPC) were collected monthly over a one-year period. Hair samples were collected quarterly and analyzed for nevirapine (NVP) levels, and plasma HIV RNA levels were collected every six months. Among children with at least one hair sample collected, we used univariable random intercept linear regression models to compare log transformed NVP concentrations with each adherence measure, and the child’s age, sex, and CD4 count percentage (CD4%). 121 children aged 2–10 years were enrolled in the study; 74 (61%) provided at least one hair sample, and the mean number of hair samples collected per child was 1.9 (standard deviation [SD] 1.0). Three-day caregiver recall, VAS, and MEMS were found to be positively associated with increasing NVP concentration in hair, although associations were not statistically significant. UPC was found to have a non-significant negative association with increasing hair NVP concentration. In conclusion, NVP drug concentrations in hair were found to have non-significant, although generally positive, associations with other adherence measures in a cohort of HIV-infected children in Uganda. Hair collection in this population proved challenging, suggesting the need for community education and buy-in with the introduction of novel methodologies.
INTRODUCTION
In 2012, an estimated 2.9 million children under age 15 were living with HIV in sub-Saharan Africa, of whom 544,000 were receiving antiretroviral therapy (ART) (WHO, 2013). While some studies report generally high adherence to ART (Barro et al., 2011; Haberer et al., 2011), others are as low as 49% (Vreeman, Wiehe, Pearce, & Nyandiko, 2008). Even in the studies with high adherence, low rates of viral suppression and the presence of drug resistance suggest current adherence assessments may be incorrect and/or important adherence challenges exist (Ahoua et al., 2011; Barro et al., 2011; Barth et al., 2011; Dehority, Deville, Lujan-Zilbermann, Spector, & Viani, 2013; Orrell et al., 2013). Accurate, reliable, and practical means of evaluating pediatric ART adherence are critical in overcoming these concerns.
Multiple approaches exist for measuring ART adherence (Berg & Arnsten, 2006); however, all have significant limitations. Self-report of adherence typically overestimates adherence (Bhattacharya & Dubey, 2011; Biressaw, Abegaz, Abebe, Taye, & Belay, 2013; Martin et al., 2009; Naar-King, Frey, Harris, & Arfken, 2005), as it may be influenced by social desirability and recall biases (Kagee & Nel, 2012). Clinic-based pill counts may be inaccurate if children or caregivers remove extra medication to appear more adherent (Simoni et al., 2006). Moreover, patients may not remember to bring their medication to appointments (Mghamba, Minzi, Massawe, & Sasi, 2013). Electronic adherence monitoring (e.g., Medication Event Monitoring System [MEMS])(Haberer, Kahane, et al., 2010; Haberer, Kiwanuka, Nansera, Wilson, & Bangsberg, 2010) is costly, and bottle openings do not always reflect medication ingestion (Martin et al., 2009). Finally, plasma antiretroviral (ARV) drug levels reflect only short-term adherence (1–3 days)(Nettles et al., 2006; Wertheimer, Freedberg, Walensky, Yazdanapah, & Losina, 2006), adherence may transiently improve before clinic visits (Cramer, Scheyer, & Mattson, 1990; Podsadecki, Vrijens, Tousset, Rode, & Hanna, 2008), and collection is resource-intensive, requiring cold chain and phlebotomy (ter Heine, Beijnen, & Huitema, 2009). These limitations highlight the need for alternative feasible, acceptable, and valid adherence measures.
Measuring ARV drug levels in hair is a promising approach for evaluating ART adherence in developing settings (Beumer, Bosman, & Maes, 2001; Gandhi, Yang, Bacchetti, & Huang, 2014; Hickey et al., 2014; Huang et al., 2008; Huang et al., 2011). Hair samples are simple to collect, can be stored at room temperature, and are processed without biohazardous precautions. Hair concentrations of ARVs reflect uptake from systemic circulation over weeks to months and have been shown to correlate well with plasma drug levels (van Zyl et al., 2011) and viral suppression (Bernard, Peytavin, Vuagnat, de Truchis, & Perronne, 1998; Bernard et al., 2002; Duval et al., 2007; Gandhi et al., 2011; Gandhi et al., 2009; Servais et al., 2001; van Zyl et al., 2011).
In this study, we sought to assess the use of ARV drug levels in hair samples from HIV-infected children in rural Uganda for evaluating ART adherence through correlation with other adherence measures.
METHODS
Study population
Between July 2008 and February 2009, HIV-infected children aged 2–10 years were recruited into a longitudinal observational cohort study (NCT00868257) from the Children’s HIV/AIDS Care Clinic in Mbarara, Uganda. Participants included children initiating and those established on ART. Both liquid and pill formulations were used; prescriptions reflected World Health Organization guidelines (e.g., nevirapine [NVP]: 160 – 200mg/m2 to max 200mg twice daily) (WHO, 2010). The clinic provides ARVs free of charge.
Data Collection
This report describes an analysis of data collected in a previously published cohort study (Haberer et al., 2012). Briefly, in the cohort study, adherence was monitored monthly for one year using caregiver interview for three-day recall (Usitalo et al., 2014), and 30-day visual analog scale (VAS) (Amico et al., 2006), MEMS (Haberer et al., 2011), and unannounced pill counts and liquid formulation weights (UPC) (Farley et al., 2008). Hair samples were collected quarterly. Plasma HIV RNA levels were assessed at baseline, six, and twelve months (Roche Amplicor HIV-1 Monitor Test, USA). Hair samples were also collected quarterly as a novel adherence measure, but were not included in the initial publication due to delays in sample processing.
Hair collection and ARV concentration determination
Research assistants cut or shaved a small thatch of hair as close as possible to the occipital scalp. Methods for extraction and analyses of ARV drug concentration in hair are reported elsewhere (Huang et al., 2008; Huang et al., 2011). Drug levels were determined only for children taking NVP-based regimens, as they comprised the majority of the cohort (77% of those providing hair samples). Our methods have been validated from 0.50 to 200 nanograms(ng)/milligrams (mg) hair for NVP with good linearity (R2>0.99) and reproducibility (coefficient of variation [CV]<10%).
Statistical analysis
Sample size was determined by the parent study as described previously (Haberer et al., 2012). Descriptive statistics were used to explore child characteristics, number of hair samples collected, summary drug concentrations, and adherence measures (reflecting the three months before hair collection). Adherence measures were not capped at 100% to avoid potential introduction of bias; however, MEMS data were treated as missing in cases of known or suspected (e.g., >30 days with no openings) device non-use.
Among children with at least one hair sample collected, we used univariable random intercept linear regression models (SAS proc mixed) to model NVP hair concentrations in terms of each adherence measure, as well as the child’s age, sex, and CD4 count percentage (CD4%). Drug concentrations were log-transformed. The association of variables with loss of viral suppression was not assessed as this event only occurred in four children.
Ethics Statement
The study was approved by the Mbarara University of Science and Technology (MUST) Research Ethics Committee, Mbarara, Uganda and the Partners Health Care Human Research Committee, Boston, MA. We received clearance from the Uganda National Council on Science and Technology and a materials transfer agreement to ship hair samples to the University of California San Francisco (UCSF) for processing was ratified by UCSF and MUST. Caregivers provided written informed consent, and children provided verbal assent when possible (typically those aged seven years and older).
RESULTS
Participant characteristics
A total of 121 HIV-infected children were enrolled in the cohort study; 74 (61%) provided at least one hair sample, and 61 (82%) of the 74 were on NVP regimens. For those 61 participants, the median age was 4.7 years (IQR 1.2–8.2) and 51% were female. Their median CD4% was 44.5 (IQR 25.5–63.5), and median weight was 17.0 kg (IQR 11.4–22.6). Of these children on NVP, 39 (64%) were established on ART, and 22 (36%) were on liquid formulations. Quarterly hair collection was as follows: 23 (16%) in Q1, 37 (26%) in Q2, 40 (28%) in Q3, 27 (19%) in Q4; 17 (12%) were missing the collection date and were excluded from the statistical analysis.
Hair collection
A total of 144 quarterly hair samples were collected, with a mean of 1.9 (standard deviation [SD] 1.0) among those who provided at least one sample.
Adherence measures
For the three-month time period prior to each collected hair sample in the children taking NVP-based ART (N=61), median and mean adherence as assessed by each measure and are listed in Table 1A. Median and mean NVP hair concentrations were 76.7 ng/mg of hair (IQR 27.7–125.7) and 104.4 ng/mg (SD 109.3), respectively. No data were available for the three-month time period in 6.6% of three-day caregiver reports, 7.2% of 30-day VAS, 9.7% of MEMS, and 11.4% of UPC.
Table 1.
Median and mean adherence and the association with hair nevirapine concentration for each adherence measure.
| Adherence measure | Median | Mean | Fold increase in hair NVP per 1.10-fold increase in adherence measure (95% CI) |
p-value |
|---|---|---|---|---|
| Three-day caregiver recall | 100% (IQR 100-100) |
99.2% (SD 6.0) |
1.10 (0.83–1.45) |
0.51 |
| 30-day visual analog scale (VAS) | 100% (IQR 98–102) |
97.6% (SD 4.8) |
1.20 (0.97–1.49) |
0.091 |
| Medication event monitoring system (MEMS) | 96.1% (IQR 87.4–104.8) |
94.6% (SD 8.0) |
1.16 (0.93–1.44) |
0.19 |
| Unannounced pill counts/liquid weights (UPC) | 97.9% (IQR 86.8–109) |
95.5% (SD 14.7) |
0.96 (0.90–1.01) |
0.11 |
Comparison of adherence measures and NVP hair concentration
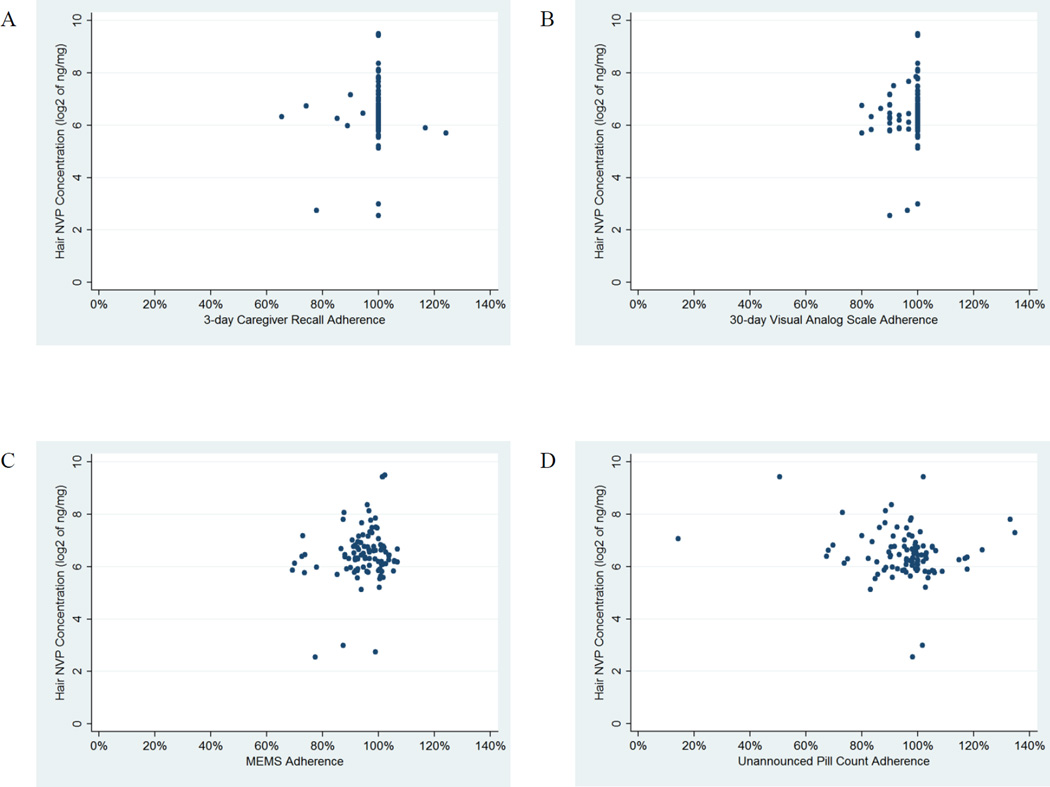
Figure 1 shows scatterplots of each adherence measure versus NVP hair concentration. Three-day caregiver recall, VAS, and MEMS were positively associated with increasing NVP concentration in hair, although not statistically significant (Table 1B). UPC was found to have a non-significant negative association with increasing hair NVP concentration.
Figure 1.
Scatterplots showing the relationship of each adherence measure to concentrations of nevirapine in hair. A. Three-day caregiver recall, B. 30-day visual analog scale (VAS), C. Medication Event Monitoring System (MEMS), and D. Unannounced pill counts and liquid formulation weights (UPC).
DISCUSSION
In this study, we compared nevirapine concentration in pediatric hair samples with multiple measures of adherence (Haberer et al., 2012). We found positive associations with all the measures of adherence aside from UPC, with proportionally or greater than proportionally higher hair levels with higher adherence, although there was substantial variability in hair levels within the same reported adherence level (Figure 1) and none of these associations reached statistical significance. Similar positive associations between NVP concentration and self-reported adherence were seen in a study among Kenyan adults (Hickey et al., 2014). The negative association with UPC is difficult to interpret. Midway through the study, we learned that clinic pharmacists were taking back and giving out extra medication in efforts to facilitate adherence among patients; this practice may have limited UPC accuracy. Liquid weight measurements may also have been inaccurate. NVP hair concentrations in this study were in the range of those in another Africa-based study (Hickey et al., 2014).
In contrast to previous studies conducted by our research group showing high acceptability and feasibility of collecting hair samples in developing settings (Bartelink et al., 2013; Baxi SM, 2014; Gandhi et al., 2013; Hickey et al., 2014; van Zyl et al., 2011), we found collection challenging among HIV-infected children in rural Uganda. Though we attempted to collect four samples per child, only 144 (30% of 484 samples) were obtained with a mean of 1.9 per child among the 61% who provided any samples. Participants readily provided data for other adherence measures, but hair collection was commonly declined or the child’s head was already clean-shaven.
While we did not design the study to elucidate hair sample refusal, we hypothesize that primary barriers were traditional practices and preference. In Uganda, caregivers shave children’s heads for reasons related to hygiene, difficulty in combing, local custom, and preference. When planning the study, we informally asked Ugandan research colleagues about appropriate frequency for hair collection and decided on quarterly collection. However, children often had no hair at the time of collection, and we learned caregivers shaved their children’s heads as often as monthly. Additionally, HIV-infected children and caregivers face significant stigma and discrimination (Amzel et al., 2013); alteration of head shaving practices could have led to fear of unintended HIV status disclosure or having the child viewed as ‘different.
Prior research on pediatric ARV drug levels in hair is limited to one study on ARV transfer from mother to infant (Gandhi et al., 2013) and another assessing ART adherence among a pediatric cohort in Asia (Prasitsuebsai W, 2013). In the latter study, acceptability of hair collection was high (90%) and a strong association between hair lopinavir levels and virologic outcomes was observed. Unlike the current study, community education and buy-in was obtained and may at least partially explain differences in feasibility. Community mobilization is integral to implementing HIV-related interventions (Kawichai et al., 2012; Tedrow et al., 2012) and likely applies to introducing research methods.
Despite the challenges encountered in this study, the use of ARV drug concentrations in hair as an adherence measure shows promise for children in developing settings. The relationships with other adherence measures should be explored in a larger sample with high baseline virologic suppression rates. Further research should also explore the costs associated with hair sample testing in resource-limited settings, as well as involve community mobilization and dedicated assessment of potential barriers and solutions for hair sample collection.
ACKNOWLEGEMENTS
The authors would like to thank the study participants, as well as the following study staff: Nneka Emenyonu, Georgina Nakafero, Jenniffer Owomuhangi, Sarah Namwanje, Ambrose Mugyenyi, Allen Kiconco, Andrew Mugumemushabe, Dan Mwehire, Mathias Orimwesiga, Constance Katabazi, Teddy Komuhangi, and Henry Kizito.
FUNDING
This study was supported by the National Institutes of Health (NIMH) (K23MH087228 to J.H), (R21MH083306 to J.H., J.K., and D.N.) and National Institute of Allergy and Infectious Diseases (NIAID) (RO1AI098472 to M.G.). Partial funding for this work was provided by NIH/NIAID (American Recovery and Reinvestment Act Funds) (3R01AI065233-05S2, Greenblatt, P.I.).
Footnotes
Disclaimers: There are no conflicts of interest.
REFERENCES
- Ahoua L, Guenther G, Rouzioux C, Pinoges L, Anguzu P, Taburet AM, Pujades-Rodriguez M. Immunovirological response to combined antiretroviral therapy and drug resistance patterns in children: 1- and 2-year outcomes in rural Uganda. BMC Pediatr. 2011;11:67. doi: 10.1186/1471-2431-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico KR, Fisher WA, Cornman DH, Shuper PA, Redding CG, Konkle-Parker DJ, Fisher JD. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr. 2006;42(4):455–459. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- Amzel A, Toska E, Lovich R, Widyono M, Patel T, Foti C, Children Promoting a combination approach to paediatric HIV psychosocial support. AIDS. 2013;27(Suppl 2):S147–S157. doi: 10.1097/QAD.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barro M, Some J, Foulongne V, Diasso Y, Zoure E, Hien H, Msellati P. Short-term virological efficacy, immune reconstitution, tolerance, and adherence of once-daily dosing of didanosine, lamivudine, and efavirenz in HIV-1-infected African children: ANRS 12103 Burkiname. J Acquir Immune Defic Syndr. 2011;57(Suppl 1):S44–S49. doi: 10.1097/QAI.0b013e31821fd64f. [DOI] [PubMed] [Google Scholar]
- Bartelink IH, Savic RM, Mwesigwa J, Achan J, Clark T, Plenty A, Aweeka F. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in tororo, uganda. J Clin Pharmacol. 2013 doi: 10.1002/jcph.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving antiretroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. Pediatr Infect Dis J. 2011;30(1):52–56. doi: 10.1097/INF.0b013e3181ed2af3. [DOI] [PubMed] [Google Scholar]
- Baxi SM, L A, Bacchetti P, Guadensia M, Sanders EJ, Kibengo FM, Haberer JE, Rooney J, Priddy F, Gandhi M. Measuring Intermittent and Daily PrEP Adherence by Hair Levels, Self-Report and MEMS Caps Openings (poster P-V4) Boston, MA: CROI; 2014. [Google Scholar]
- Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S79–S87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard L, Peytavin G, Vuagnat A, de Truchis P, Perronne C. Indinavir concentrations in hair from patients receiving highly active antiretroviral therapy. Lancet. 1998;352(9142):1757–1758. doi: 10.1016/S0140-6736(05)79831-7. [DOI] [PubMed] [Google Scholar]
- Bernard L, Vuagnat A, Peytavin G, Hallouin MC, Bouhour D, Nguyen TH, Perronne C. Relationship between levels of indinavir in hair and virologic response to highly active antiretroviral therapy. Ann Intern Med. 2002;137(8):656–659. doi: 10.7326/0003-4819-137-8-200210150-00009. [DOI] [PubMed] [Google Scholar]
- Beumer JH, Bosman IJ, Maes RA. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract. 2001;55(6):353–357. [PubMed] [Google Scholar]
- Bhattacharya M, Dubey AP. Adherence to antiretroviral therapy and its correlates among HIV-infected children at an HIV clinic in New Delhi. Ann Trop Paediatr. 2011;31(4):331–337. doi: 10.1179/1465328111Y.0000000031. [DOI] [PubMed] [Google Scholar]
- Biressaw S, Abegaz WE, Abebe M, Taye WA, Belay M. Adherence to Antiretroviral Therapy and associated factors among HIV infected children in Ethiopia: unannounced home-based pill count versus caregivers' report. BMC Pediatr. 2013;13:132. doi: 10.1186/1471-2431-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med. 1990;150(7):1509–1510. [PubMed] [Google Scholar]
- Dehority W, Deville JG, Lujan-Zilbermann J, Spector SA, Viani RM. Effect of HIV genotypic drug resistance testing on the management and clinical course of HIV-infected children and adolescents. Int J STD AIDS. 2013;24(7):549–553. doi: 10.1177/0956462412473958. [DOI] [PubMed] [Google Scholar]
- Duval X, Peytavin G, Breton G, Ecobichon JL, Descamps D, Thabut G, Leport C. Hair versus plasma concentrations as indicator of indinavir exposure in HIV-1-infected patients treated with indinavir/ritonavir combination. AIDS. 2007;21(1):106–108. doi: 10.1097/QAD.0b013e3280118486. [DOI] [PubMed] [Google Scholar]
- Farley JJ, Montepiedra G, Storm D, Sirois PA, Malee K, Garvie P, Team PP. Assessment of adherence to antiretroviral therapy in perinatally HIV-infected children and youth using self-report measures and pill count. J Dev Behav Pediatr. 2008;29(5):377–384. doi: 10.1097/DBP.0b013e3181856d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, Greenblatt RM. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52(10):1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Ameli N, Bacchetti P, Gange SJ, Anastos K, Levine A Women's Interagency, H. I. V. S. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23(4):471–478. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Mwesigwa J, Aweeka F, Plenty A, Charlebois E, Ruel TD study, H. I. V. d. i. T. Hair and Plasma Data Show That Lopinavir, Ritonavir, and Efavirenz All Transfer From Mother to Infant In Utero, But Only Efavirenz Transfers via Breastfeeding. J Acquir Immune Defic Syndr. 2013;63(5):578–584. doi: 10.1097/QAI.0b013e31829c48ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Yang Q, Bacchetti P, Huang Y. Short communication: A low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses. 2014;30(1):25–28. doi: 10.1089/aid.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Cook A, Walker AS, Ngambi M, Ferrier A, Mulenga V, Bangsberg DR. Excellent adherence to antiretrovirals in HIV+ Zambian children is compromised by disrupted routine, HIV nondisclosure, and paradoxical income effects. PLoS One. 2011;6(4):e18505. doi: 10.1371/journal.pone.0018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, Bangsberg DR. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–1346. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Kiwanuka J, Nansera D, Ragland K, Mellins C, Bangsberg DR. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS One. 2012;7(5):e36737. doi: 10.1371/journal.pone.0036737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Kiwanuka J, Nansera D, Wilson IB, Bangsberg DR. Challenges in using mobile phones for collection of antiretroviral therapy adherence data in a resource-limited setting. AIDS Behav. 2010;14(6):1294–1301. doi: 10.1007/s10461-010-9720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MD, Salmen CR, Tessler RA, Omollo D, Bacchetti P, Magerenge R, Gandhi M. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(21):3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang Q, Yoon K, Lei Y, Shi R, Gee W, Gandhi M. Microanalysis of the antiretroviral nevirapine in human hair from HIV-infected patients by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(6):1923–1933. doi: 10.1007/s00216-011-5278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24(11):1448–1452. doi: 10.1080/09540121.2012.687816. [DOI] [PubMed] [Google Scholar]
- Kawichai S, Celentano D, Srithanaviboonchai K, Wichajarn M, Pancharoen K, Chariyalertsak C Project Accept Study, T. NIMH Project Accept (HPTN 043) HIV/AIDS community mobilization (CM) to promote mobile HIV voluntary counseling and testing (MVCT) in rural communities in Northern Thailand: modifications by experience. AIDS Behav. 2012;16(5):1227–1237. doi: 10.1007/s10461-011-0099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Elliott-DeSorbo DK, Calabrese S, Wolters PL, Roby G, Brennan T, Wood LV. A comparison of adherence assessment methods utilized in the United States: perspectives of researchers, HIV-infected children, and their caregivers. AIDS Patient Care STDS. 2009;23(8):593–601. doi: 10.1089/apc.2009.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mghamba FW, Minzi OM, Massawe A, Sasi P. Adherence to antiretroviral therapy among HIV infected children measured by caretaker report, medication return, and drug level in Dar Es Salaam, Tanzania. BMC Pediatr. 2013;13:95. doi: 10.1186/1471-2431-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar-King S, Frey M, Harris M, Arfken C. Measuring adherence to treatment of paediatric HIV/AIDS. AIDS Care. 2005;17(3):345–349. doi: 10.1080/09540120412331299753. [DOI] [PubMed] [Google Scholar]
- Nettles RE, Kieffer TL, Parsons T, Johnson J, Cofrancesco J, Jr, Gallant JE, Flexner C. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42(8):1189–1196. doi: 10.1086/501458. [DOI] [PubMed] [Google Scholar]
- Orrell C, Levison J, Ciaranello A, Bekker LG, Kuritzkes DR, Freedberg KA, Wood R. Resistance in pediatric patients experiencing virologic failure with first-line and second-line antiretroviral therapy. Pediatr Infect Dis J. 2013;32(6):644–647. doi: 10.1097/INF.0b013e3182829092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. "White coat compliance" limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9(4):238–246. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- Prasitsuebsai WKS, Khanh TH, Ananworanich J, Viet DC, Van LN, Kurniati N, Kosalaraksz P, Sirisanthana V, Chokephaibulkit K, Thammajaruk N, Singtoroj T, Teeraananchai S, Gandhi M, Sohn AH. Lopinavir Hair Concentrations Predict Virological Failure Among Asian Children. 5th International Workshop on HIV Pediatrics; Kuala Lumpur, Malaysia. 2013. [Google Scholar]
- Servais J, Peytavin G, Arendt V, Staub T, Schneider F, Hemmer R, Schmit JC. Indinavir hair concentration in highly active antiretroviral therapy-treated patients: association with viral load and drug resistance. AIDS. 2001;15(7):941–943. doi: 10.1097/00002030-200105040-00019. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedrow VA, Zelaya CE, Kennedy CE, Morin SF, Khumalo-Sakutukwa G, Sweat MD, Celentano DD. No "magic bullet": exploring community mobilization strategies used in a multi-site community based randomized controlled trial: Project Accept (HPTN 043) AIDS Behav. 2012;16(5):1217–1226. doi: 10.1007/s10461-011-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Heine R, Beijnen JH, Huitema AD. Bioanalytical issues in patient-friendly sampling methods for therapeutic drug monitoring: focus on antiretroviral drugs. Bioanalysis. 2009;1(7):1329–1338. doi: 10.4155/bio.09.124. [DOI] [PubMed] [Google Scholar]
- Usitalo A, Leister E, Tassiopoulos K, Allison S, Malee K, Paul ME, Mellins CA. Relationship between viral load and self-report measures of medication adherence among youth with perinatal HIV infection. AIDS Care. 2014;26(1):107–115. doi: 10.1080/09540121.2013.802280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, Maartens G. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56(4):333–339. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeman RC, Wiehe SE, Pearce EC, Nyandiko WM. A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. Pediatr Infect Dis J. 2008;27(8):686–691. doi: 10.1097/INF.0b013e31816dd325. [DOI] [PubMed] [Google Scholar]
- Wertheimer BZ, Freedberg KA, Walensky RP, Yazdanapah Y, Losina E. Therapeutic drug monitoring in HIV treatment: a literature review. HIV Clin Trials. 2006;7(2):59–69. doi: 10.1310/hct.2006.7.2.004. [DOI] [PubMed] [Google Scholar]
- WHO. Antiretroviral therapy for HIV infection in infants and children: Towards universal access 206. 2010 [PubMed]
- WHO. Global update on HIV treatment 2013: Results, impact and opportunities. 2013