Abstract
Background
Cognitive impairment is common in hemodialysis patients and associated with significant morbidity. Limited information exists on whether cognitive impairment is associated with survival, and whether type of cognitive impairment is important.
Study Design
Longitudinal cohort.
Setting & Participants
Cognitive function was assessed at baseline and yearly using a comprehensive battery of cognitive tests in 292 prevalent hemodialysis patients.
Predictor
Using principal component analysis, individual test results were reduced into 2 domain scores, representing memory and executive function. By definition, each score carried a mean of 0 and SD of 1.
Outcomes
Association of each score with all-cause mortality was assessed using Cox proportional hazards models adjusted for demographics as well as dialysis and cardiovascular (CV) risk factors.
Results
Mean age of participants was 63 years, 53% were male, 23% were African American and 90% had at least a high school education. During median follow up of 2.1 (IQR, 1.1–3.7) years, 145 deaths occurred. Each 1-SD better executive function score was associated with 35% lower hazard of mortality (HR, 0.65; 95% CI, 0.55–0.76). In models adjusting for demographics and dialysis-related factors, this relationship was partially attenuated but remained significant (HR, 0.81; 95% CI, 0.67–0.98), while adjustment for CV disease and heart failure further attenuated it (HR, 0.87; 95% CI, 0.72–1.06). Use of time-dependent models showed a similar unadjusted association (HR, 0.62; 95% CI, 0.54–0.72), with the relationship remaining significant after adjustment for demographics, dialysis, and CV risk factors (HR, 0.79; 95% CI, 0.66–0.94). Better memory was associated with lower mortality in univariate analysis (HR per 1 SD, 0.82 [95% CI, 0.69–0.96]), but not when adjusting for demographics (HR, 1.00; 95% CI, 0.83–1.19).
Limitations
Patients with dementia were excluded from the full battery, perhaps underestimating strength of the association.
Conclusions
Worse executive function and memory are associated with increased risk of mortality. For memory, this association is explained by patient demographics, while for executive function this relationship may be partly explained by CV disease burden.
Keywords: cognition, cognitive impairment, executive function, memory, neurocognitive testing, cardiovascular disease, mortality, hemodialysis, end-stage renal disease (ESRD)
Cognitive impairment is common among patients treated with maintenance hemodialysis.1,2 Reflecting an aging dialysis population with increased comorbidity, the burden of cognitive impairment in the dialysis population may continue to rise, potentially affecting various areas of patient care, such as patient adherence to treatment plans and quality of life.3,4 Hemodialysis patients have impairment in multiple domains of cognitive function, although executive function, which is critical to planning and carrying out tasks, appears to be more affected than other domains such as memory.5 While the etiology of cognitive impairment in dialysis patients appears to be multi-factorial,1 cerebrovascular disease likely is an important cause,6 as cerebrovascular disease more often affects brain structures related to executive function.7
Mortality among dialysis patients is high, in part due to the high prevalence of comorbid conditions, including cardiovascular disease.8 In individuals without kidney disease, cognitive impairment is independently associated with increased mortality9,10, with both executive and memory dysfunction contributing to the increased risk.11,12 Advanced dementia, classified based on administrative codes or medical chart review and therefore likely only identifying individuals with severe disease, is also a risk for increased mortality in dialysis patients;13,14 however, few studies have explored whether more moderate deficits in cognitive function are associated with mortality in hemodialysis patients and, if so, whether memory, executive function, or both are complicit.
Therefore, we assessed cognitive performance using a detailed neuro-cognitive battery of tests that maps to multiple cognitive domains and examined the association between cognitive performance and mortality in a cohort of prevalent maintenance hemodialysis patients.
Methods
Patient Characteristics
Outpatients aged 18 years or older receiving maintenance in-center hemodialysis at five Dialysis Clinic Inc (DCI) units and one hospital-based unit (St. Elizabeth’s Medical Center) in the greater Boston area were screened for the Cognition and Dialysis Study1 with study enrollment occurring from January 28 2004 through May 31 2012. Reflecting the nature of the cognitive test battery, eligibility criteria included English fluency as well as sufficient visual and hearing acuity to complete neurocognitive testing. To minimize floor effects and reflecting inability to provide consent, individuals with MMSE score ≤10 and/or advanced dementia based on medical record review were excluded. Temporary exclusion criteria included non–access-related hospitalization within 1 month, receipt of hemodialysis for less than 1 month, and single-pool Kt/V <1.0. The Tufts Medical Center/Tufts University Institutional Review Board approved the study, and all participants who completed the detailed cognitive testing signed informed consent.
Baseline Demographics and Clinical Characteristics
Demographic, clinical and laboratory factors were ascertained at the time of cognitive testing. Demographic data (age, sex, and race) were obtained via participant report, medical charts, and the DCI and St. Elizabeth’s Medical Center databases. Education (<12th grade, high school graduate to less than 2 years of college, and ≥2 years of college) was obtained via patient questionnaire. Medical history, including history of cardiovascular disease (a composite of either coronary artery disease and/or peripheral vascular disease), stroke, heart failure, diabetes, hypertension, and smoking, were defined by patient history or documentation in the patient’s electronic or paper chart. Patients were queried about personal history of myocardial infarction and coronary revascularization (which were used to define coronary disease) and intermittent claudication and peripheral vascular disease (which were used to define peripheral vascular disease). Additionally, DCI electronic medical and paper records were reviewed for a history of these conditions, with specific focus on problem lists, hospital discharge summaries, cardiac test results, and procedure results. Similarly, stroke was defined using patient history or documentation in the patient’s electronic or paper chart. Cause of end-stage renal disease and dialysis vintage were obtained from the DCI or St. Elizabeth’s electronic record, as were mean monthly systolic and diastolic blood pressures and body mass index (BMI). Medication lists from the time of cognitive testing were obtained from DCI electronic records. Pre-dialysis blood tests included serum albumin, hematocrit, phosphorus, intact parathyroid hormone, and white blood cell count. High-sensitivity C-reactive protein was measured at a later date from frozen stored samples obtained at study enrollment. Single-pool Kt/V was calculated using blood urea nitrogen before and after cognitive testing. All DCI laboratory tests were measured in a central laboratory in Nashville, TN.
Neurocognitive Assessment
At study enrollment, participants were administered a battery of cognitive tests by research assistants following training and direct observation by the study neuropsychologist (T.S.). The same battery of tests was administered yearly to study participants whenever possible. To maintain quality and inter-rater reliability, testing was observed by the study neuropsychologist at 3- to 6-month intervals. To limit subject fatigue, all testing was completed during the first hour of hemodialysis. Additionally, in a prior study using the same battery of tests, we demonstrated similar performance regardless of whether testing was performed during the first hour of dialysis or before the start of a dialysis session.15 When possible, neurocognitive testing was performed in a private room or in as quiet an environment as possible. The neurocognitive battery included well-validated commonly used cognitive tests that possess high inter- and intra-rater reliability and often have established age-, sex-, and/or education-matched normative scores. The MMSE16 was used as a screening test and the North American Adult Reading Test (NAART) served as a measure of premorbid verbal IQ.17 The neurocognitive battery consisted of the Wechsler Memory Scale III (WMS-III) Word List Learning Subtest,18 the Wechsler Adult Intelligence Scale III (WAIS-III) Block Design18 and Digit Symbol-Coding Subtests,18 and Trail Making Tests A and B19 (Trails A and B). For the Trails B test, a 300 second time limit was imposed, with those unable to complete the test during this time period considered “non-completers”. During the last two years of the study, the cognitive panel was expanded to include additional verbal tests assessing both memory and executive functions, including Digit Span (forwards and backwards),18 the Mental Alternation Test,20 and the Controlled Oral Word Association Test (COWAT).21 The overall battery assesses a broad range of functioning including global ability, supraspan learning, auditory retention, visual retention, attention/mental processing speed, visual construction/fluid reasoning, and motor speed.
Principal component analysis with varimax rotation was used as a data reduction technique to derive composite scores for separate cognitive domains in the entire study population.22 For 14 individuals with missing scores on one cognitive test (up to 2 scores if derived from the same test), imputation was performed, incorporating linear regression models based on results of the available cognitive tests. These results were incorporated into the data for principal components analysis. Two principal components with eigenvalues greater than 2 were obtained, and the resulting component scores subsequently were used for primary statistical analyses. Using this method, all component scores have a mean of 0 and standard deviation (SD) of 1. The first component was interpreted to reflect executive functioning, attention, and processing speed (referred to as executive function in the Results section), with the Trails A and B, Block Design, and Digit Symbol-Coding tests contributing significantly (Table S1, available as online supplementary material). The second component primarily was composed of Word List Learning Recall and Recognition and was interpreted to reflect memory. Formulas for deriving the principal component score at the baseline examination were used to calculate the principal component scores for follow-up testing. Digit Span, mental alternations and the COWAT were not used to calculate the principal component analysis because of the smaller number of individuals who completed these tests.
Study Outcome
The primary outcome was all-cause mortality. We obtained survival status on all patients through periodic electronic medical record monitoring as well as contact with each patient’s dialysis unit. Survival time was defined as the period of time elapsed from initial study enrollment until death, kidney transplantation, or March 31, 2013.
Statistical Analysis
Descriptive characteristics of the study population were reported as proportions for categorical and binary variables, means with standard deviations for continuous normally distributed variables, and medians with inter-quartile ranges for skewed variables. To better assess differences by level of cognitive function, the study population was sorted into equally sized quartiles by factor score for both memory and executive function. Linear trends across quartiles were assessed using linear regression for continuous variables and the Cochrane-Armitage test for binary variables. Differences between categorical variables were assessed using Chi-squared tests. Mortality rates were calculated by taking the number of deaths in each group divided by the total accrued person-time in each group.
Mortality rates were then adjusted for age using Poisson regression. Kaplan-Meier plots for time to death were constructed by quartiles of executive or memory function. Log-rank test was used to test differences in survival between the quartiles. Cox proportional hazards regression models were used to assess the association between memory and executive function and all-cause mortality, with nested models adjusting for demographics (age, sex, race, and education)—model 1; additional adjustment for dialysis-related factors (cause of end-stage renal disease [diabetes vs other], dialysis vintage, dialysis access type, serum albumin, diastolic blood pressure and Kt/V)—model 2; additional adjustment for cardiovascular disease (CVD; either coronary artery disease or peripheral vascular disease) and heart failure—model 3; and finally adjustment for stroke—model 4. To assess for differences by CVD status, an interaction term for executive/memory score and CVD was included in the final multivariable model. Additionally, as cognitive function was assessed longitudinally at one year increments, Cox regression models with the same covariates described above were created using principal component score (memory or executive function) as a time-dependent variable. All models evaluated memory and executive function as a continuous variable per standard deviation change. In secondary analyses, the association between performance on individual neurocognitive tests and mortality was assessed. For Trails B, scores were sorted into four ordered categories: non-completers and completers stratified into three equal sized tertiles. Using the same models described above, the relationship between the Trails B categories and mortality was then determined. All analyses were performed using SAS, version 9.2 (SAS Institute Inc, Cary, NC).
Sensitivity Analyses
As patients reported to have a history of dementia or MMSE score of 10 or lower were excluded from entry into the study, we obtained survival status on this subgroup to allow for comparison with our study population. Mortality rates were calculated using the methods described above, both unadjusted and adjusted for age. As depression may be associated with both decreased cognitive function and mortality, we included adjustment23 for a Center for Epidemiologic Studies Depression (CES-D) Scale score > 16, which was obtained at study enrollment. Finally, we also included adjustment for use of psychotropic medications (yes or no), including use of benzodiapenes, antidepressants, and antihistamines.
Results
Study Participants
Three hundred fourteen patients met inclusion criteria and had cognitive testing performed, with 292 having sufficient data to create memory and executive factors.1 The mean age of the study population was 63.1 ± 16.7 (SD) years, 53% were male, 23% percent African American and 90% had a high school education or higher. Sixty-four percent had an arteriovenous fistula at baseline as their form of vascular access, while diabetes was the most common cause of end-stage renal disease (35%).
Patients with better executive functioning (higher score) were more likely to be younger and have a higher education level and diastolic blood pressure, but less likely to have diabetes, heart failure, stroke, and cardiovascular disease (Table 1). Similar trends were seen across memory quartiles. In addition, women performed better on tests of memory (Table S2).
Table 1.
Baseline Demographics and Clinical Characteristics by Quartiles of Executive Performance
| Q1 (n = 73) | Q2 (n = 73) | Q3 (n = 73) | Q4 (n = 73) | P for Trend | |
|---|---|---|---|---|---|
| Executive factor | −1.16 ± 0.65 | −0.21 ± 0.16 | 0.32 ± 0.14 | 1.06 ± 0.34 | NA |
| Age (y) | 73.0 ± 11.1 | 67.9 ± 14.7 | 59.5 ± 16.0 | 52.0 ± 16.4 | <0.001 |
| Female sex | 30 (41%) | 39 (53%) | 40 (55%) | 27 (37%) | 0.7 |
| Black race | 14 (19%) | 18 (25%) | 19 (26%) | 15 (21%) | 0.8 |
| Education | 0.001 | ||||
| < HS | 13 (18%) | 8 (11%) | 7 (10%) | 1 (1%) | |
| HS to <2 y college | 38 (52%) | 47 (64%) | 38 (52%) | 39 (53%) | |
| ≥2 y college | 22 (30%) | 18 (25%) | 28 (38%) | 33 (45%) | |
| Hypertension | 69 (95%) | 67 (92%) | 66 (90%) | 61 (84%) | 0.03 |
| Heart failure | 34 (47%) | 32 (44%) | 25 (34%) | 14 (19%) | <0.001 |
| Stroke | 18 (25%) | 14 (19%) | 13 (18%) | 6 (8%) | 0.01 |
| CVD | 46 (63%) | 44 (60%) | 27 (37%) | 13 (18%) | <0.001 |
| Primary cause of ESRD | <0.001 | ||||
| Diabetes | 32 (44%) | 30 (41%) | 26 (36%) | 13 (18%) | |
| Hypertension | 19 (26%) | 18 (25%) | 13 (18%) | 6 (8%) | |
| Other | 5 (7%) | 14 (19%) | 12 (16%) | 18 (25%) | |
| Unknown | 11 (15%) | 5 (7%) | 11 (15%) | 10 (14%) | |
| Glomerulonephritis | 6 (8%) | 6 (8%) | 11 (15%) | 26 (36%) | |
| Smoking History | 0.2 | ||||
| Never | 25 (34%) | 26 (36%) | 23 (32%) | 38 (52%) | |
| Past | 41 (56%) | 38 (53%) | 41 (56%) | 31 (42%) | |
| Current | 7 (10%) | 8 (11%) | 9 (12%) | 4 (5%) | |
| Dialysis Access | 0.7 | ||||
| Fistula | 46 (63%) | 41 (56%) | 51 (70%) | 48 (66%) | |
| Graft | 6 (8%) | 3 (4%) | 4 (5%) | 5 (7%) | |
| Catheter | 21 (29%) | 29 (40%) | 18 (25%) | 20 (27%) | |
| Systolic BP* (mmHg) | 141.1 ± 21.1 | 138.5 ± 20.7 | 144.1 ± 21.0 | 141.5 ± 20.2 | 0.8 |
| Diastolic BP* (mmHg) | 71.7 ± 10.4 | 70.1 ± 11.3 | 75.1 ± 12.9 | 76.9 ± 12.8 | 0.001 |
| Single-pool Kt/V | 1.50 ± 0.24 | 1.49 ± 0.23 | 1.53 ± 0.24 | 1.52 ± 0.25 | 0.5 |
| Body Mass Index (kg/m2) | 27.6 ± 5.8 | 28.7 ± 6.2 | 28.1 ± 7.2 | 27.6 ± 6.6 | 0.5 |
| Hematocrit (%) | 35.4 ± 4.2 | 35.6 ± 3.2 | 35.7 ± 3.5 | 35.8 ± 3.3 | 0.8 |
| Albumin (mg/dL) | 3.8 ± 0.4 | 3.8 ± 0.3 | 3.8 ± 0.4 | 3.9 ± 0.4 | 0.06 |
| Phosphorus (mg/dL) | 5.4 ± 1.5 | 5.3 ± 1.6 | 5.4 ± 1.4 | 5.7 ± 1.5 | 0.1 |
| Dialysis vintage (mo) | 14.9 [8.9–32.8] | 14.2 [5.5–35.4] | 15.4 [6.5–32.5] | 13.5 [7.0–48.3] | 0.9 |
| WBC (103/μL) | 7.2 ± 2.2 | 7.9 ± 2.3 | 7.4 ± 2.4 | 7.3 ± 2.4 | 0.5 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Conversion factor for phosphorus in mg/dL to mmol/L, ×0.3229.
Abbreviations and definitions: HF = heart failure, CVD=Cardiovascular disease and is a composite of coronary artery disease and/or peripheral vascular disease, ESRD=end-stage renal disease, BP=blood pressure, WBC=white blood cell count; NA, not applicable; Q, quartile
Before dialysis.
Ouctomes
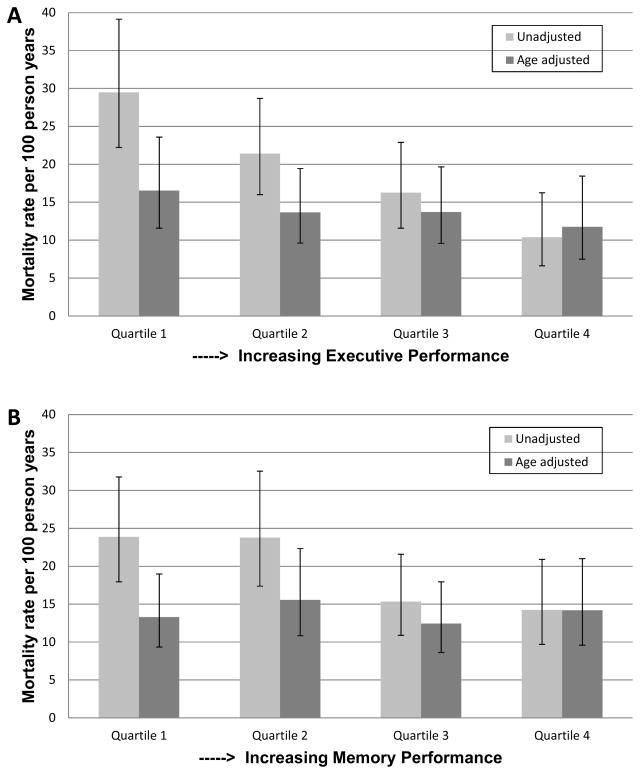
During median follow up of 2.1 (interquartile range, 1.1–3.7) years, 196 participants were re-administered the cognitive battery of tests at least one additional time (median number of visits, 3). During this time period, 145 deaths were observed, yielding an event rate of 19.1 deaths per 100 person-years. When stratified by quartiles of executive performance, mortality rates increased with worse executive function, such that the lowest executive function quartile had a mortality rate three times that of the highest quartile (29.4 vs 10.4 deaths per 100 person-years, respectively) (Figure 1A). This difference was partially attenuated when adjusting for age (16.5 vs 11.8 deaths per 100 person-years in highest versus lowest quartile). In contrast, the relationship between quartiles of memory and all-cause mortality was fully attenuated following adjustment for age (Figure 1B).
Figure 1.
Figures 1A and 1B Unadjusted and age adjusted mortality rates by quartiles of executive and memory function. * = significant trend p value
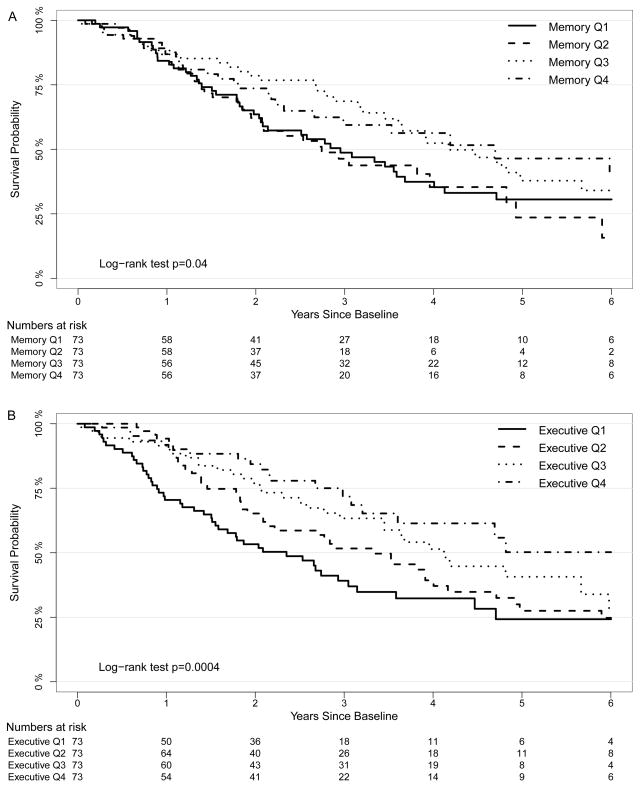
Kaplan-Meier plots stratified by quartiles of baseline cognitive function demonstrated an association between better executive function and memory and all-cause mortality (global log-rank p <0.001 and p = 0.04 respectively) (Figures 2A and 2B). Using Cox proportional hazards models, each 1-SD higher baseline executive score was associated with a 35% lower hazard for mortality (hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.55–0.76) (Table 2). Following adjustment for age, sex, race and education (model 1), the association was somewhat reduced but remained significant (HR, 0.78; 95% CI, 0.65–0.94). An expanded model with further adjustment for cause of end-stage renal disease, dialysis vintage, access type, serum albumin, diastolic blood pressure, and Kt/V (model 2) yielded similar findings (HR, 0.81; 95% CI, 0.67–0.98). Further adjustment for a baseline history of cardiovascular disease and heart failure attenuated the relationship (HR, 0.87; 95% CI, 0.72–1.06), while additional adjustment for previous stroke resulted in no further change (HR, 0.87; 95% CI, 0.72–1.06). Better baseline memory function was associated with decreased mortality in univariate analysis (HR per 1-SD higher score, 0.82; 95% CI, 0.69–0.96). This relationship was eliminated following adjustment for demographics (model 1: HR, 1.00; 95% CI, 0.83–1.19). In final multivariable models, there was no interaction between either executive function or memory with CVD status and mortality (p values each > 0.9).
Figure 2.
Figures 2A and 2B Kaplan-Meier plots by quartiles of executive and memory function. A global p-value is provided (log rank test) for each plot.
Table 2.
Association between Executive and Memory Performance and All-Cause Mortality
| Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI]) | p | |
| Executive* | 0.65 [0.55, 0.76] | <0.001 | 0.78 [0.65, 0.94] | 0.008 | 0.81 [0.67, 0.98] | 0.03 | 0.87 [0.72, 1.06] | 0.2 | 0.87 [0.72, 1.06] | 0.2 |
| Executive time dependent | 0.62 [0.54, 0.72] | <0.001 | 0.74 [0.62, 0.88] | 0.001 | 0.75 [0.63, 0.89] | 0.001 | 0.79 [0.66, 0.94] | 0.008 | 0.79 [0.66, 0.94] | 0.01 |
| Memory* | 0.82 [0.69, 0.96] | 0.02 | 1.00 [0.83, 1.19] | 0.9 | 0.97 [0.80, 1.17] | 0.7 | 0.93 [0.77, 1.13] | 0.5 | 0.94 [0.77, 1.14] | 0.5 |
| Memory time dependent | 0.84 [0.71, 0.99] | 0.03 | 1.05 [0.87, 1.26] | 0.6 | 1.02 [0.85, 1.24] | 0.8 | 1.01 [0.84, 1.22] | 0.9 | 1.02 [0.84, 1.24] | 0.8 |
Note: For each model, the HR is per 1 standard deviation higher score, with higher scores indicates better performance. Model 1: adjusted for age, sex, race, and education. Model 2: additional adjustment for cause of ESRD, dialysis vintage, access type, albumin, diastolic blood pressure and kt/V. Model 3: additional adjustment for cardiovascular disease and heart failure. Model 4: additional adjustment for stroke.
Baseline principal components
CI, confidence interval HR, hazard ratio
When longitudinal executive function scores were examined in time-dependent Cox models, a similar relationship between executive function and all-cause mortality was observed (HR, 0.62; 95% CI, 0.54–0.72). Partial attenuation was seen when adjusting for demographics (HR, 0.74; 95% CI, 0.62–0.88), whereas little change was seen when adjusting for dialysis-related factors, CVD and heart failure, and finally stroke (HR for final multivariable model, 0.79; 95% CI, 0.66–0.94). Time-dependent models utilizing longitudinal memory scores demonstrated a pattern very similar to the baseline memory models, with an association seen in univariate analysis (HR, 0.84; 95% CI, 0.71–0.99), and complete attenuation when adjusting for demographics (HR, 1.05; 95% CI, 0.87–1.26).
Analysis of individual cognitive tests demonstrated that the MMSE was not associated with mortality (Table 3). Poor performance on the Word List Learning Subtest, Block Design, Digit Symbol-Coding Subtests, and Trail Making Tests were associated with decreased survival in unadjusted analyses. In adjusted analyses, worse performance on the Blocks Design and Trails A tests remained significantly associated with mortality. For the Trails B test, non-completers showed the highest hazard for mortality (HR of 2.39 [95% CI, 1.40–4.09] compared to the first tertile of completers); this association was attenuated in adjusted analyses.
Table 3.
Association between Individual Cognitive Test Scores and All-Cause Mortality
| Cognitive Test | No. | Score | Unadjusted | Model 1 | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |||
| MMSE | 314 | 26.7 ± 2.8 | 0.89 [0.77, 1.03] | 0.12 | 0.93 [0.80, 1.08] | 0.3 |
| Delayed Recall | 309 | 4.4 ± 2.7 | 0.80 [0.68, 0.95] | 0.01 | 0.97 [0.81, 1.17] | 0.8 |
| Short Recall | 310 | 4.8 ± 2.9 | 0.76 [0.64, 0.90] | <0.001 | 0.94 [0.78, 1.13] | 0.5 |
| Recall Total | 312 | 23.8 ± 7.2 | 0.74 [0.63, 0.87] | <0.001 | 0.97 [0.81, 1.16] | 0.7 |
| Delayed Recognition | 310 | 20.7 ± 3.0 | 0.85 [0.74, 0.98] | 0.02 | 0.92 [0.78, 1.08] | 0.3 |
| Blocks Design | 307 | 26.1 ± 10.6 | 0.72 [0.59, 0.86] | <0.001 | 0.78 [0.63, 0.96] | 0.02 |
| Digit Symbol | 282 | 40.1 ± 17.0 | 0.62 [0.50, 0.77] | <0.001 | 0.82 [0.64, 1.05] | 0.1 |
| Trails A | 293 | 61.3 ± 39.6 | 1.36 [1.20, 1.53] | <0.001 | 1.18 [1.03, 1.35] | 0.01 |
| Trails B | 229 | 136.9 ± 64.7 | - | - | - | - |
| First tertile* | 77 | - | 1.00 (reference) | - | 1.00 (reference) | - |
| Second tertile* | 75 | - | 1.24 [0.71, 2.16] | 0.5 | 0.82 [0.46, 1.46] | 0.5 |
| Third tertile* | 77 | - | 1.90 [1.13, 3.19] | 0.02 | 1.07 [0.61, 1.88] | 0.8 |
| Non-completers | 60 | - | 2.39 [1.40, 4.09] | 0.002 | 1.42 [0.78, 2.57] | 0.2 |
| Digits Forward | 116 | 9.9 ± 2.4 | 0.94 [0.68, 1.30] | 0.7 | 0.97 [0.69, 1.37] | 0.9 |
| Digits Backward | 116 | 5.8 ± 2.5 | 0.99 [0.71, 1.39] | 0.9 | 1.00 [0.68, 1.45] | 0.9 |
| Mental Alternations | 116 | 21.4 ± 7.9 | 0.79 [0.55, 1.12] | 0.2 | 0.72 [0.48, 1.08] | 0.1 |
| COWAT animal | 117 | 16.2 ± 6.0 | 0.83 [0.57, 1.21] | 0.3 | 1.10 [0.72, 1.68] | 0.7 |
| COWAT supermarket items | 116 | 21.3 ± 6.7 | 1.00 [0.70, 1.44] | 0.9 | 1.15 [0.77, 1.71] | 0.5 |
Note: Scores shown as Mean ± SD. All models represent the HR for mortality per 1-SD change in score for each cognitive test. For all tests except Trails A and B, higher score is consistent with better performance and therefore an HR below 1 suggests that better performance is associated with lower mortality risk. Model 1: Adjusted for age, sex, race, and education.
Tertile of completers.
CI, confidence interval HR, hazard ratio; MMSE, Mini-Mental State Examination; COWAT, Controlled Oral Word Association Test; SD, standard deviation
Sensitivity Analyses
Out of 929 patients screened1, 40 had dementia as at least one of their exclusion criteria (4.3%). Survival status was available for 39 of 40 patients; this subgroup had a mortality rate of 31.8 deaths per 100 person-years and an age-adjusted mortality rate of 16.0 (95% CI, 10.3–24.8) deaths per 100 person-years. Both rates were similar to the mortality rate of the worst performing executive function quartile. Additional adjustment for depression (CESD score >16) or psychotropic medications (yes or no) yielded results similar to the final multivariable models for both memory and executive function (data not shown).
Discussion
In this longitudinal study of maintenance hemodialysis patients examining the association of baseline cognitive function with mortality, impairment in both executive function and memory were associated with increased risk of mortality. The relationship between memory and all-cause mortality was attenuated after adjusting for demographics, while for executive function, the relationship remained significant until adjustment for baseline history of CVD and heart failure. When longitudinal cognitive scores were considered, executive function remained significantly associated with all-cause mortality even after adjustment for CVD, heart failure, and stroke. The Trails A and Blocks Design tests were the individual tests most strongly associated with mortality, while the commonly used MMSE had no association with mortality.
There are few data relating cognitive function to mortality in dialysis patients. Two studies explored the association between dementia, defined by either review of the medical chart or by billing codes, and mortality, with both noting that dementia was an independent risk factor for mortality.13,14 It is important to note that dementia defined by chart review or billing codes is a relatively crude metric for identifying individuals with cognitive impairment, leading to a prevalence of dementia in only 1%–4% of dialysis patients. Therefore, these administrative codes only represent a small proportion of dialysis patients with cognitive impairment. In support of this, we noted a similar prevalence of clinically recognized dementia (4.3%) in our screened sample. This subgroup experienced a similar mortality rate to the lowest performing executive quartile, indicating that risks of cognitive impairment are underestimated by only considering overt dementia. Another report of 145 patients from a combined peritoneal dialysis, incenter hemodialysis, and home hemodialysis cohort noted that cognitive impairment, defined by performing below 1 SD on two or more cognitive tests, was associated with mortality.24 In that study, survival rates were fairly high, likely reflecting the young age (mean, 50 years) of the cohort as well as a high proportion of patients treated with peritoneal dialysis and home hemodialysis. Our study builds on these previous results by performing detailed cognitive testing, evaluating a range of cognitive function (rather than considering cognitive function as a binary exposure variable) and testing different domains of cognitive function (memory and executive function).
There are several possible explanations for our findings. First, cognitive impairment may be a marker of illness and therefore identify individuals at greater risk for mortality. Indeed, patients with worse cognitive function were older, less educated, and more likely to have CVD, diabetes, and hypertension. Adjusted analyses showed complete attenuation for memory function after adjusting for demographic factors, while for executive function, partial attenuation occurred when adjusting for CVD and heart failure. The attenuation after adjustment for CVD and heart failure may be consistent with the hypothesis that impairment in executive function reflects generalized subclinical vascular disease5, including poor cerebrovascular health. A high burden of subclinical cerebrovascular disease in hemodialysis patients is consistent with this hypothesis.25 The results of the time dependent analyses, however, suggest that executive function may be an independent risk factor for mortality. Reflecting this possibility, impaired cognitive function can impact a patient’s ability to adhere to treatment plans including the ability to take medications as prescribed. As many dialysis patients are required to follow complex treatment regimens with frequent medication and dietary recommendation changes, impairment in executive function, which is critical for planning and carrying out tasks, may be particularly associated with adverse outcomes. In either case, our results indicate that measurement of executive function could be useful for identifying those at high risk for mortality (i.e. screening for executive dysfunction). Screening for cognitive impairment may also help providers tailor complex instructions and medical regimens that are prescribed for patients, possibly motivating alternative care practices. For example, if cognitive impairment is recognized, additional efforts to involve family members and enhance home supports could be engaged.
We acknowledge several limitations to our study. First, several tests were added to our cognitive battery midway through our study and we were unable to incorporate these tests into the principal component analyses, possibly leading to less precision within the principal component analysis. Second, by design the study excluded those with most advanced cognitive impairment (dementia), which likely underestimates the risk associated with poor cognitive function. However, even in this select group of patients, worse cognitive function was associated with increased mortality. We also performed cognitive testing during hemodialysis, which in theory may influence cognitive performance through fluid shifts and changes in electrolytes. Addressing this, we previously conducted a randomized, cross-over study in hemodialysis patients utilizing the same battery of tests and found no difference in performance based on the timing of testing.15 Furthermore, even if there was an effect of the procedure on level of cognitive function, we have no a priori reason to believe that it should influence the association between cognitive function and mortality. Finally, this remains an observational study, and, as such, we cannot eliminate residual and unmeasured confounding. In particular, although we have attempted to comprehensively adjust for prevalent vascular disease, we are not able to fully adjust for its severity. The strengths include the detailed ascertainment of cognitive function, and assessment of two different domains of cognitive function. We also have detailed measurement of comorbid conditions, a moderate period of patient follow-up, and have used principal component analysis to limit multiple testing in our primary analyses.
In conclusion, we have shown that worse cognitive function, in particular worse executive function, is associated with a higher risk of mortality. The relationship of executive function with mortality may partially reflect the association of executive function with vascular disease. Novel strategies that may improve or stabilize cognitive impairment in dialysis patients, including different dialysis techniques, cognitive training and treatment of depression, should be investigated.
Supplementary Material
Table S1: Principal component analysis pattern.
Table S2: Baseline demographics and clinical characteristics by quartiles of memory performance.
Acknowledgments
We acknowledge the tremendous assistance of DCI and, in particular, the staff and patients at the five DCI units in the Boston area and St. Elizabeth’s Dialysis unit, whose generous cooperation made this study possible.
Support: The study was funded through the American Society of Nephrology Research Fellowship Grant (Dr Drew), National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grants R21 DK068310 and R01 DK078204 (Dr Sarnak), NIDDK grant K23 DK71636 (Dr Weiner), National Institutes of Health Clinical and Translational Science Awards grant UL1 TR000073, and NIDDK grant K24 DK078204.
Footnotes
N SECTION: Because an author of this article is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Cheuk-Chen Szeto, MD) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Information for Authors & Editorial Policies.
An abstract based on this manuscript was presented the 2013 American Society of Nephrology Kidney Week in Atlanta, GA.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: DAD, DEW, HT, TS, MJS; data acquisition: TS, KL, AK; data analysis/interpretation: DAD, DEW, HT, TS, LF, MJS; statistical analysis: HT; supervision or mentorship: DEW, TS, JAS, AKS, MJS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. MJS takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013 Jan 29;80(5):471–480. doi: 10.1212/WNL.0b013e31827f0f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006 Jul 25;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen EP, Sarnak MJ, Tighiouart H, et al. The kidney disease quality of life cognitive function subscale and cognitive performance in maintenance hemodialysis patients. American Journal of Kidney Diseases. 2012;60(3):417–426. doi: 10.1053/j.ajkd.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. American Journal of Kidney Diseases. 1997 Jul;30(1):41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 5.Weiner DE, Scott TM, Giang LM, et al. Cardiovascular Disease and Cognitive Function in Maintenance Hemodialysis Patients. American Journal of Kidney Diseases. 2011;58(5):773–781. doi: 10.1053/j.ajkd.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira AA, Weiner DE, Scott T, et al. Subcortical cognitive impairment in dialysis patients. Hemodialysis International. 2007;11(3):309–314. doi: 10.1111/j.1542-4758.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 7.Román GC, Sachdev P, Royall DR, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226(1):81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 8.System USRD. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 9.Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ. 1996 Mar 09;312(7031):608–611. doi: 10.1136/bmj.312.7031.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smits CHM, Deeg DJH, Kriegsman DMW, Schmand B. Cognitive Functioning and Health as Determinants of Mortality in an Older Population. American Journal of Epidemiology. 1999 Nov 1;150(9):978–986. doi: 10.1093/oxfordjournals.aje.a010107. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JK, Lui L-Y, Yaffe K. Executive Function, More Than Global Cognition, Predicts Functional Decline and Mortality in Elderly Women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007 Oct 1;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gussekloo J, Westendorp RGJ, Remarque EJ, Lagaay AM, Heeren TJ, Knook DL. Impact of mild cognitive impairment on survival in very elderly people: cohort study. BMJ. 1997 Oct 25;315(7115):1053–1054. doi: 10.1136/bmj.315.7115.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakowski DA, Caillard S, Agodoa LY, Abbott KC. Dementia as a Predictor of Mortality in Dialysis Patients. Clinical Journal of the American Society of Nephrology. 2006 Sep 1;1(5):1000–1005. doi: 10.2215/CJN.00470705. [DOI] [PubMed] [Google Scholar]
- 14.Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrology Dialysis Transplantation. 2006 Sep 1;21(9):2543–2548. doi: 10.1093/ndt/gfl275. [DOI] [PubMed] [Google Scholar]
- 15.Drew DA, Tighiouart H, Scott TM, et al. Cognitive Performance before and during Hemodialysis: A Randomized Cross-Over Trial. Nephron Clinical Practice. 2013;124(3–4):151–158. doi: 10.1159/000356393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Blair J, Spreen O. Predicting Premorbid IQ: a revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;3(2):129–136. [Google Scholar]
- 18.Tulsky D, Zhu J, Lebetter M. Technical Manual. San Antonio: Harcourt Brace & Company; 1997. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III), Wechsler Memory Scale-Third Scale (WMS-III) [Google Scholar]
- 19.Heaton R, Grant I, Mathews C. Comprehensive Norms for an Expanded Halstead-Reitan Battery. Odessa: Psychological Assessment Resources Inc; 1991. [Google Scholar]
- 20.Salib E, McCarthy J. Mental Alternation Test (MAT): a rapid and valid screening tool for dementia in primary care. International journal of geriatric psychiatry. 2002 Dec;17(12):1157–1161. doi: 10.1002/gps.738. [DOI] [PubMed] [Google Scholar]
- 21.Benton A, Hamsher K. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1978. [Google Scholar]
- 22.Heyer NJ, Bittner AC, Jr, Echeverria D. Analyzing multivariate neurobehavioral outcomes in occupational studies: a comparison of approaches. Neurotoxicology and teratology. 1996 Jul-Aug;18(4):401–406. doi: 10.1016/0892-0362(96)00026-8. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 24.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive Impairment and 7-Year Mortality in Dialysis Patients. American Journal of Kidney Diseases. 2010;56(4):693–703. doi: 10.1053/j.ajkd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Drew DA, Bhadelia R, Tighiouart H, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013 Feb;61(2):271–278. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorsuch RL. Factor Analysis. Erlbaum; 1983. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Principal component analysis pattern.
Table S2: Baseline demographics and clinical characteristics by quartiles of memory performance.