Abstract
Purpose
While estrogens are important in prostate growth and play a role in benign prostatic hyperplasia (BPH), no current therapies directly target estrogen action. Estrogens act primarily via estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). Using a mouse model, we evaluated the relative contribution of these receptors to bladder complications of BPH. We also evaluated prevention of these bladder complications by selective estrogen receptor modulators (SERMs), raloxifene and tamoxifen (ERα selective antagonists) and (R,R)-5,11-Diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol (R,R-THC, ERβ selective antagonist).
Materials and Methods
Adult male C57bl/6 mice received implants of 25 mg testosterone (T) and 2.5 mg 17β-estradiol (E2) slow release pellets and untreated controls underwent sham surgery. We used ERα and ERβ knockout (KO) mice compared to their respective wild type (WT) littermates to probe contributions of ER subtypes. WT mice treated with T+E2 were compared to mice treated with T+E2 and 25 mg SERM to evaluate prevention of BPH complications with SERMs.
Results
While ERαWT and ERβWT littermates treated with T+E2 developed large bladders with urinary retention, ERαKO mice treated with T+E2 did not. ERβKO mice treated with T+E2 developed large bladders with urinary retention and increased bladder mass. Co-treatment with the ERα antagonist raloxifene resulted in decreased bladder mass compared to WT mice treated with T+E2, while bladders from mice treated with the ERβ antagonist R,R-THC were similar to T+E2-treated mice.
Conclusions
ERα, but not ERβ, is a key mediator of bladder complications of BPH, and is a potential target for future therapies.
Keywords: mice, testosterone, 17β-estradiol, BPH, LUTS
Introduction
Benign prostatic hyperplasia (BPH) is the most common cause of bothersome lower urinary tract symptoms (LUTS) in older men, which include irritative symptoms (urinary frequency, urge incontinence, nocturia, painful urination, small voided volumes) and obstructive symptoms (hesitancy, straining, weak flow, prolonged voiding, urinary retention, and ultimately, overflow incontinence).1 It is clear the prostate enlarges in virtually all men as they age, and the prevalence of histologic BPH increases.2 LUTs likely result from a complex interaction of prostate pathology, outlet obstruction, and bladder dysfunction.3 Although androgens are necessary for development of BPH, as men age, serum androgens decline as the prevalence of BPH-LUTS increases.4 Estrogens are increasingly recognized as important male sex steroids and serum 17β-estradiol (E2) increases or remains stable as men age, resulting in an increased E2 to testosterone (T) ratio.4 Administration of T+E2 to dogs and rats leads to prostate enlargement and voiding dysfunction, underscoring the importance of the E2 to T ratio in BPH-LUTS.5 We have recently reported that treatment of male mice with T+E2 to model the increased E2 to T ratio in older men causes prostatic glandular growth, bladder outlet obstruction, and urinary voiding dysfunction, mimicking many of the clinical aspects of BPH.6,7
Estrogens primarily mediate their effects via two cognate ligand-activated transcription factors: estrogen receptor alpha (ERα) and beta (ERβ). In mice and men, they are encoded by unique genes from two different chromosomes, but have considerable overlap in tissue distribution and function.8 ERs are expressed throughout the male lower urinary tract and reproductive system, including the prostate,9–11 urethra,9 bladder,12 epididymis,11 and testes.11 While the central DNA-binding domains of human ERα and ERβ are highly conserved (97% homology) the carboxyl terminal ligand-binding domains and amino-terminals differ substantially (60% and 18% homology, respectively).8 This likely accounts for differences in ligand affinity and biological function.
There is growing recognition that there are many estrogen responsive tissues in the male urinary tract and that estrogens play an important role in BPH-LUTS, but which ER mediates BPH-associated bladder dysfunction remains unknown. Increased ERα expression has been reported in bladder tissues from men with bladder outlet obstruction12 and there is increased ERα expression in prostate epithelium from symptomatic BPH patients compared to men without LUTS.12,13 ERα mediates prostatic epithelial,14 urothelial15 and bladder fibroblast12 proliferation in response to exogenous estrogens. Based on these observations, we hypothesized that ERα, but not ERβ, mediates bladder enlargement in male mice treated with T+E2.
Understanding the relative roles of ERα and ERβ is critical to the development of estrogen-directed therapies for BPH-LUTS. Selective estrogen receptor modulators (SERMs) interact directly with ERs to either promote or inhibit transcription of target genes. Several SERMs are in clinical use, including raloxifene, tamoxifen, toremifene and clomiphene. These compounds act as relatively selective ERα antagonists and could readily be tested for clinical efficacy in BPH-LUTS patients. Therefore, we used raloxifene and tamoxifen to test the hypothesis that bladder enlargement in response to T+E2 is prevented by co-treatment with ERα antagonist SERMs, but not by co-treatment with an experimental ERβ selective antagonist 5,11-Diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol (R,R-THC).
Materials and Methods
Hormone and Drug Implantation
All animal experiments and procedures were conducted under protocols approved by the University of Wisconsin Animal Care and Use Committee and the University of Rochester’s University Committee on Animal Resources. Mice heterozygous for ERα (Esr1) and ERβ (Esr2) on a C57bl/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred to generate knockout mice (ERαKO and ERβKO) for comparison with respective wild type littermates (ERαWT and ERβWT). Genotypes of offspring were determined by polymerase chain reaction as previously described.10 For uroflow, urine spot assay and SERMs experiments, adult male wild type C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA) and Harlan Laboratories (Madison, WI). Mice were reared under standard laboratory conditions (12:12 light/dark cycle) and provided with food and water ad libitum. T, E2, raloxifene and tamoxifen were obtained from Sigma Chemical Co. (St. Louis, MO) and (R,R)-5,11-Diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol (R,R-THC) was obtained from Tocris Bioscience (Minneapolis, MN). Compressed hormone pellets containing 25 mg T and 2.5 mg E2 were surgically implanted in adult (6–8 weeks old) male mice for four months as previously described.16,17 Untreated (UNT) mice underwent the surgical procedure but no pellet was implanted. To evaluate prevention of bladder complications, mice were simultaneously implanted with T, E2 and 25 mg SERM for one month. Mice were euthanized with carbon dioxide followed by cardiac puncture, and intact urogenital tracts, including seminal vesicles, bladder, prostate lobes and urethra, were carefully dissected and photographed. Bladders were emptied of urine, carefully dissected from the urogenital tract and bladder and prostate lobe masses were determined with a precision balance.
Urinary Voiding Assays
Uroflow studies utilized wild type mice (UNT n = 2, T+E2 n = 5). One month prior to pellet implantation, we began noninvasive assessment of uroflow as previously described6 and continued until one month after steroid hormone implantation. For each urinary void, peak uroflow (g/sec), mass (g) and duration (sec) were determined. Voids were classified as droplet (mass < 0.1 g and duration < 3 sec) or sustained (mass ≥ 0.1 g and duration ≥ 3 sec) and the median proportion of droplet voids was expressed for each mouse at baseline and after 1 month of T+E2 treatment or sham surgeries. We then conducted a urine void spot assay at the same time points with an independent cohort of wild type mice (UNT n =11, T+E2 n = 13) using a previously described method.18 Briefly, mice were placed into an empty cage lined with grade 540 filter paper (Healthcare Life Sciences, #1540–320 GE) for four hours without water. Filter papers were photographed under UV light and the size of each void spot was quantified using Image J particle analysis (Image J version 1.37a). Each void was classified as droplet (< 6.7 cm2, equivalent to urine mass < 0.1 g in uroflow assay) or sustained (≥ 6.7 cm2) and the proportion of droplet voids was calculated. Normal mice typically void in the corners19 and spatially distributed patterns have been associated with voiding dysfunction; we therefore quantified the percentage of voided urine in the corners.
Data Analysis
To compare continuous variables, t-tests and one-way ANOVA with Bonferroni post hoc comparisons were used. Repeated measures two-way ANOVA was used for within-subjects experiments (uroflow and urine spot assay). Graphs show means ±SEM. GraphPad Prism (La Jolla, CA) was used for statistical analysis and P < 0.05 was considered statistically significant in all analyses.
Results
ER α is required for bladder complications in male mice treated with T+E2
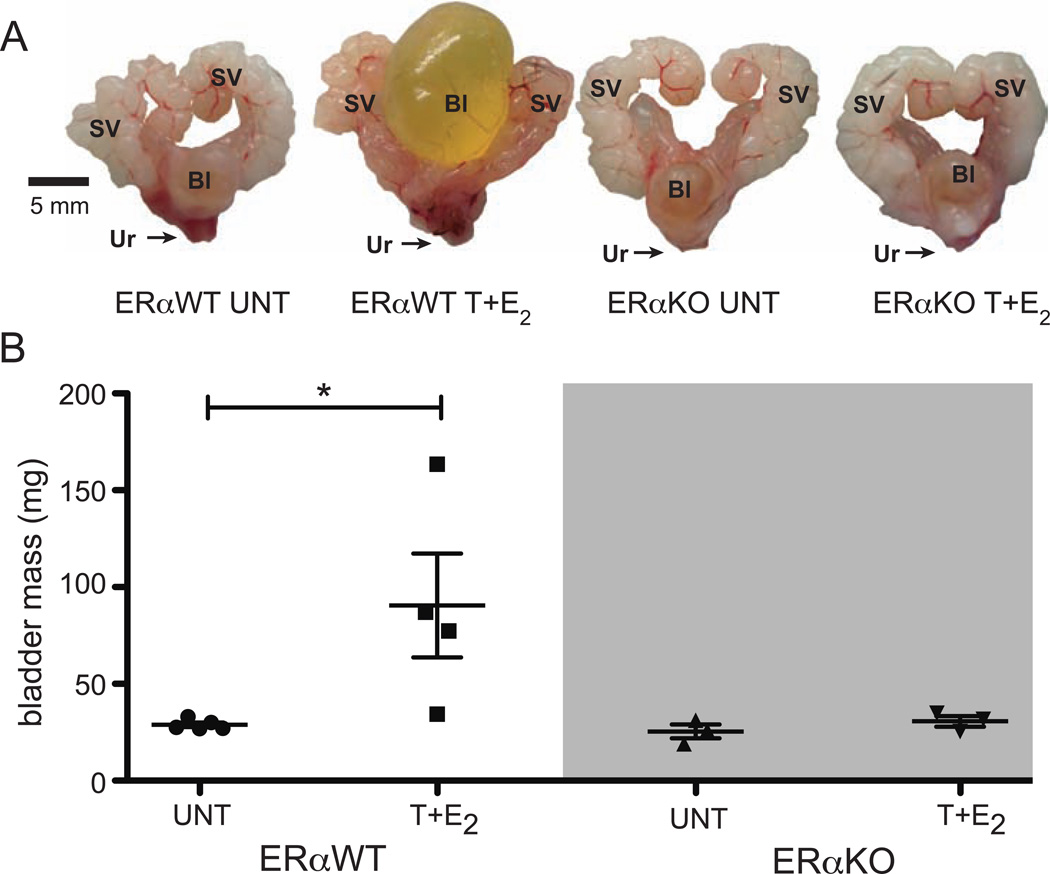
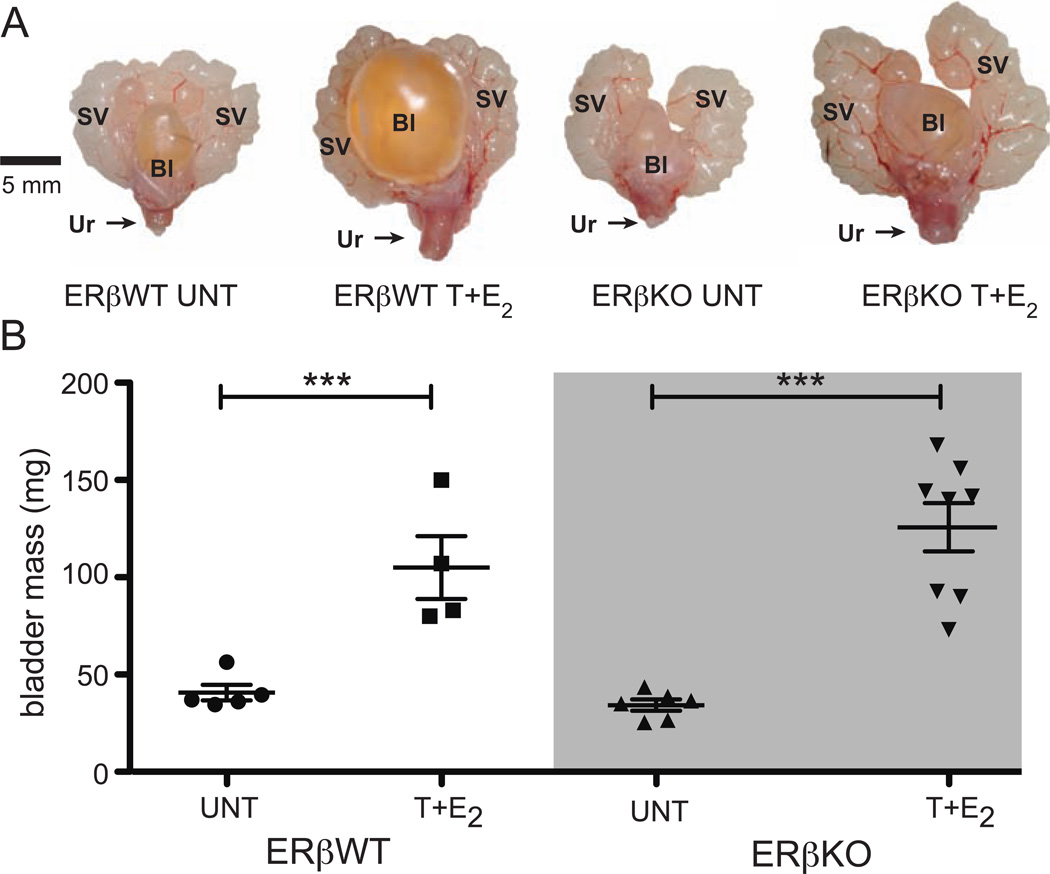
Bladder enlargement with urinary retention was observed in ERαWT littermates treated with T+E2 for four months at the time of euthanasia (Figure 1A). ERαKO mice treated with T+E2 did not exhibit bladder enlargement (Figure 1A). Bladder mass increased in ERαWT littermates treated with T+E2 relative to UNT ERαWT littermates, but there was not change in bladder mass in ERαKO mice treated with T+E2 (Figure 1B). Bladders from ERβWT littermates were enlarged with urinary retention after four months of treatment with T+E2 (Figure 2A). ERαKO mice treated with T+E2 also developed urinary retention (Figure 2A), and bladder mass increased significantly in ERβKO mice treated with T+E2 compared to UNT ERβKO mice (Figure 2B).
Figure 1.
Bladder enlargement in male mice treated T+E2 is mediated by ERα. (A) Urogenital tracts from UNT and T+E2-treated ERα WT and ERαKO mice treated for four months. (B) Bladder masses for UNT and T+E2-treated ERαWT and ERαKO mice. SV = seminal vesicles, Bl = bladder, Ur = urethra. *P < 0.05.
Figure 2.
Bladder enlargement in male mice treated with T+E2 is not mediated by ERβ. α. (A) Urogenital tracts from UNT and T+E2-treated ERβWT and ERβKO mice treated for four months. (B) Bladder masses for UNT and T+E2-treated ERβWT and ERβKO mice. SV = seminal vesicles, Bl = bladder, Ur = urethra. ***P < 0.001
Early manifestations of bladder dysfunction are present in male mice treated with T+E2
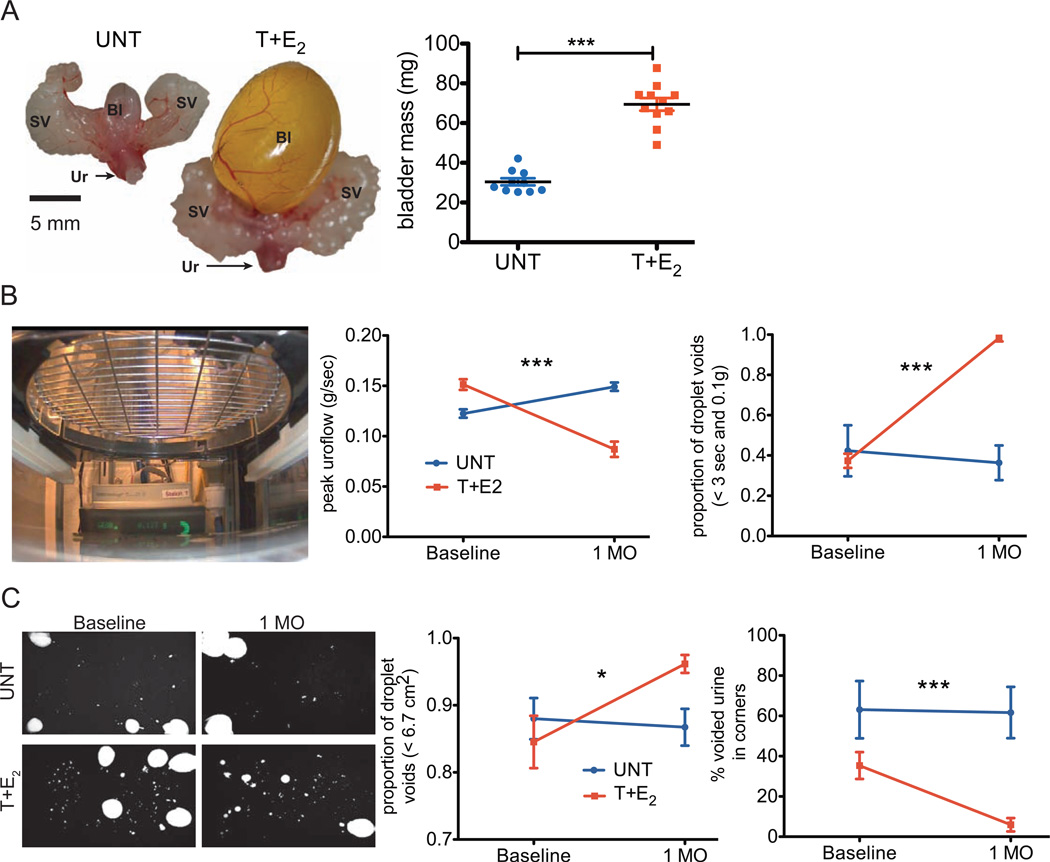
We have previously shown that detrusor hypertrophy and urinary voiding dysfunction in male mice treated with T+E2 manifests after 2–4 months of hormone treatment.6 We evaluated bladder dysfunction after one month of treatment to determine an optimal time point for prevention of these complications. Bladder enlargement with urinary retention and increased bladder mass were present after one month of T+E2 treatment in WT mice (Figure 3A). A metabolic cage system was used to assess uroflow (Figure 3B, image). We found a significant decrease in peak uroflow in these animals (Figure 3B, left graph, P < 0.0001). The change in peak uroflow we observed in T+E2 treated mice was accounted for by a predominance of droplet voiding (defined as < 3 sec and < 0.10 g; Figure 3B, right graph, P < 0.0001). There was no change in peak uroflow or the proportion of droplet voids in untreated mice from baseline to 1 month after sham surgery (Figure 3B). Using the urine spot assay, we found a differences in the proportion of droplet voids (defined as < 6.7 cm2 which corresponded to droplet voids from the uroflow assay) in mice treated with T+E2 for one month (Interaction of time point by treatment, P = 0.0358, Figure 3C, left graph) as well as a change in the percentage of voided urine in the corners (Interaction of time point by treatment, P = 0.0347, Figure 3C, right graph). The proportion of droplet voids and the percentage of voided urine in the corners did not change from baseline to 1 month after sham surgeries in untreated mice (Figure 3C).
Figure 3.
Bladder enlargement and urinary voiding dysfunction is present after one month of treatment with T+E2. (A) Urogenital tracts and bladder masses (graph) from UNT and T+E2-treated wild type mice for one month. (B) Image shows mouse placed in metabolic cage suspended over a precision balance to assess uroflow. Graphs show peak uroflow (left) and proportion of droplet voids (right) at baseline in T+E2 and UNT. (C) Representative urination patterns from urine void spot assay, observed on filter paper viewed with ultraviolet light (image). Proportion of droplet voids (left) and percentage of voided urine in corners (right) of the cage at baseline and one month after T+E2 implant or UNT. SV = seminal vesicles, Bl = bladder, Ur = urethra, 1 MO = one month. ***P < 0.001, *P < 0.05.
ER α antagonist SERMs prevent bladder complications of BPH
Based on early bladder dysfunction observed in our mouse model (after 1 month of treatment), we then evaluated prevention of bladder complications with ERα antagonists (raloxifene and tamoxifen) vs. ERβ antagonist (R,R,-THC) SERMs at this time point. Co-treatment of mice with T+E2+raloxifene, but not T+E2+tamoxifen or ERβ antagonist SERM R,R-THC, prevented bladder enlargement when compared to mice treated with T+E2 only (Figure 4A). Bladder mass was significantly decreased among mice treated with T+E2+raloxifene compared to mice treated with T+E2 only (P < 0.01, Figure 4B, left graph). There was no difference in mice treated with T+E2+tamoxifen or T+E2+R,R-THC compared to T+E2 only (Figure 4B, left graph). Raloxifene (P < 0.0001) and tamoxifen (P < 0.01) prevented the increased prostate mass observed in mice treated with T+E2, but R,R-THC did not affect prostate mass relative to mice treated with T+E2. (Figure 4B, right graph).
Figure 4.
ERα antagonist but not ERβ antagonist SERM prevents bladder enlargement and prostate growth in male mice treated with T+E2. (A) Urogenital tracts from UNT, T+E2, T+E2+RALOX, T+E2+TAMOX, T+E2+R,R-THC wild type mice treated for one month. (B) Graphs show bladder masses (left) and hemiprostate masses (right). SV = seminal vesicles, Bl = bladder, Ur = urethra,1 MO = one month. ***P < 0.001, **P < 0.01.
Discussion
We have previously shown that treatment of male mice with a combination of T+E2, to mimic the dynamic hormonal environment of aging men, recreates many aspects of BPH, including the bladder complications of urinary retention and bladder hypertrophy.6,10 In the present study, we determined the relative contribution of ERα and ERβ to bladder complications in this mouse model. While ERβKO mice developed large bladders in response to T+E2 treatment, ERαKO mice treated did not. This indicates that hormone-induced bladder enlargement and bladder mass depends on intact ERα, but not on ERβ. We also sought to prevent bladder complications of prostate changes in the mouse model and demonstrated that male mice display urinary retention, increased bladder mass and urinary voiding dysfunction one month after treatment with T+E2. Co-treatment with the ERα antagonist SERM raloxifene prevented bladder enlargement and prostate growth in male mice treated with T+E2 for one month, while the ERβ antagonist SERM R,R-THC did not. Thus we conclude that ERα is a potential target for medication development focused on preventing the bladder complications of BPH.
Diversity in ERα and ERβ function depends both on the organ and cell type, as well as the capacity to bind different co-activators and co-repressors. 17β-estradiol interacts with both receptors, primarily activating transcription of estrogen responsive genes by binding ERα, but also when binding ERβ.20 While both ERα and ERβ act as ligand-activated transcription factors, estrogens also induce rapid, so-called non-genomic effects by activation of ERs or other signaling pathways, such as mitogen-activated protein kinases outside of the nucleus. Importantly, extranuclear and intranuclear estrogen receptor signaling cascades are tightly linked and relevant to many human disease processes.21 While the present results demonstrate a key role of ERα in the bladder enlargement in the male mouse treated with T+E2, we do not yet know whether ERα in this context is acting as classic ligand-activated transcription factor or via extranuclear signaling.
When we compared untreated ERαKO or ERβKO mice with their respective WT littermates, we did not observe any differences in bladder mass. This is in agreement with other investigations that did not find bladder hypertrophy or histologic changes in untreated ERαKO or ERβKO mice.22,23 We did not observe bladder enlargement in ERαKO male mice treated with T+E2, indicating that ERα is necessary for hormone-induced bladder enlargement. While the specific cell types and target tissues mediating this effect remain unknown, bladder outlet obstruction is related to prostatic enlargement in male mice treated with T+E2, and the bladder responds with hypertrophy and eventually decompensates. In the untreated wild type mouse prostate, stromal cells primarily express ERα while ERβ is expressed in luminal epithelial cells.10 We have previously shown that T+E2 treatment increases ERα expression in prostate epithelium while decreasing ERβ expression.10 Moreover, ERαKO mice treated with T+E2 have fewer hyperplastic prostate cells.10 Therefore, when is ERα is not present there is likely a protective effect on prostate growth and resulting bladder outlet obstruction due to T+E2 treatment, and bladder complications are also prevented.
Based on our finding that ERαKO mice did not develop hormone-induced bladder enlargement, we sought to prevent bladder complications of hormone treatment in the male mouse with SERMs. We chose raloxifene and tamoxifen as they act as relatively selective ERα antagonists, are approved for other indications, and could be readily translated to clinical use. Raloxifene prevented increased bladder mass due to hormone treatment, but there was no difference in bladder mass in mice co-treated with tamoxifen compared to T+E2 alone. Tamoxifen did appear to prevent urinary retention similarly to raloxifene. Moreover, both ERα antagonist SERMs partially prevented the increased prostate mass caused by treatment with T+E2. This observation is consistent with earlier work that showed raloxifene-induced regression of the ventral prostate in intact male rats.24 Supportive of the concept that ERα inhibition is key to prevention, the ER03B2 antagonist SERM R,R-THC did not prevent increased bladder or prostate mass. This is consistent with genetic experiments utilizing ERβKO mice, in which mice did develop bladder enlargement with hormone treatment. While antagonism of ERα is promising for therapeutic application in BPH-LUTS, another strategy could be activation of ERβ, perhaps in combination with ERα inhibition.
Estrogen action has long been considered an attractive therapeutic target in BPH-LUTS. Aromatase inhibitors were shown to be effective at inhibiting estrogen-induced hyperplastic changes in dog and monkey BPH models.25 However, a randomized clinical trial did not demonstrate clinical efficacy superior to placebo in BPH patients.26 While the aromatase inhibitor atamestane reduced serum 17β-estradiol by about 30%, it also caused substantial increases in serum T and dihydrotestosterone26 which may have counteracted the beneficial reduction in 17β-estradiol. Moreover, while 17β-estradiol is the predominant endogenous estrogen found in men (in men 40–80 years old, means range from 30.3–54.5 pg/mL)4 there are clinically relevant levels of other non-aromatizable estrogens, such as bisphenol-A, found in the ng/mL range in men.27 The role of other estrogens in BPH-LUTS remains to be explored and warrants further study.
While aromatase inhibition has been abandoned as a treatment strategy, there has been renewed interest in SERMs as BPH-LUTS therapies. SERMs such as tamoxifen are commonly used to treat male breast cancer and are well tolerated.28 The SERM clomiphene citrate stimulates endogenous serum T and E2 and improves symptoms in hypogonadal men.29 Taneja et al. tested the SERM toremifene for prevention of prostate cancer progression after biopsy detection of high-grade prostatic intraepithelial neoplasia in a recent trial.30 Toremifene affected neither the primary endpoint of prostate cancer free survival nor detection of prostate cancer after a three year follow up, but this study reported a low prevalence of adverse events related to BPH (3.7% in placebo arm and 4.1% in toremifene). In addition, there was no increased risk of thromboembolic events with toremifene, a potential concern with SERM therapy.30 While 62% of the study population had a history of BPH, this trial did not address whether toremifene affected LUTS or prostate volume. Taken together, these studies show that SERMs are well tolerated in men. While it is unknown whether SERM therapy targeting ERα will result in an improvement in lower urinary tract symptoms in men, this is an enticing area for future study.
Conclusions
The T+E2-treated male mouse mirrors many clinical features of BPH-LUTS and is useful for genetic approaches to uncover key mediators of the BPH phenotype. The present results suggest that ERα may play a key role bladder enlargement accompanying BPH, and highlight the potential of ERα antagonist SERMs for future therapies targeting bladder complications in BPH-LUTS.
Acknowledgements
We thank Emily Ricke, Tyler Bauman, Jonathan Ewald, Jalissa Wynder, Ashleigh Theberge, Pam Weller, Nikesha Haynes and other Ricke lab members for animal collection assistance and feedback on this manuscript. We also acknowledge support from Dr. Edward Messing and the Department of Urology at the University of Rochester. We would like to thank the NIDDK and NIEHS for their financial support for these studies: R01DK093690 (WAR), RC2ESO18764 (WAR), R01DK075036 (RWW), R01DK088806 (DEB) and R01DK09932 (CMV). TMN is a trainee in the Medical Scientist Training Program at the University of Rochester funded by NIH T32 GM07356 and Ruth L. Kirschstein National Research Service Award F30DK093173. This project was supported by the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS) grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or NIH.
Abbreviations
- BPH
Benign prostatic hyperplasia
- E2
17β-estradiol
- ERαWT
Estrogen receptor alpha (Esr1) wild type littermate
- ERαKO
Estrogen receptor alpha (Esr1) null/knockout
- ERβWT
Estrogen receptor beta (Esr2) wild type littermate
- ERβKO
Estrogen receptor beta (Esr2) null/knockout
- LUTS
Lower urinary tract symptoms
- R,R-THC
(R,R)-5,11-Diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol
- SERM
Selective estrogen receptor modulator
- T
Testosterone
- UNT
Untreated
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7(Suppl 9):S3. [PMC free article] [PubMed] [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 3.Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49:651. doi: 10.1016/j.eururo.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Belanger A, Candas B, Dupont A, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 5.Bernoulli J, Yatkin E, Konkol Y, et al. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate. 2008;68:1296. doi: 10.1002/pros.20791. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson TM, Ricke EA, Marker PC, et al. Testosterone and 17beta-Estradiol Induce Glandular Prostatic Growth, Bladder Outlet Obstruction, and Voiding Dysfunction in Male Mice. Endocrinology. 2012 doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricke WA, Ishii K, Ricke EA, et al. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118:2123. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- 8.Enmark E, Pelto-Huikko M, Grandien K, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 9.Bodker A, Bruun J, Balslev E, et al. Estrogen receptors in the human male prostatic urethra and prostate in prostatic cancer and benign prostatic hyperplasia. Scand J Urol Nephrol. 1999;33:237. doi: 10.1080/003655999750015844. [DOI] [PubMed] [Google Scholar]
- 10.Ricke WA, McPherson SJ, Bianco JJ, et al. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22:1512. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 11.Couse JF, Lindzey J, Grandien K, et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 12.Lin W, Rahman NA, Lin J, et al. Molecular mechanisms of bladder outlet obstruction in transgenic male mice overexpressing aromatase (Cyp19a1) Am J Pathol. 2011;178:1233. doi: 10.1016/j.ajpath.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson TM, Sehgal PD, Drew SA, et al. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 2013;178:1233. doi: 10.1016/j.diff.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risbridger G, Wang H, Young P, et al. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001;229:432. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- 15.Teng J, Wang ZY, Jarrard DF, et al. Roles of estrogen receptor alpha and beta in modulating urothelial cell proliferation. Endocr Relat Cancer. 2008;15:351. doi: 10.1677/erc.1.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricke WA, Wang Y, Kurita T, et al. Hormonal and stromal regulation of normal and neoplastic prostatic growth. Prog Mol Subcell Biol. 2005;40:183. doi: 10.1007/3-540-27671-8_8. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson TM, Uchtmann KS, Valdez CD, et al. Renal capsule xenografting and subcutaneous pellet implantation for the evaluation of prostate carcinogenesis and benign prostatic hyperplasia. J Vis Exp. 2013 doi: 10.3791/50574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu W, Ackert-Bicknell C, Larigakis JD, et al. Spontaneous Voiding by Mice Reveals Strain-Specific Lower Urinary Tract Function to be a Quantitative Genetic Trait. Am J Physiol Renal Physiol. 2014 doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanasaki K, Yu W, von Bodungen M, et al. Loss of beta1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J. 2013;27:1950. doi: 10.1096/fj.12-223404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 21.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont S, Krust A, Gansmuller A, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 23.Hsu I, Chuang KL, Slavin S, et al. Suppression of ERbeta signaling via ERbeta knockout or antagonist protects against bladder cancer development. Carcinogenesis. 2014;35:651. doi: 10.1093/carcin/bgt348. [DOI] [PubMed] [Google Scholar]
- 24.Neubauer BL, Best KL, Clemens JA, et al. Endocrine and antiprostatic effects of raloxifene (LY156758) in the male rat. Prostate. 1993;23:245. doi: 10.1002/pros.2990230307. [DOI] [PubMed] [Google Scholar]
- 25.el Etreby MF. Atamestane: an aromatase inhibitor for the treatment of benign prostatic hyperplasia. A short review. J Steroid Biochem Mol Biol. 1993;44:565. doi: 10.1016/0960-0760(93)90260-4. [DOI] [PubMed] [Google Scholar]
- 26.Radlmaier A, Eickenberg HU, Fletcher MS, et al. Estrogen reduction by aromatase inhibition for benign prostatic hyperplasia: results of a double-blind, placebo-controlled, randomized clinical trial using two doses of the aromatase-inhibitor atamestane. Atamestane Study Group. Prostate. 1996;29:199. doi: 10.1002/(SICI)1097-0045(199610)29:4<199::AID-PROS1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Vandenberg LN, Chahoud I, Heindel JJ, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley KL, Tyldesley S, Speers CH, et al. Contemporary systemic therapy for male breast cancer. Clin Breast Cancer. 2014;14:31. doi: 10.1016/j.clbc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Katz DJ, Nabulsi O, Tal R, et al. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110:573. doi: 10.1111/j.1464-410X.2011.10702.x. [DOI] [PubMed] [Google Scholar]
- 30.Taneja SS, Morton R, Barnette G, et al. Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene. J Clin Oncol. 2013;31:523. doi: 10.1200/JCO.2012.41.7634. [DOI] [PubMed] [Google Scholar]