Abstract
We sought to examine the course of adherence and cognition in HIV-infected individuals with either cocaine or heroin dependence and investigate independent predictors of cognition change. A prospective study over six months was undertaken in which adherence was measured by monthly electronic pill cap monitoring (MEMS), while a comprehensive neuropsychological battery resulting in a composite score (NPZ8) was performed at baseline and six months. Multivariable regression models were performed in order to determine independent associations with change in cognition. There were 101 subjects at baseline, of whom 62% were male and 83% were non-Hispanic black. 46.6% of subjects at baseline had completed high school, 36.6% reported active cocaine use during the course of the study, and 0% reported active heroin use during the course of the study. 66 subjects completed the final cognitive assessment at 6 months. Subjects had markedly impaired cognitive function at baseline (NPZ8 −1.49) which persisted at six months (NPZ8 −1.47) in the group of study completers. There was an average monthly decrease in adherence of −2.91% overall (p= 0.008). In the multivariable model, each of the following variables: baseline cognition (R2change= 0.121, p= 0.006), cocaine use during the study (R2change= 0.059, p= 0.046), and monthly adherence change (R2change= 0.078, p= 0.018) independently contributed to NPZ8 change with an overall R2change= 0.219 (p= 0.001). This study shows an overall decrease in adherence over time in this population of subjects with a history of drug dependence. Active cocaine use, baseline cognition, and temporal adherence changes independently contributed to changes in cognition. Further study on enhancing adherence, cognition, and limiting drug abuse are warranted in this subgroup of HIV-infected individuals.
Keywords: HIV, neurocognition, adherence, cocaine, HIV-associated neurocognitive disorder
Introduction
The course of HIV infection is often complicated by cocaine and heroin use. Adherence to combination antiretroviral therapy (ART) by individuals who actively use these substances is significantly compromised(Arnsten et al., 2002; Baum et al., 2009; Wood et al., 2003). While a recent six month longitudinal study in HIV-infected individuals with no history of drug dependence (Ettenhofer, Foley, Castellon, & Hinkin, 2010) found that global cognition at baseline predicted better adherence over six months and that adherence reciprocally predicted cognition at six months, a clearer understanding of these relationships in HIV-infected substance abusers is needed.
Methods
A prospective study of six months was performed to measure changes in adherence and cognition among HIV-infected subjects with cocaine or heroin dependence. Participants were recruited between 2005 and 2009 in South Florida and the study was IRB approved. Inclusion criteria were: 1) HIV-infected on verified antiretroviral therapy, 2) use of heroin or cocaine in the past 12 months, 3) diagnosed Heroin or Cocaine Dependence (past or current), and 4) proficient in English. Exclusion criteria were: 1) schizophrenia or bipolar disorder and 2) history of loss of consciousness > 30 minutes.
For study eligibility, current or past heroin/cocaine dependence was assessed using the substance abuse section of the Structured Clinical Interview for DSM-IV diagnoses (First et al., 1995). Those who met basic inclusion/exclusion criteria underwent a baseline visit that included a neuropsychological evaluation and plasma HIV RNA and CD4 count. Study visits to monitor medication adherence and alcohol/substance use were conducted at 4-week intervals for 6-months. Finally, at 6-months, participants repeated neuropsychological evaluation. In total, the study consisted of 7 visits. Eight tests were completed at baseline and 6 months: California Verbal Learning Test, California Computerized Assessment Package, Rey Complex Figure Test, Trail Making Test A/B, the Digit Span subtest from the Wechsler Adult Intelligence Scale – III (WAIS-III), Symbol Digit Modalities, Grooved Pegboard, and Verbal Fluency test. The NPZ-8 was calculated by averaging the scores on each measure. The Beck Depression Inventory –II was included using only items measuring non-somatic symptoms of depression. Substance use was measured at baseline and 4-week intervals using the Addiction Severity Index (ASI). The Central Nervous System Penetration Effectiveness (CPE) rank for each participant’s antiretroviral regimen was calculated.(Letendre, 2011)
Adherence was measured using an electronic device (Medication Event Monitoring System [MEMS], Aprex, Union City, CA). The cap was placed on only one medication (a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor if a protease inhibitor was not part of the regimen). “Pocket dosing” (taking out more than one dose when opening the bottle), was assessed and adherence recalculated based on the number of reported pocket doses. Cap openings that exceeded the prescribed doses were not included. Self-report adherence was assessed by an interviewer-administered questionnaire for one week preceding the study visit (Arnsten et al., 2001). Adherence was defined as percent of doses taken during the previous seven days.
One hundred seventy-one individuals met prescreening criteria and of these, 139 completed the screening interview. Thirty-seven did not meet the criteria for cocaine/heroin dependence while one subject was not included in the analyses due to a data entry error, for a final sample of 101. Distributions of descriptive statistics were assessed for normality, outliers and missing data. Subjects were defined as completers if they finished cognitive assessments at baseline and 6 months. Depending on normality, comparisons between completers and non-completers used parametric t-tests or non-parametric Mann Whitney tests. Chi-square tests were performed for categorical variables.
Adherence (% doses taken) was assessed monthly. Slopes (average change in MEMS% per month) were calculated for all subjects with two or more time points who had been assessed initially at either the 1st or 2nd month. Changes in cognition were calculated as the difference between the NPZ8 scores at baseline and six months. One-sample t-tests were used to test whether slopes differed significantly from zero. While each of the NPZ cognitive domains and global functioning measures were evaluated, only the composite score of NPZ8 was significantly associated with changes in adherence and used in a final model. Bivariate correlations were assessed for the variables listed in Table 1. Only age, income, cocaine use and homelessness were significantly associated with changes in cognition. Age and income predicted study completion and were also significantly associated with better adherence over time (age r = 0.297, p=.009; income r = 0.277, p=.016). Cocaine use and homelessness were associated with declines in cognition from baseline to 6 months (cocaine use r= −0.248, p=.047; homelessness r= −0.270 p=.028). Thus, age, income, cocaine use and homelessness were all considered in the final regression model. The final model was created using a three-block “sequential” (hierarchical) linear regression.
Table 1.
Demographics and Baseline Measures
| Overall n=101 (unless noted) | ||
|---|---|---|
| Continuous Measures | Mean (SD) | [Range] |
| Age | 43.95 (6.9) | [22, 62] |
| Years School Completed | 11.24 (2.1) | [5, 20] |
| Income | $5,961 ($5,467) | [$0, $36,000] |
| Plasma HIV copies/mL | 10,174.0 (26,936.3) N=69 | [0, 100,000] |
| HIV CD4 Count | 367.5 (226.9) N=85 | [15, 952] |
| Non-somatic Depression | 10.6 (8.5) | [0, 39] |
| Adherence % (1mo) | 78.3% (30.5%) N=85 | [0%, 113.8%] |
| CPE Rank | 7.73 (1.7) N=99 | [3, 12] |
| NPZ.8 | −1.46 (1.0) N=100 | [−5.01, 0.26] |
| Categorical Measures | n | % |
| Gender | ||
| Male | 63 | 62.4% |
| Female | 35 | 34.7% |
| Transgender | 3 | 3.0% |
| Race/Ethnicity | ||
| NH-White | 6 | 5.9% |
| Hispanic | 11 | 10.9% |
| NH-Black | 84 | 83.2% |
| Sexual Orientation | ||
| Homosexual | 16 | 15.8% |
| Heterosexual | 73 | 72.3% |
| Bisexual/Unsure | 12 | 11.9% |
| Education Level | ||
| < HS | 47 | 46.6% |
| HS Diploma/GED | 32 | 31.7% |
| More than HS | 22 | 21.8% |
| Employment (yes) | 3 | 3.0% |
| Disability (yes) | 56 | 55.4% |
| Regular Place to Stay (yes) | 92 | 91.1% |
| Homeless in Last Year (yes) | 56 | 55.4% |
| Cocaine Use During Study (yes) | 37 | 36.6% |
NH Non-Hispanic
HS High School
Results
The majority of subjects was male (62.4%), non-Hispanic black (83.2%), heterosexual (72.3%), and completed high school or more education (53.4%). Ninety-four subjects had used cocaine for one year or more in the past and 13 had used heroin for one year or more in the past. Thirty-six percent of baseline subjects reported cocaine use during the study, while no subjects reported heroin use. Neuropsychological scores were below average at baseline (mean NPZ8 of −1.49). Of 101 subjects, 66 completed both baseline and 6-month cognitive assessment. The 35 who did not complete both were younger (completers’ mean age 45.3 years (SD 7.1), non-completers’ mean age 41.4 years (SD 5.9), t(99)=2.79, p=.006); had lower incomes (completers’ mean income $6531 (SD $4762), non-completers’ mean income $4885 (SD $6537), Mann Whitney Z=−2.28, p=.023); and had higher plasma HIV RNA (completers’ mean 6567 (copies/mL) (SD 21,775), non-completers’ mean 16,937 (SD 34,128), Mann Whitney Z=−2.24, p=.025).
Seventy-six subjects had ≥2 adherence measurements over the 6 months. The average monthly change in adherence (table 2) from the 1st month to the 6th month was negative: −2.91% (SD 9.4%), significantly different from zero (t(75)= −2.71, p=.008). In contrast, changes in cognition (NPZ8 change scores) for study completers were not significant with average change scores of 0.05 (SD 0.5) (t(65)=0.75, p=.459). However, there was a weak bivariate correlation between changes in adherence and changes in cognition (r= 0.239, p=.061), which was further investigated with the final model.
Table 2.
Adherence and Cognition Summary Statistics Across the Six Months
| Timepoint | Adherence % Prescribed Doses Taken N, Mean (SD) [range] |
Cognitive Scores NPZ.8 N, Mean (SD) [range] |
|---|---|---|
| Baseline | 101, −1.49 (1.1) [−5.01, 0.26] |
|
| 1 mo | 85, 78.3% (30.5%) [0%, 113.8%] |
|
| 2 mo | 74, 68.6% (35.2%) [0%, 107.4%] |
|
| 3 mo | 64, 66.9% (34.5%) [0%, 114.3%] |
|
| 4 mo | 65, 66.6% (37.3%) [0%, 115.6%] |
|
| 5 mo | 61, 71.8% (34.2%) [0%, 110.7%] |
|
| 6 mo | 55, 67.6% (33.6%) [0%, 120.8%] |
66, −1.47 (1.1) [−4.73, −0.24] |
|
| ||
| Slopes (adherence change per month) | 76, −2.91 (9.4) | 66, 0.05 (0.5) |
| Change Scores for NPZ.8 (6mo – BL) | [−39.7, 16.4] | [−1.35, 1.62] |
| Test Ho: Slope=0 or Ho: Change scores=0 | t=−2.71 (df=75) p=.008 | T=0.75 (df=65) p=.459 |
| Correlation between adherence Slopes and NPZ.8 change scores | r=0.239 (n=62) p=.061 | |
In order to account for practice effects, baseline NPZ8 scores were included in the first block of the regression model (Table 3). Baseline NPZ8 scores were significant (p=.006) and accounted for 12.1 % of the variance in NPZ8 change scores. After accounting for practice effects and other covariates, only cocaine use during the study was significant (p=.046), accounting for 5.9% of the variance. After adjusting for practice effects and cocaine use, changes in adherence were significant (p=.018) and accounted for an additional 7.8% of the variance in NPZ8 change scores, with the final model explaining 21.9% of the variability in NPZ8 change scores.
Table 3.
Sequential Linear Regression Model for Changes in Cognition associated with Changes in Adherence Controlling for Cocaine Use and Cognition at Baseline
| Variable | B | 95% CI for B | β | p-value | F(df1,df2) | p-value | Adj R2 | Δ R2 (p-value) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BLOCK 1 | F(1,60)=8.132 | .006 | .106 | .121 (.006) | ||||||
| (Constant) | −.173 | −.377 | .031 | .096 | ||||||
| NPZ.8 (baseline) | −.152 | −.260 | −.045 | −.348 | .006 | |||||
|
| ||||||||||
| BLOCK 2 | F(2,60)=6.373 | .003 | .152 | .059 (.046) | ||||||
| (Constant) | −.069 | −.252 | −.044 | .537 | ||||||
| NPZ.8 (baseline) | −.148 | −.485 | −.005 | −.338 | .006 | |||||
| Cocaine Use During Study | −.245 | −.276 | .153 | −.243 | .046 | |||||
|
| ||||||||||
| BLOCK 3 | F(3,60)=6.603 | .001 | .219 | .078 (.018) | ||||||
| (Constant) | −.061 | −.276 | .153 | .570 | ||||||
| NPZ.8 (baseline) | −.158 | −.259 | −.057 | −.361 | .003 | |||||
| Cocaine Use During Study | −.251 | −.482 | −.020 | −.249 | .033 | |||||
| Change in Adherence % per mo | .022 | .004 | .040 | .280 | .018 | |||||
- Outcome variable: Change in Cognition (NPZ.8 at 6mo – NPZ.8 at Baseline, positive change scores indicate improved cognition)
-
Variables included were:
-
○Block 1: NPZ.8 (baseline)
-
○Block 2: Age, Income, Cocaine Use During Study and Homeless in the last year (Stepwise variable selection used (Criteria: Probability-of-F-to-enter <= .05, Probability-of-F-to-remove >= .10))
-
○Block 3: Change in Adherence % per mo (change in MEMS Percents per month, positive change scores indicate better adherence)
-
○
- N=61 with complete data on all variables considered
The multivariable model yielded a significant positive correlation between increases in monthly adherence and positive NPZ8 change scores between baseline and 6-months (p=0.018). Fitted regression lines are shown in figure 1 for average NPZ8 cognitive scores at baseline (mean NPZ8 baseline −1.54). The slopes were homogenous indicating that the relationship was the same on average for subjects who used (dashed line, white stars) and did not use (solid line, black filled circles) cocaine during the study. However, there was an overall separation in regression lines (subjects who actively used cocaine had consistently less positive improvement in NPZ8).
Figure 1.
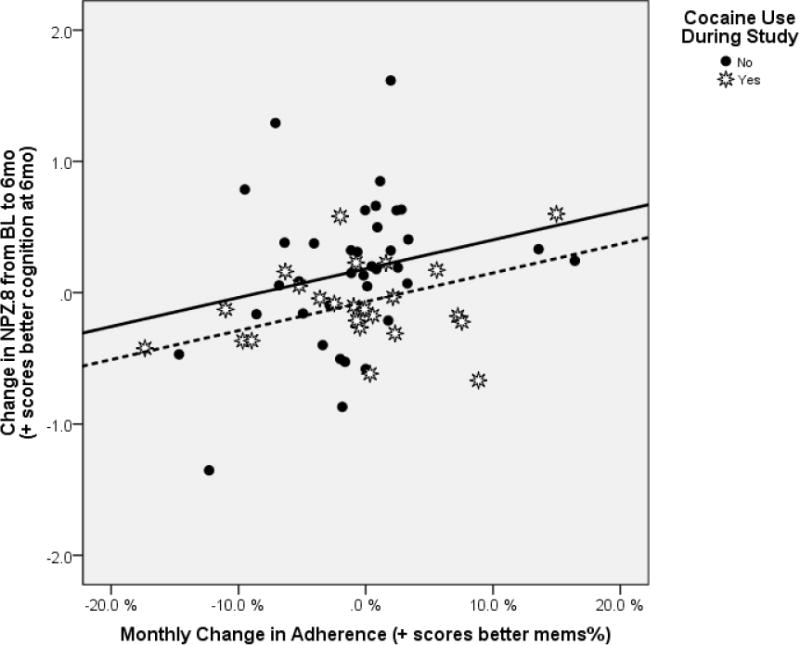
Changes in Cognition Associated with Changes in Adherence Controlling for Cocaine Use and Cognition at Baseline
- Cocaine NO: Black Circles and Solid line [equation Y = 0.182 + 0.022*X]
- Cocaine YES: White Stars and Dashed Line [equation Y = −0.068 + 0.022*X]
Discussion
The goal of this study was to evaluate the relation of medication adherence to changes in neurocognitive functioning among HIV-infected subjects with a history of dependence on heroin or cocaine. Findings showed that changes in adherence over the 6-month study period were related to changes in neurocognitive functioning. These findings are similar to those recently reported in HIV-infected individuals without significant substance use histories.(Andrade et al., 2013; Ettenhofer et al., 2010) Our study provides an extension of this knowledge to the HIV-infected substance abusers group. Over the six months of observation, monthly adherence rates showed a downward trend. Combined with existing evidence showing that HIV-infected individuals with decline in adherence over time are more likely to be active drug users (Becker, Thames, Woo, Castellon, & Hinkin, 2011), intensified efforts to maximize consistent ART adherence in this population are justified.
Limitations of this study include inconsistent subject followup and lack of longitudinal plasma HIV RNA results to assess adherence over the course of the study. Controlling for other substances also may have strengthened this study, though use of other substances was minimal compared to cocaine. Studies have shown that targeted cognitive and behavioral interventions may improve adherence in HIV-infected drug abusers.(Copenhaver, Lee, Margolin, Bruce, & Altice, 2011; Gross et al., 2013) Further study is warranted in order to potentially bring such therapies into the mainstream of comprehensive HIV care.
Acknowledgments
Sources of support:
NIH K23MH095679
NIH R01DA18066
Footnotes
This research was presented in part as:
Abstract/Poster 80035. 7th International Conference on HIV Treatment and Prevention Adherence. Miami, Florida. June 3–5, 2012.
Financial Disclosure
Each author reports no conflict of interest.
Contributor Information
Melinda K. Higgins, Email: mkhiggi@emory.edu.
Raymond L. Ownby, Email: ro71@nova.edu.
Drenna Waldrop-Valverde, Email: drenna.waldrop-valverde@emory.edu.
References
- Andrade AS, Deutsch R, S AC, Duarte NA, Marcotte TD, Umlauf A, Collier AC. Relationships among neurocognitive status, medication adherence measured by pharmacy refill records, and virologic suppression in HIV-infected persons. J Acquir Immune Defic Syndr. 2013;62(3):282–292. doi: 10.1097/QAI.0b013e31827ed678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50(1):93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav. 2011;15(8):1888–1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver MM, Lee IC, Margolin A, Bruce RD, Altice FL. Testing an optimized community-based human immunodeficiency virus (HIV) risk reduction and antiretroviral adherence intervention for HIV-infected injection drug users. Subst Abus. 2011;32(1):16–26. doi: 10.1080/08897077.2011.540466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010;74(15):1217–1222. doi: 10.1212/WNL.0b013e3181d8c1ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R, Bellamy SL, Chapman J, Han X, O’Duor J, Palmer SC, Strom BL. Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA Intern Med. 2013;173(4):300–306. doi: 10.1001/jamainternmed.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19(4):137–142. [PMC free article] [PubMed] [Google Scholar]
- Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169(7):656–661. [PMC free article] [PubMed] [Google Scholar]


