Abstract
Purpose
Phase III studies of bevacizumab in advanced pancreas cancer (APCA) demonstrated no improvement in outcome. No validated biomarkers for bevacizumab efficacy exist. We evaluated bevacizumab-related hypertension (B-HTN) as a biomarker in APCA patients in a pooled analysis from 4 prospective clinical trials of gemcitabine-based therapy combined with bevacizumab.
Materials and Methods
Data were collected from individual databases from 4 prospective, single-arm phase II trials. Patients were grouped according to B-HTN or no hypertension (HTN), and patients with HTN were further grouped according to highest Common Terminology Criteria for Adverse Events grade of HTN: grade 1-2 or grade 3-4. Clinical outcomes of overall survival, time to progression, overall response rate (ORR), and disease control rate (ORR + SD > 16 wk) were compared.
Results
A total of 163 patients with stage IV APCA and Eastern Cooperative Oncology Group 0-1 were included. Median age was 59 years (range, 33 to 85 y). Thirty-four patients had B-HTN, and 129 patients had no HTN. Prognostic factors were balanced between groups. Patients with any grade B-HTN had a significantly improved median overall survival (13.1 vs. 8.1 mo, P = 0.0006), median time to tumor progression (7.6 vs. 5.5 mo, P = 0.0074), ORR (47% vs. 16%, P = 0.0001), and disease control rate (85% vs. 59%, P = 0.004). There were no differences in outcomes according to HTN grade (1-2 [N = 16] vs. 3-4 [N = 18]).
Conclusions
APCA patients who develop any grade of B-HTN appear to derive benefit from bevacizumab. Additional investigation is needed to identify subgroups of patients who develop B-HTN and are more likely to benefit from bevacizumab.
Keywords: pancreas cancer, pharmacodynamic marker, hypertension, bevacizumab
Pancreas cancer (PCA) remains the fourth leading cause of cancer death in the United States.1 The prognosis for patients with advanced disease is poor, with most surviving <6 months with standard gemcitabine therapy.2 A 4-month survival benefit was recently reported with the combination of 5-fluorouracil, oxaliplatin, and irinotecan (FOLFIRINOX)3; however, this regimen was associated more toxicities. Recently published data indicate a 1.8-month survival benefit from the addition of nab-paclitaxel to gemcitabine in metastatic PCA.4 Despite these advances, there is a continuous need to further improve survival through the investigation of molecularly targeted agents.
Bevacizumab (Avastin; Roche/Genentech Inc.) is a recombinant humanized monoclonal IgG1 antibody that binds to vascular endothelial growth factor (VEGF) and prevents it from interacting with its receptors.5 Preclinical data suggest VEGF as a promising therapeutic target in PCA6–8; however, phase III trials of gemcitabine plus antiangiogenic therapy with bevacizumab9,10 or the VEGF receptor tyroskine kinase inhibitor axitinib11 failed to reach their primary endpoint of overall survival in unselected patients.
Efforts have been made to identify predictive biomarkers for bevacizumab efficacy in PCA, however, none have yet been validated. Exploratory analyses from the AViTA trial10 suggested that a subset of patients with elevated VEGFA or VEGFR2 levels may benefit from bevacizumab.12 These results suggest that angiogenesis remains an interesting therapeutic target in PCA and further investigation is needed to identify subsets of patients who may benefit from this treatment approach.
Bevacizumab-related hypertension (B-HTN) has been suggested as a pharmacodynamic marker for improved clinical outcome in other advanced malignancies where its use represents standard practice.13–15 In advanced pancreas cancer (APCA), individual phase II studies have suggested a relationship between B-HTN and improved clinical outcomes,16–18 but these findings have been limited by small patient numbers and not been explored in a larger patient population.
In order to further investigate the utility of B-HTN as a biomarker for bevacizumab efficacy in APCA, we evaluated clinical outcomes according to B-HTN using pooled data from 4 prospective studies of gemcitabine-based therapy with bevacizumab.
Materials and Methods
Study Design
This was an analysis of pooled data from 4 prospective single-arm phase II trials of gemcitabine-based regimens combined with bevacizumab, conducted at The Ohio State University, University of Michigan, Roswell Park Cancer Institute, University of California San Francisco, MD Anderson Cancer Center, and Oklahoma University (Table 1). Raw data were collected from each clinical trial database before pooled analysis, including patient demographics, known prognostic factors including disease stage, Eastern Cooperative Oncology Group (ECOG) performance status, baseline CA19-9, change of CA19-9 with treatment, treatment-related hypertension (HTN), and Common Terminology Criteria for Adverse Events (CTCAE) grade. CTCAE grading was predetermined according to respective CTCAE versions used in each individual trial. Three of the 4 studies16,17,20 used CTCAE version 3.0, and 1 study19 used version 2.0. Clinical outcome measures including overall response rate (ORR), disease control rate (DCR) (DCR = ORR + SD > 16 wk), time to progression (TTP), and overall survival (OS) were collected for all patients. Patients were grouped according to B-HTN (group 1) or no HTN (group 2) and clinical outcomes of interest were compared between groups. Patients in group 1 were further grouped according to CTCAE HTN grade: grade 1-2 or grade 3-4, based on the highest grade HTN experienced by each patient on each respective clinical trial. Clinical outcomes were compared according to HTN grade (1-2 vs. 3-4). The primary aims of the study were to determine of B-HTN as a pharmacodynamic biomarker in APCA patients treated with bevacizumab.
Table 1. Studies Included in Pooled Analysis.
| References | Phase | Patients (n) | Chemotherapy | Median (mo) TTP/PFS | Median OS (mo) |
|---|---|---|---|---|---|
| Ko et al19 | II | 52 | FDR gemcitabine (1000 mg/m2) day 1 + cisplatin (20 mg/m2) day 1 + bevacizumab (10 mg/kg) day 1; every 2 wk | 6.6 (TTP) | 8.2 |
| Javle et al20 | II | 50 | Gemcitabine (1000 mg/m2) day 1-8 + capecitabine (1300 mg/m2) day 1-14 + bevacizumab (15 mg/kg) day 1; every 3 wk | 5.8 (PFS) | 4.8 |
| Fogelman et al17 | II | 50 | FDR gemcitabine (1000 mg/m2) day 1 + oxaliplatin (100 mg/m2) day 2 + bevacizumab (10 mg/kg) day 1; every 2 wk | 4.9 (PFS) | 11.9 |
| Martin et al16 | II | 42 | FDR gemcitabine (1000 mg/m2) day 1 + infusional 5-fluorouracil (2400 mg/m2) day 1 + bevacizumab (10 mg/kg) day 1; every 2 wk | 5.9 (PFS) | 7.4 |
FDR indicates fixed dose rate; OS, overall survival; PFS, progression free survival; TTP, time to progression.
Eligibility
Studies selected for pooled analysis were required to include patients with advanced pancreatic adenocarcinoma proven by cytology or histology. To limit potential confounding factors for clinical outcomes, patients included in the raw database were required to have stage IV disease, EGOG performance status 0-1, and no prior treatment for metastatic disease. Patients were required to have raw toxicity data available and HTN was required to be graded according to CTCAE criteria used on each respective study. Treatment was required to be gemcitabine-based for inclusion in our analyses and would include bevacizumab at the equivalent of 5 mg/kg/wk at the various dosing schedules. All studies included were approved by the respective institutional review boards.
Statistical Analysis
Clinical outcomes were defined as follows: OS was defined as the time from first treatment until death from any cause; TTP was defined as the time from first treatment until disease progression; ORR was defined as the percentage of patients achieving complete or partial response by Response Evaluation Criteria in Solid Tumors criteria used in each individual study; and DCR was defined as percentage of patients achieving objective response or stable disease >16 weeks. Patients who were lost to follow-up or still alive were censored at the date of last visit. Patient characteristics were summarized using descriptive statistics and graphical analyses as part of exploratory data analyses. Factors were compared between groups of interest using 2-sample t tests for continuous measures and χ2 tests for categorical markers or their nonparametric equivalents in the cases where assumptions did not hold. Clinical outcomes described above were compared between groups of interest. For dichotomous outcomes such as ORR and DCR, univariate and multivariable logistic regression models were used to evaluate differences. Kaplan-Meier methods were also used to assess differences in these distributions graphically and quantitatively in the univariate setting. Statistical significance was declared for P < 0.05.
Results
Characteristics of Included Clinical Trials
Four prospective clinical trials were included in these analyses.16,17,19,20 Trials were conducted between 2004 and 2008 and all included patients with APCA. All studies included bevacizumab at an equivalent dose of 5 mg/kg/wk in combination with a gemcitabine doublet. In 2 studies, the doublet contained a fluoropyrimidine,16,20 and in 2 studies the doublet contained a platinum.17,19 Only 2 of these trials met their primary endpoint. Median age and CA19-9 were similar among studies.
A total of 167 patients with complete data available were identified in the pooled database. Four patients were excluded for the following reasons: stage III disease (N = 3), or ECOG performance status 2 (N = 1). One hundred sixty-three patients were included in the raw data analysis. Patient characteristics are outlined in Table 2, and were balanced between groups 1 and 2.
Table 2. Patient Characteristics (N = 163).
| N (%) | Group 1: B-HTN (N = 34) | Group 2: No HTN (N = 129) | P |
|---|---|---|---|
| Age | |||
| Median | 60 | 59 | 0.3 |
| Range | 36-85 | 33-78 | |
| Sex | |||
| Male | 15 (44) | 69 (53) | 0.33 |
| Female | 19 (56) | 60 (47) | |
| Performance status | |||
| 0 | 15 (45) | 54 (42) | 0.71 |
| 1 | 18 (53) | 75 (58) | |
| 0 or 1 (not specified) | 1 (2) | 0 (0) | |
| Stage | |||
| IV | 34 (100) | 129 (100) | 1.00 |
| CA19-9 ≥ 2 × ULN | |||
| Yes | 23 (68) | 97 (76) | 0.34 |
| No | 11 (32) | 32 (24) | |
| Baseline albumin (g/dL) | |||
| ≥ 3.4 | 28 (82) | 100 (78) | 0.78 |
| < 3.4 | 6 (18) | 29 (22) |
B-HTN indicates bevacizumab-related hypertension; HTN, hypertension; ULN, Single upper limit of normal.
Clinical Outcomes According to B-HTN
Patients who experienced any grade of B-HTN had significantly prolonged OS (median 13.1 vs. 8.1 mo; hazard ratio = 0.50, P = 0.0006, Fig. 1A), longer TTP (median 7.6 vs. 5.5 mo; hazard ratio = 0.53, P = 0.0074, Fig. 1B), improved ORR (47% vs. 16%, P = 0.0001), and DCR (85% vs. 59%, P = 0.004) (Table 3 and Fig. 1). Median time to development of B-HTN was 37 days (range, 5 to 226 d), suggesting a true predictive effect rather than selection of patients who had continued treatment beyond their first restaging scan. Furthermore, 80% of patients who were destined to develop HTN did so by day 91. There were no significant differences in clinical outcomes between patients who developed grade 1-2 versus grade 3-4 HTN (Table 3), however, there was a trend toward improved TTP with grade 3-4 B-HTN which may have been limited by small sample size. No predefined clinical factors were found to be predictive of an increased risk of B-HTN.
Figure 1.
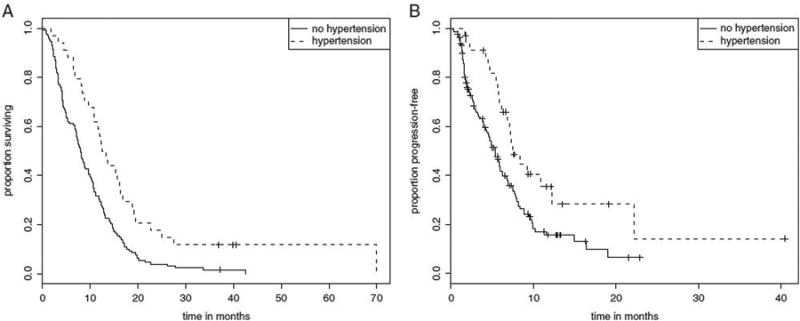
A, Overall survival depending on hypertension in patients treated with bevacizumab. B, Time to progression depending on hypertension in patients treated with bevacizumab.
Table 3. Clinical Outcomes According to B-HTN (N = 163).
| Outcomes (95% CI) | ||||
|---|---|---|---|---|
|
|
||||
| Groups | mOS (mo) | mTTP (mo) | ORR (%) | DCR* (%) |
| Group 1 (B-HTN) (N = 34) | 13.1 (9.8-16.5) | 7.6 (5.9-12.4) | 47 | 85 |
| Group 2 (no HTN) (N = 129) | 8.1 (6.9-9.7) | 5.5 (4.3-6.3) | 16 | 59 |
| P | 0.0006 | 0.0074 | 0.0001 | 0.004 |
| CTCAE HTN grade 1-2 (N = 16) | 13.1 (6.6-19.1) | 6.4 (4.3-22.5) | 44 | 81 |
| CTCAE HTN grade 3-4 (N = 18) | 13 (8.9-17.1) | 11 (7.4-NA) | 50 | 89 |
| P | 0.7 | 0.13 | 0.72 | 0.4 |
DCR = PR + SD ≥ 16 weeks.
B-HTN indicates bevacizumab-related hypertension; CTCAE, Common Terminology Criteria for Adverse Events; CI, confidence interval; DCR, disease control rate; mOS, median overall survival; mTTP, median time to tumor progression; HTN, hypertension; ORR, overall response rate.
Discussion
APCA has proven to be a relatively chemoresistant disease and new approaches with targeted therapies are needed. Phase III studies of antiangiogenic agents including bevacizumab have been negative in unselected patients,9–11 however, a recent pooled analysis of phase II trials of gemcitabine-containing doublets plus bevacizumab21 demonstrated a median OS of 9.1 months (95% confidence interval, 7.6-13.2); greater than the median OS of 5.8 months (95% confidence interval, 4.9-6.6) reported in the Cancer and Leukemia Group B (CALGB) phase III study.
Increasing evidence suggests that proper patient selection through identification and use of biomarkers may maximize the efficacy of targeted anticancer therapies. No such predictive biomarkers for bevacizumab have been validated in any advanced malignancy, although recent exploratory data suggest baseline VEGFA levels may correlate with clinical out-comes.10 Our previous investigations indicated that B-HTN may be predictive for bevacizumab efficacy and warranted further investigation.
APCA patients who developed any grade of B-HTN while receiving first-line therapy with a bevacizumab-containing regimen demonstrated a statistically significant improvement in OS compared to those who did not develop HTN. There was a trend toward improved TTP with grade 3-4 B-HTN and there were no differences in outcomes according to HTN grade. It is possible that we did not see a greater effect with grade 3-4 HTN as the sample size was limited. This suggests that B-HTN may be a potential pharmacodynamic biomarker for the efficacy of bevacizumab in patients with APCA.
Small exploratory analyses from individual phase II studies of APCA have suggested a. relationship between B-HTN16–18 or axitinib-HTN11 and improvement in clinical outcome. In our analysis, we confirmed the presence of a significant relationship between B-HTN and improved clinical outcomes in a larger patient population. Furthermore, patients with B-HTN had a median time of 37 days to onset of HTN, which suggests a true pharmacodynamic effect of bevacizumab in these patients. Therefore although possible, it is unlikely that improved outcomes observed in the B-HTN group were a result of other undetected factors, such as imbalances in baseline prognostic factors, which may have allowed patients in this group to remain on treatment longer and thus more likely to develop B-HTN.
In other advanced malignancies where the use of anti-angiogenic agents represents standard practice, exploratory analyses from previously conducted studies have generally suggested an association between treatment-related HTN and clinical outcomes including improved response rates, prolonged time to progression and improved overall survival.13–15,22–26 In contrast, no relationship between B-HTN and outcomes was observed in patients with glioblastoma multiforme27 and a recently published systematic review of all placebo-controlled phase III trials of bevacizumab found no predictive or prognostic relationship between early B-HTN (within the first 60d of treatment) and clinical outcomes.28 Although this study represents the largest existing analysis of previously conducted randomized trials, results should be interpreted with caution in the context of our findings, as this analysis was conducted across all disease sites with varying chemotherapy backbones, and was not specific to pancreas cancer.
Interpretation of our findings is limited by their retrospective nature and the relatively small sample size of the B-HTN group; however, our overall results are strengthened by the relatively large sample size of the entire study population enrolled at multiple participating institutions and by our choice of study design. Unlike a meta-analysis, a pooled analysis includes individual patient data that was prospectively collected in the context of a clinical trial, which improves the strength and statistical significance of the final results.
It is important to acknowledge that in this retrospective analysis, we were unable to control for adjustments to anti-hypertensive agents made by treating physicians, which may have prohibited capture of all hypertensive events and affected maximum grade of HTN developed by patients.
Although patients with uncontrolled HTN were excluded from enrollment on the individual trials, we did not have information on underlying pretreatment HTN for patients on these trials, which could have predisposed them to development of B-HTN. CTCAE toxicity grading was not uniform across all studies included, however, CTCAE scales used in each study were nearly identical with respect to HTN grading (Table 4) and therefore probably did not influence our results. Finally, while all therapy was gemcitabine-based across trials, treatment was not uniform with regard to dosing of gemcitabine or addition of other chemotherapeutic agents, although all studies included either platinum or fluoropyrimidines. However, bevacizumab dosing was uniform in all 4 studies included in our analysis.
Table 4. Comparison of HTN Grading by Common Terminology Criteria for Adverse Events (CTCAE).
| HTN Grade Definition | |||||
|---|---|---|---|---|---|
|
|
|||||
| CTCAE Version | 1 | 2 | 3 | 4 | 5 |
| Version 2.0 (1999) | Asymptomatic; transient increase by >20 mm Hg (diastolic) or to >150/100 if previously WNL; not requiring treatment | Recurrent or persistent or symptomatic increase by >20 mm Hg (diastolic) or >150/100 if previously WNL, not requiring treatment | Requiring therapy or more intensive therapy than previously | Hypertensive crisis | Not applicable |
| Version 3.0 (2003) | Asymptomatic; transient (<24 h) increase by >20 mm Hg (diastolic) or to >150/100 if previously WNL, intervention not indicated | Recurrent or persistent (≥ 24h) or symptomatic increase by >20 mm Hg (diastolic) or to >150/100 if previously WNL; monotherapy may be indicated | Requiring >1 drug or more intensive therapy than previously | Life-threatening consequences (eg, hypertensive crisis) | Death |
CTCAE indicates Common Terminology Criteria for Adverse Events; HTN, hypertension; WNL, Sngle within normal limits.
In conclusion, we identified B-HTN as a potential pharmacodynamic biomarker for the efficacy of bevacizumab in APCA. Although phase III data from CALGB 80303 conclusively demonstrated that the addition of bevacizumab to gemcitabine did not result in a survival benefit for patients with APCA, future studies evaluating antiangiogenesis agents in the management of APCA should include biomarker analyses to study HTN as a predictor of treatment response, including the possibility of pharmacodynamic titration to HTN. Finally, although our evaluations focused on APCA, they have potential applicability to other advanced malignancies where bevacizumab is currently under study or represents a standard of care.
Acknowledgments
T.B.-S. is a compensated consultant for Genentech and Sanofi-Aventis. A.H.K. is a compensated consultant for Genentech and has received prior honoraria. S.P. received prior honoraria from Genentech. R.I. discloses research funding from Genentech.
Footnotes
The remaining authors declare no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avastin prescribing information. Genentech I; 2011. [Google Scholar]
- 6.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 7.Bruns CJ, Shrader M, Harbison MT, et al. Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer [Journal international du cancer] 2002;102:101–108. doi: 10.1002/ijc.10681. [DOI] [PubMed] [Google Scholar]
- 8.Buchler P, Reber HA, Ullrich A, et al. Pancreatic cancer growth is inhibited by blockade of VEGF-RII. Surgery. 2003;134:772–782. doi: 10.1016/S0039-6060(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 9.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 11.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Jayson G, Dive C, et al. Analysis of blood plasma factors in the AVITA phase III randomized study of bevacizumab (bev) with gemcitabine-erlotinib (GE) in patients (pts) with metastatic pancreatic cancer (mPC) Eur J Cancer. 2011;45:95. Abstract. [Google Scholar]
- 13.Ryanne WuR, Lindenberg PA, Slack R, et al. Evaluation of hypertension as a marker of bevacizumab efficacy. J Gastrointest Cancer. 2009;40:101–108. doi: 10.1007/s12029-009-9104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlberg SE, Sandler AB, Brahmer JR, et al. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–954. doi: 10.1200/JCO.2009.25.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 16.Martin LK, Li X, Kleiber B, et al. VEGF remains an interesting target in advanced pancreas cancer (APCA): results of a multi-institutional phase II study of bevacizumab, gemcitabine, and infusional 5-fluorouracil in patients with APCA. Ann Oncol. 2012;23:2812–2820. doi: 10.1093/annonc/mds134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogelman D, Jafari M, Varadhachary GR, et al. Bevacizumab plus gemcitabine and oxaliplatin as first-line therapy for metastatic or locally advanced pancreatic cancer: a phase II trial. Cancer Chemother Pharmacol. 2011;68:1431–1438. doi: 10.1007/s00280-011-1601-4. [DOI] [PubMed] [Google Scholar]
- 18.Friberg G, Kasza K, Vokes EE, et al. Early hypertension (HTN) as a potential pharmacodynamic (PD) marker for survival in pancreatic cancer (PC) patients (pts) treated with bevacizumab (B) and gemcitabine (G) J Clin Oncol. 2005;23(16S) [Google Scholar]
- 19.Ko AH, Dito E, Schillinger B, et al. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Investig New Drugs. 2008;26:463–471. doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 20.Javle M, Yu J, Garrett C, et al. Bevacizumab combined with gemcitabine and capecitabine for advanced pancreatic cancer: a phase II study. Br J Cancer. 2009;100:1842–1845. doi: 10.1038/sj.bjc.6605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Loon K, Espinoza AM, Fogelman DR, et al. Should combination chemotherapy serve as the backbone in clinical trials of advanced pancreatic cancer?: a pooled analysis of phase II trials of gemcitabine-containing doublets plus bevacizumab. Pancreas. 2013;43:343–349. doi: 10.1097/MPA.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Stefano A, Carlomagno C, Pepe S, et al. Bevacizumab-related arterial hypertension as a predictive marker in metastatic colorectal cancer patients. Cancer Chemother Pharmacol. 2011;68:1207–1213. doi: 10.1007/s00280-011-1604-1. [DOI] [PubMed] [Google Scholar]
- 24.George S, Reichardt P, Lechner T, et al. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann Oncol. 2012;23:3180–3187. doi: 10.1093/annonc/mds179. [DOI] [PubMed] [Google Scholar]
- 25.Lombardi G, Zustovich F, Farina P, et al. Hypertension as a biomarker in patients with recurrent glioblastoma treated with antiangiogenic drugs: a single-center experience and a critical review of the literature. Anticancer Drugs. 2013;24:90–97. doi: 10.1097/CAD.0b013e32835aa5fd. [DOI] [PubMed] [Google Scholar]
- 26.Estfan B, Byrne M, Kim R. Sorafenib in advanced hepatocellular carcinoma: hypertension as a potential surrogate marker for efficacy. Am J Clin Oncol. 2013;36:319–324. doi: 10.1097/COC.0b013e3182468039. [DOI] [PubMed] [Google Scholar]
- 27.Wick A, Schafer N, Dorner N, et al. Arterial hypertension and bevacizumab treatment in glioblastoma: no correlation with clinical outcome. J Neurooncol. 2010;97:157–158. doi: 10.1007/s11060-009-0003-5. [DOI] [PubMed] [Google Scholar]
- 28.Hurwitz HI, Douglas PS, Middleton JP, et al. Analysis of early hypertension and clinical outcome with bevacizumab: results from seven phase III studies. Oncologist. 2013;18:273–280. doi: 10.1634/theoncologist.2012-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]


