Abstract
Purpose
We evaluated whether prostate cancer patients receiving “Best Care” according to a set of five nationally endorsed quality measures had decreased treatment related morbidity and improved cancer control.
Methods
In this retrospective cohort study, we included 38,055 men from the Surveillance Epidemiology and End Results–Medicare database treated for localized prostate cancer between 2004 and 2010. For each patient, we determined whether he received “Best Care”, defined as care adherent to all applicable measures. We measured associations of “Best Care” with need for interventions addressing treatment related morbidity and with need for secondary cancer therapy using Cox proportional hazards models.
Results
Only 3,412 men (9.0%) received “Best Care”. Five years after treatment, these men had a similar likelihood as men who did not receive “Best Care” to undergo procedures for urinary morbidity (e.g., 10.7% vs. 12.9%, p=0.338, for the subset of men who underwent prostatectomy) and secondary cancer therapy (e.g., 40.9% vs. 37.3%, p=0.522, for the subset of men who underwent prostatectomy for high-risk prostate cancer). However, they were more likely to have a procedure for sexual morbidity (e.g., 17.3% vs. 10.8%, p<0.001, for men who underwent prostatectomy). Similar trends were observed among men treated with radiotherapy.
Conclusions
Overall, men receiving “Best Care” did not fare better with regards to treatment related morbidity and cancer control. Collectively, our findings suggest that the current process of care measures are not tightly linked to outcomes and that further research is needed to identify better measures that are meaningful and important to patients.
Keywords: prostate cancer, quality of care, outcomes
Introduction
Given the aging population in the United States, the prevalence and costs for prostate cancer care are expected to rise more than 35% during this decade.1 Thus, it is highly important to provide efficient and high-quality prostate cancer care. To assess the quality of prostate cancer care, a group at RAND recommended a set of quality measures in 2000.2 These recommendations were primarily based on expert consensus and a review of the available evidence base. Subsequently, several of these measures have been endorsed by the National Quality Forum (NQF) and the Physician Consortium for Quality Improvement and a few have been incorporated into the Centers for Medicare and Medicaid Services Physician Quality Reporting system.3–5
Despite the development of these measures more than a decade ago, it is not known whether “Best Care” according to these measures is associated with outcomes that are important and meaningful for patients. On the one hand, it is conceivable that patients who receive “Best Care” have better outcomes, as they receive what is currently considered the highest quality of care. On the other hand, the quality measures assess structure and processes of care and the extent to which they are tightly linked to outcomes such as treatment-related morbidity and cancer control remains uncertain.
For this reason, we examined whether patients who received the “Best Care” — defined as care according to all applicable measures out of a set of 5 nationally endorsed structure and process of care measures — have decreased treatment-related morbidity and improved cancer control compared to those not receiving “Best Care”.
Methods
Study population
We identified all men with newly diagnosed localized prostate cancer between 2004 and 2009 within the Surveillance, Epidemiology, and End Results (SEER) – Medicare linked database. Using standard criteria, we limited our study to men 66 years of age and older in the fee-for-service program eligible for Parts A and B of Medicare for at least 12 months before and after prostate cancer diagnosis. Because the quality measures only apply to men treated with radical prostatectomy or radiotherapy,3,4 we only included men who received these treatments. Finally, we excluded patients who could not be assigned a D’Amico prostate cancer risk category (n=5,135) or who had missing information on quality measure adherence for other reasons (e.g., inability to assign a treating provider, n=4,316). Using these criteria, our study population consisted of 38,055 patients who were followed through December 31, 2010.
Defining “Best Care”
As previously described,6 we used the following five nationally endorsed measures to assess quality of care:4,7 (1) was the patient seen by both a urologist and a radiation oncologist between diagnosis and treatment, (2) for patients with low-risk8 cancer, was a non-indicated bone scan avoided, (3) for patients with high-risk8 cancer undergoing radiotherapy, was adjuvant androgen deprivation therapy administered, (4) was the patient treated by a high volume (upper tertile) provider, and (5) did the patient have at least two follow-up visits within one year after treatment with his treating surgeon or radiation oncologist.
Using an “all-or-none” approach,9,10 we categorized a patient as receiving “Best Care”, if all the applicable quality measures were successfully met. Patients who had one or more applicable quality measures not met were categorized as not receiving “Best Care”.
Prostate cancer outcomes
As previously described,11,12 we used well defined algorithms to evaluate (1) treatment-related morbidity (categorized as urinary, sexual, or gastrointestinal) and (2) receipt of secondary cancer therapy. Treatment-related morbidity was defined as morbidity requiring an intervention (e.g., dilation of urethral stricture, procedure to treat incontinence, intracavernosal injections, penile prosthesis implantation, colonoscopy with biopsy or resection, etc.),11 because diagnosis codes alone lack specificity.13 Patients with claims indicating pre-existing urinary (e.g., incontinence, strictures), sexual (e.g., erectile dysfunction), or gastrointestinal (eg., hematochezia, fistulae) morbidity in the year prior to their prostate cancer diagnosis were excluded from analysis of the index morbidity to avoid misclassification of pre-existing conditions.12
Using established methods, we defined receipt of secondary cancer therapy as follows: (1) for patients undergoing primary surgical treatment, receipt of either radiotherapy or hormonal therapy after radical prostatectomy;14 (2) for patients undergoing radiotherapy without concurrent androgen deprivation therapy, receipt of androgen deprivation therapy 6 or more months after completion of radiotherapy; (3) for patients undergoing radiotherapy with concurrent androgen deprivation therapy, receipt of androgen deprivation therapy 36 or more months after completion of radiotherapy.12
Statistical analyses
We first compared patient and regional (from the Area Resource File) characteristics between men who did and did not receive “Best Care” using chi-squared tests. Next, we evaluated risk for treatment-related morbidity and secondary cancer therapy. We modeled these time-dependent outcomes with Cox proportional hazards models. For each patient, we used an indicator of whether or not he had received “Best Care”. We then adjusted these models for all patient and regional covariates noted in Table 1 and used Huber/White sandwich estimators of variance to account for heteroscedasticity. These models were then back-transformed to calculate the adjusted risk for treatment-related morbidity at 5 years, comparing patients who did to those who did not receive “Best Care”.
Table 1.
Patient and regional characteristics overall and according to whether patient received “Best Care” or not.
| “Best care” (column %) | ||||
|---|---|---|---|---|
| N | No | Yes | p | |
| Overall number of patients | 38,055 | 34,643 | 3,412 | |
| Age | <0.001* | |||
| 66–69 | 12,940 | 34.4 | 29.9 | |
| 70–74 | 13,929 | 36.5 | 37.3 | |
| 75–79 | 8,180 | 21.2 | 24.2 | |
| 80–84 | 2,582 | 6.7 | 7.6 | |
| 85+ | 424 | 1.1 | 1.0 | |
| Race | 0.060** | |||
| White | 31,597 | 83.0 | 83.1 | |
| Black | 3,646 | 9.5 | 10.1 | |
| Hispanic | 640 | 1.7 | 1.1 | |
| Asian | 1,031 | 2.7 | 2.7 | |
| Other/Unknown | 1,141 | 3.0 | 3.0 | |
| Comorbidity | 0.269* | |||
| 0 | 25,297 | 66.6 | 65.2 | |
| 1 | 8,586 | 22.5 | 23.4 | |
| 2 | 2,621 | 6.8 | 7.6 | |
| 3+ | 1,551 | 4.1 | 3.8 | |
| Clinical stage | <0.001* | |||
| T1 | 22,447 | 58.8 | 61.2 | |
| T2 | 14,594 | 38.5 | 36.9 | |
| T3/T4*** | 1,014 | 2.7 | 1.9 | |
| Gleason Grade | 0.663* | |||
| ≤6 | 15,613 | 41.2 | 38.8 | |
| 7 | 16,335 | 42.5 | 46.8 | |
| ≥8 | 6,107 | 16.2 | 14.4 | |
| Prostate Specific Antigen | 0.008* | |||
| low (≤10 ng/ml) | 29,066 | 76.3 | 77.4 | |
| intermediate | 6,036 | 15.8 | 16.5 | |
| high (>20 ng/ml) | 2,953 | 7.9 | 6.1 | |
| D’Amico risk | <0.001** | |||
| Low | 11,758 | 31.3 | 27.3 | |
| Intermediate | 15,144 | 38.9 | 48.6 | |
| High | 11,153 | 29.8 | 24.1 | |
| Year of diagnosis | 0.019* | |||
| 2004 | 6,738 | 17.9 | 15.6 | |
| 2005 | 6,332 | 16.7 | 16.3 | |
| 2006 | 6,831 | 17.8 | 19.6 | |
| 2007 | 7,130 | 18.7 | 18.9 | |
| 2008 | 6,264 | 16.4 | 16.7 | |
| 2009 | 4,760 | 12.5 | 12.9 | |
| 25% or more of adults in census tract with a college education | 18,477 | 48.2 | 53.2 | <0.001* |
| Median annual household income of census tract | <0.001* | |||
| Low (≤$40,784) | 12,484 | 33.7 | 24.7 | |
| Intermediate | 12,654 | 33.2 | 34.7 | |
| High (≥$59,938) | 12,855 | 33.2 | 40.6 | |
| Residing in urban area | 34,465 | 90.1 | 95.6 | <0.001* |
| Number of urologists per 100,000 men 65 and over | <0.001* | |||
| Low (≤52) | 12,127 | 32.5 | 25.7 | |
| Intermediate | 11,677 | 30.4 | 33.3 | |
| High (≥87) | 14,247 | 37.1 | 41.0 | |
| Number of radiation oncologists per 100,000 men 65 and over | 0.429* | |||
| Low (≤21) | 12,394 | 32.5 | 33.7 | |
| Intermediate | 12,200 | 32.2 | 30.8 | |
| High (≥37) | 13,457 | 35.4 | 35.5 | |
| Number of hospital beds per 100,000 men 65 and over | 0.528* | |||
| Low (≤4,735) | 12,004 | 32.1 | 26.2 | |
| Intermediate | 12,790 | 32.6 | 43.4 | |
| High (≥6,854) | 13,257 | 35.3 | 30.4 | |
| Medicare managed care penetration | <0.001* | |||
| Low (≤4.8%) | 12,565 | 33.6 | 27.6 | |
| Intermediate | 12,685 | 31.8 | 49.3 | |
| High (≥16.0%) | 12,801 | 34.7 | 23.1 | |
| Treatment received | <0.001** | |||
| Open prostatectomy | 5,531 | 15.8 | 1.4 | |
| Robotic prostatectomy | 4,496 | 12.2 | 7.5 | |
| Brachytherapy | 12,903 | 29.9 | 74.4 | |
| IMRT | 13,004 | 36.0 | 15.5 | |
| EBRT | 2,121 | 6.0 | 1.1 | |
Mantel-Haenszel chi-squared test;
Chi-squared test;
T3 and T4 were combined, because the SEER-Medicare data use agreement stipulates that cells with <11 cases must not be derivable (<100 patients had T4 disease).
Because time to treatment-related morbidity and to secondary cancer therapy depends on the primary treatment received, we fitted three separate models for each outcome: (1) only including prostatectomy patients, (2) only including patients receiving radiotherapy without concurrent androgen-deprivation therapy, and (3) only including patients receiving radiotherapy with concurrent androgen-deprivation therapy. These models were then further sub-stratified by type of prostatectomy (open or minimally invasive) or type of radiotherapy (external-beam, intensity-modulated, or brachytherapy with or without external radiotherapy boost).15 In order to evaluate need for secondary cancer therapy according to prostate cancer risk, we fitted separate models including only low-risk or high-risk patients (according to the D’Amico risk classification8).
We performed all analyses using Stata (Version 12SE) and SAS (Version 9.3) and considered p≤0.05 as statistically significant. The University of Michigan Medical School Institutional Review Board exempted this study from review.
Results
Overall, only 3,412 men (9.0%) received “Best Care”. Those receiving “Best Care” were more likely to be older and to have intermediate-risk disease (Table 1). Patients receiving “Best Care” were more likely to live in a census tract with a higher proportion of college educated adults and with a higher median household income (Table 1). In addition, these patients were more likely to live in a region with higher urologist density. Finally, the distribution of treatments differed by receipt of “Best Care”. Specifically, as the quality measures value close collaboration between urologists and radiation oncologists, brachytherapy was significantly more common among men receiving “Best Care” (Table 1).
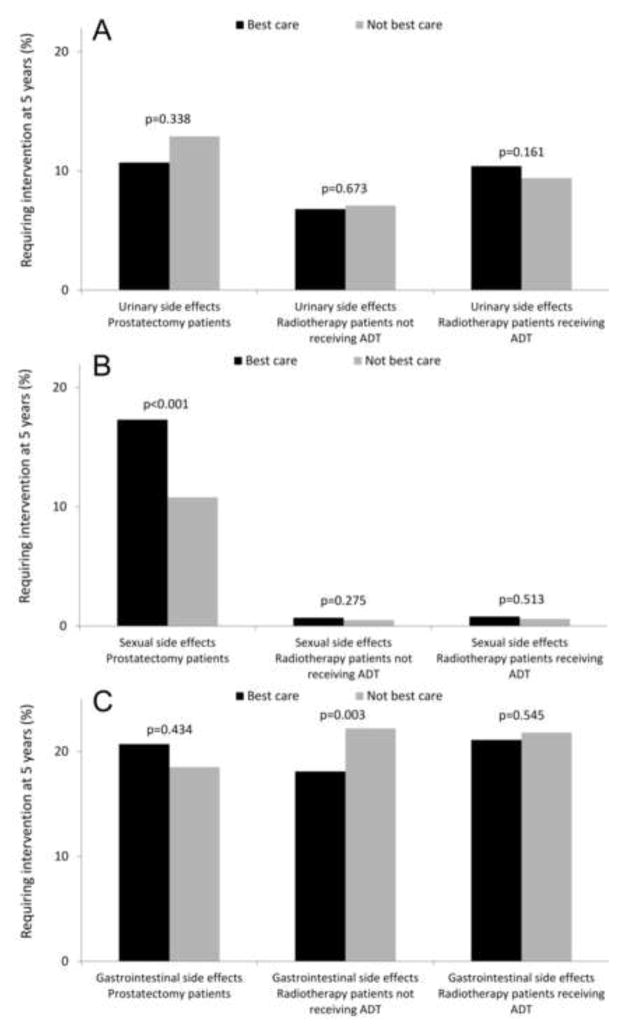
When evaluating the association between receipt of “Best Care” and treatment-related morbidity, our findings were mixed. “Best care” was not associated with the risk of requiring an intervention for urinary morbidity (Figure 1A). However, prostatectomy patients receiving “Best Care” were more likely to receive an intervention for treatment of sexual morbidity (17.3% vs. 10.8%, p<0.001, Figure 1B), while among men undergoing radiotherapy without concurrent androgen-deprivation therapy, “Best Care” was associated with a lower risk for gastrointestinal morbidity (18.1% vs. 22.2%, p=0.003, Figure 1C).
Figure 1.
Association of receipt of “Best Care” with need for an intervention addressing urinary (panel A), sexual (panel B), or gastrointestinal (panel C) morbidity of prostate cancer treatment at 5 years. All values are back transformed from Cox regression models and adjusted for the covariates shown in Table 1. ADT: androgen-deprivation therapy.
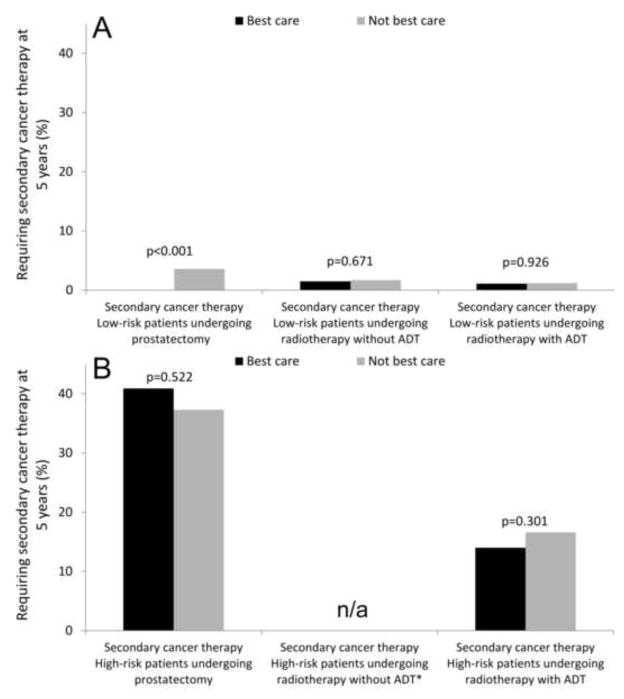
For most men, “Best Care” was not associated with a significantly lower risk of requiring secondary cancer therapy (Figure 2). For example, among high-risk patients undergoing radiotherapy with adjuvant androgen deprivation, 14.0% of those receiving “Best Care” underwent a secondary cancer therapy compared to 16.6% of those not receiving “Best Care” (p=0.301). One notable exception to this were men undergoing prostatectomy for low-risk cancer. In this subgroup, none of the men receiving “Best Care” required secondary cancer therapy, while a small proportion of men not receiving “Best Care” (3.6%) did require such an intervention at 5 years (p<0.001, Figure 2B).
Figure 2.
Association of receipt of “Best Care” with secondary cancer therapy at 5 years among prostate cancer patients with low-risk (panel A) and high-risk (panel B) disease. All values are back transformed from Cox regression models and adjusted for the covariates shown in Table 1. * Receipt of radiotherapy without concurrent androgen-deprivation therapy (ADT) for high-risk disease is not adherent to quality measures and therefore no patients in this group received “Best Care”.
Discussion
We evaluated whether patients receiving “Best Care” according to a set of nationally endorsed quality measures have better outcomes than those not receiving “Best Care”. We found that only a minority of patients (9.0%) received “Best Care”, defined as care adherent to all applicable quality measures. Men receiving “Best Care” were older, more likely to harbor intermediate-risk disease, and tended to be of higher socioeconomic status. When examining the association between receipt of “Best Care” and outcomes, our findings were mixed. “Best Care” was associated with a decreased risk for gastrointestinal morbidity among radiotherapy patients not receiving concurrent androgen-deprivation therapy and with a slightly decreased risk for secondary cancer therapy among prostatectomy patients with low-risk cancer. However, for most patient groups and outcomes, “Best Care” was not associated with better outcomes.
While some may feel that the quality measures evaluated in this study represent a low bar and are easy to achieve, the fact that only nine percent of patients received “Best Care” underlines how difficult it can be to consistently adhere to recommended processes of care. For each patient, there may be multiple factors at multiple levels potentially deterring from receipt of “Best Care”. For example, patients with low-risk cancer may have a high-level of anxiety and demand a bone scan to rule out metastatic disease. At the physician-level, not all providers may be aware of current guideline recommendations. At the clinic or facility level, it may be difficult to effectively coordinate care between urologists and radiation oncologists if these physicians practice in different locations or not within the same integrated delivery system. Thus, efforts to increase the number of patients receiving “Best Care” will likely require multi-level interventions.16
However, prior to implementing multi-level quality improvement efforts, it appears to be worthwhile to consider how relevant the current quality measures are for outcomes that are meaningful and important for patients. This is the first study to assess whether individual patients receiving “Best Care” according to nationally endorsed measures have better outcomes. In a prior study, we assessed healthcare system performance on these measures and found that patients treated in healthcare systems with higher performance do not necessarily have better outcomes.11 We now expand on these findings by evaluating whether individual patients who received the “Best Care” actually fared better with regards to treatment related morbidity and cancer control. In the current study, we do not find any consistent associations between “Best Care” and outcomes, which is in line with our previous findings at the healthcare system level.
There are several potential reasons for these findings. First, the quality measures used in this study are primarily process measures of care that do not necessarily have to be tightly linked to the outcomes evaluated. For example, whether a patient is counseled by both a radiation oncologist and a urologist may not make a difference in his cancer control or treatment-related morbidity. Second, the outcomes that could be evaluated in this study were limited, given the nature of claims data. It is entirely possible, that receipt of “Best Care” does matter for other outcomes which are important for patients but could not be evaluated in the current study. For instance, thorough pre-treatment counseling and post-treatment follow-up may increase satisfaction with treatment and decrease regret of treatment choice.17
It is noteworthy that men with prostate cancer receiving “Best Care” were of higher socioeconomic status. This is in line with previous reports indicating that access to high-quality cancer care varies by socioeconomic status.18 For example, women with early-stage breast cancer were significantly less likely to receive breast conserving therapy if they resided in poorer census tracts.19 Similarly, patients of lower socioeconomic status with prostate, bladder, or kidney cancer were significantly less likely to have their surgery performed at a high-volume center than those of higher socioeconomic status.20 Thus, our findings are in line with previous work indicating that socioeconomic status is linked to the quality of cancer care.
Despite these findings, there are several limitations that warrant discussion. First, it is harder for low-risk and high-risk patients to receive “Best Care” than for intermediate-risk patients, because some of the quality measures specifically apply to low-risk or high-risk patients. This is reflected in the data, as intermediate-risk disease was significantly more common among men receiving “Best Care” compared to those not receiving “Best Care”. However, we accounted for this fact by adjusting all models for cancer risk and by evaluating need for secondary cancer therapy separately among low- and high-risk patients. Second, the current analyses are based on claims data, which have their own well described limitations.21 For example, we were only able to identify interventions for treatment related morbidity, but did not have detailed quality of life data. As such, it is hard to judge whether more interventions for sexual dysfunction reflect a higher occurrence rate of this morbidity or a more aggressive treatment approach chosen by patients or their physicians. It is entirely plausible that physicians who provide “best care” have a more aggressive treatment approach towards sexual dysfunction which could explain some of our findings. In addition, the claims data did not allow us to determine whether urinary, sexual, and gastrointestinal quality of life had been measured, a process of care RAND had recommended as a potential quality indicator. Third, the volume measure was based on the number of Medicare beneficiaries treated. However, previous work has shown high correlation between surgical volume measured in Medicare and overall surgical volume (r=0.91).22 Lastly, generalizability of our findings is limited by the fact that only men older than 65 were included. However, prostate cancer is very common in the Medicare population (more than half of all prostate cancer diagnoses occur in men older than 65)23 and quality measurement and improvement are high-priority topics within the Centers for Medicare & Medicaid Services.24
These limitations notwithstanding, our study has important implications. We show that among men who receive the “Best Care”, defined by adherence to a set of nationally endorsed quality measures, outcomes are not necessarily better than among men not receiving “Best Care”. This implies that the current quality measures – while relatively easy to capture in claims data – may not be of high importance for patients. Therefore, further research is needed to identify better measures which are more tightly linked to outcomes or clearly meaningful to patients.
Acknowledgments
Grant support: FRS is supported in part by Postdoctoral Fellowship PF-12-118-01-CPPB from the American Cancer Society. BLJ is supported in part by grant KL2 TR000146 from the NIH. BKH is supported in part by Research Scholar Grant RSGI-13-323-01-CPHPS from the American Cancer Society.
Abbreviations
- ADT
androgen deprivation therapy
- NQF
National Quality Forum
- SEER
Surveillance Epidemiology and End Results
Footnotes
Disclaimer: The views expressed in this article do not necessarily reflect the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwin M, Steinberg M, Malin J, et al. [accessed October 7, 2013];Prostate Cancer Patient Outcomes and Choice of Providers: Development of an Infrastructure for Quality Assessment. 2000 Available at: http://www.rand.org/pubs/monograph_reports/MR1227.html.
- 3.National Quality Forum (NQF): NQF-endorsed standards. [accessed January 26, 2013];2012 Available at: www.qualityforum.org/QPS/QPSTool.aspx?Exact=false&Keyword=prostatecancer.
- 4.Thompson IM, Clauser S. [accessed September 13, 2011];Prostate Cancer Physician Performance Measurement Set. 2007 Available at: http://www.ama-assn.org/apps/listserv/x-check/qmeasure.cgi?submit=PCPI.
- 5.Centers for Medicare & Medicaid Services: Physician Quality Reporting System. Measure Specifications Manual for Claims and Registry Reporting of Individual Measures. 2011 Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/downloads/2011_PhysQualRptg_MeasureSpecificationsManual_033111.pdf.
- 6.Schroeck FR, Kaufman SR, Jacobs BL, et al. Regional Variation in Quality of Prostate Cancer Care. J Urol. 2014;191:957–963. doi: 10.1016/j.juro.2013.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer BA, Steinberg M, Malin J, et al. Quality-of-care indicators for early-stage prostate cancer. J Clin Oncol. 2003;21:1928–1936. doi: 10.1200/JCO.2003.05.157. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 9.Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295:1168–1170. doi: 10.1001/jama.295.10.1168. [DOI] [PubMed] [Google Scholar]
- 10.Reeves D, Campbell SM, Adams J, et al. Combining multiple indicators of clinical quality: an evaluation of different analytic approaches. Med Care. 2007;45:489–496. doi: 10.1097/MLR.0b013e31803bb479. [DOI] [PubMed] [Google Scholar]
- 11.Schroeck FR, Kaufman SR, Jacobs BL, et al. Adherence to Performance Measures and Outcomes among Men Treated for Prostate Cancer. J Urol. 2014 doi: 10.1016/j.juro.2014.03.091. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs BL, Zhang Y, Skolarus TA, et al. Comparative Effectiveness of External-Beam Radiation Approaches for Prostate Cancer. Eur Urol. 2014;65:162–168. doi: 10.1016/j.eururo.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy EP, Iezzoni LI, Davis RB, et al. Does clinical evidence support ICD-9-CM diagnosis coding of complications? Med Care. 2000;38:868–876. doi: 10.1097/00005650-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Hu JC, Gu X, Lipsitz SR, et al. Comparative Effectiveness of Minimally Invasive vs Open Radical Prostatectomy. JAMA. 2009;302:1557–1564. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 15.Cleves M, Gutierrez R, Gould W, et al. An introduction to survival analysis using Stata. 2. Stata Press; 2008. [Google Scholar]
- 16.Zapka J, Taplin SH, Ganz P, et al. Multilevel Factors Affecting Quality: Examples From the Cancer Care Continuum. J Natl Cancer Inst Monogr. 2012;2012:11–19. doi: 10.1093/jncimonographs/lgs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry DL, Wang Q, Halpenny B, et al. Decision preparation, satisfaction and regret in a multi-center sample of men with newly diagnosed localized prostate cancer. Patient Educ Couns. 2012;88:262–267. doi: 10.1016/j.pec.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward E, Jemal A, Cokkinides V, et al. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA: A Cancer Journal for Clinicians. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 19.Singh GK, Miller BA, Hankey BF, et al. Area socioeconomic variations in US cancer incidence, mortality, stage, treatment, and survival, 1975–1999. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda (MD): 2003. [accessed April 8, 2014]. Available at: http://open-dev.umms.med.umich.edu/sites/default/files/1232/reading-resources-1/Singh-fullarticle.pdf. [Google Scholar]
- 20.Trinh Q-D, Sun M, Sammon J, et al. Disparities in access to care at high-volume institutions for uro-oncologic procedures. Cancer. 2012;118:4421–4426. doi: 10.1002/cncr.27440. [DOI] [PubMed] [Google Scholar]
- 21.Finlayson E, Birkmeyer JD. Research based on administrative data. Surgery. 2009;145:610–616. doi: 10.1016/j.surg.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 23.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009. Bethesda, MD: National Cancer Institute; 2012. [accessed November 27, 2012]. Available at: http://seer.cancer.gov/csr/1975_2009_pops09. [Google Scholar]
- 24.Centers for Medicare & Medicaid Services: Quality Initiatives - General Information. [accessed April 10, 2014];2014 Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/index.html.