Abstract
Electroencephalography (EEG) is a powerful method of studying the electrophysiology of the brain with high temporal resolution. Several analytical approaches to extract information from the EEG signal have been proposed. One method, termed microstate analysis, considers the multichannel EEG recording as a series of quasi-stable “microstates” that are each characterized by a unique topography of electric potentials over the entire channel array. Because this technique simultaneously considers signals recorded from all areas of the cortex, it is capable of assessing the function of large-scale brain networks whose disruption is associated with several neuropsychiatric disorders. In this review, we first introduce the method of EEG microstate analysis. We then review studies that have discovered significant changes in the resting-state microstate series in a variety of neuropsychiatric disorders and behavioral states. We discuss the potential utility of this method in detecting neurophysiological impairments in disease and monitoring neurophysiological changes in response to an intervention. Finally, we discuss how the resting-state microstate series may reflect rapid switching among neural networks while the brain is at rest, which could represent activity of resting-state networks described by other neuroimaging modalities. We conclude by commenting on the current and future status of microstate analysis, and suggest that EEG microstates represent a promising neurophysiological tool for understanding and assessing brain network dynamics on a millisecond timescale in health and disease.
Keywords: Microstates, Electroencephalography, Global Field Potential, Resting State, Schizophrenia, Dementia, Aging
Introduction
The pathophysiology of several neuropsychiatric and neurodegenerative disorders is linked to neurophysiological impairments which may be detectable well before manifestation of severe clinical symptoms (Ponomareva et al., 1998, Jelic et al., 2000, Avila et al., 2002). This suggests that longitudinal monitoring of brain's neurophysiological health may offer a valuable diagnostic and disease management strategy. To achieve this goal, however, we need to identify and establish cost-effective and reliable neurophysiological markers that have potential for translation to clinical practice.
With advancements in neurophysiological techniques and computational power, neurophysiologists continue to gain more insight into how the brain functions in health, and how function is disrupted in disease. Some of these techniques, such as functional magnetic resonance imaging (fMRI), have elucidated functional connectivity among specific brain regions organized into networks (Heuvel et al., 2010). The dynamic of these networks drives various classes of the brain's functions, and their disruption may be associated with pathophysiology of various neuropsychiatric illnesses (Heuvel et al., 2010). Electroencephalography (EEG) is a powerful and popular method that can also be used to examine network activity across the cortex in health and disease. EEG is inexpensive, and enables non-invasive assessment of neural activity resulting from both local and long-range neural coordination (Ingber and Nunez, 2011). In addition to low cost, EEG has millisecond temporal resolution, which is orders of magnitude finer than other neuroimaging modalities such as fMRI.
More than 80 years ago, Hans Berger coined the term EEG and for the first time recorded cortical oscillatory activity from the surface of the skull in humans (Berger, 1929). He described the potentiation and emergence of specific brain waves, today referred to as alpha oscillations (8-12 Hz), in posterior brain regions when human subjects were instructed to close their eyes. Since then, numerous studies have explored the association between various cortical frequency bands of delta (1-4 Hz), theta (4-7 Hz), alpha (8-12 Hz), beta (12-28 Hz), and gamma (>30 Hz) oscillations with different behavioral and disease states. Furthermore, due to the stochastic and multidimensional nature of EEG signals, a wide variety of analytical approaches have been proposed to quantify and discover different features of cortical oscillatory activity and the functional roles they serve. One common approach is to consider the EEG signal as a dynamical system that can be described in terms of its state and dynamics. The system state is the combination of all variables that describe the system at any given time t, and the system dynamics describe how the state changes over time.
One method of studying EEG, therefore, is by defining momentary states of the system based on variables of interest and describing changes in brain activity in terms of the modification of state characteristics, such as the duration or frequency of occurrence of specific states. For example, in nonlinear dynamical analysis, the EEG signal can be embedded into a so-called “state space” to derive values for the chaotic complexity (Wackermann et al., 1993) or synchronicity (Carmeli et al., 2005) of particular states. Several methods to define the entropy as a state characteristic of the EEG signal have been proposed to recognize ictal patterns (Kannathal et al., 2005). Microstate analysis, reviewed in this article, is another such method where states are defined by topographies of electric potentials over a multichannel electrode array.
In a seminal paper, Lehmann et al. demonstrated that the alpha frequency band (8-12 Hz) of the multichannel resting-state EEG signal can be parsed into a limited number of distinct quasi-stable states (Lehmann et al., 1987). These discrete states, called “microstates,” are defined by topographies of electric potentials recorded in a multichannel array over the scalp, which remain stable for 80-120 ms before rapidly transitioning to a different microstate. Unlike some other techniques, microstate analysis simultaneously considers the signal from all electrodes to create a global representation of a functional state. The rich syntax of the microstate time series offers a variety of new quantifications of the EEG signal with potential neurophysiological relevance. Indeed, many studies have since illustrated that characteristics of the EEG microstate time series vary across behavioral states (Stevens and Kircher, 1998, Lehmann et al., 2010), personality types (Schlegel et al., 2012), and neuropsychiatric disorders (Dierks et al., 1997, Lehmann et al., 2005, Kikuchi et al., 2011). Converging lines of evidence suggest that the microstate time series may provide insight about the neural activity of the brain in the resting state (Britz et al., 2010, Musso et al., 2010, Yuan et al., 2012). Microstate analysis of EEG may be a powerful, inexpensive, and clinically translatable neurophysiological method to study and assess global functional states of the brain in health and disease.
In this review, we first introduce the method of microstate analysis as it applies to resting-state EEG. We define resting-state EEG as recording from subjects that are not actively engaged in sensory or cognitive processing. A number of studies have examined EEG microstates during active tasks such as motor function and auditory processing (e.g. Günther et al., 1996), or during cognitive tasks (e.g. Stevens et al., 1997), in addition to event-related studies examining microstates time-locked to a stimulus (e.g. Ott et al., 2011); these are not reviewed here. Second, we discuss the functional interpretation of EEG microstates, both as reflections of resting-state brain activity and indicators of states of the brain. We then describe changes in resting-state microstates that have been associated with neuropsychiatric diseases and other altered brain states. We describe factors that can affect microstate dynamics, analysis, and interpretation. Finally, we conclude by summarizing future directions of microstate analysis.
Introduction to Microstate Analysis
Global brain activity can be described by the global field power (GFP), which is the root of the mean of the squared potential differences at all K electrodes (i.e. Vi(t)) from the mean of instantaneous potentials across electrodes (i.e. Vmean(t)) (Lehmann and Skrandies, 1980).
| [2] |
The GFP represents the strength of the electric field over the brain at each instant, and so is often used to measure the global brain response to an event or to characterize the rapid changes in brain activity. Local maxima of GFP curve represent instants of strongest field strength and highest topographic signal-to-noise ratio. In microstate analysis, the topographies of the electric field at local maxima of the GFP curve are considered discrete states of the EEG, and the evolution of the signal is considered as a series of these states (Figure 1a). In early studies, the alpha frequency band (8-12 Hz) of the original signal was used in the analysis. However, most microstates studies are based on larger bandwidths such as 2-20 Hz (Koenig et al., 2002) or 1-40 Hz (Van De Ville et al., 2010).
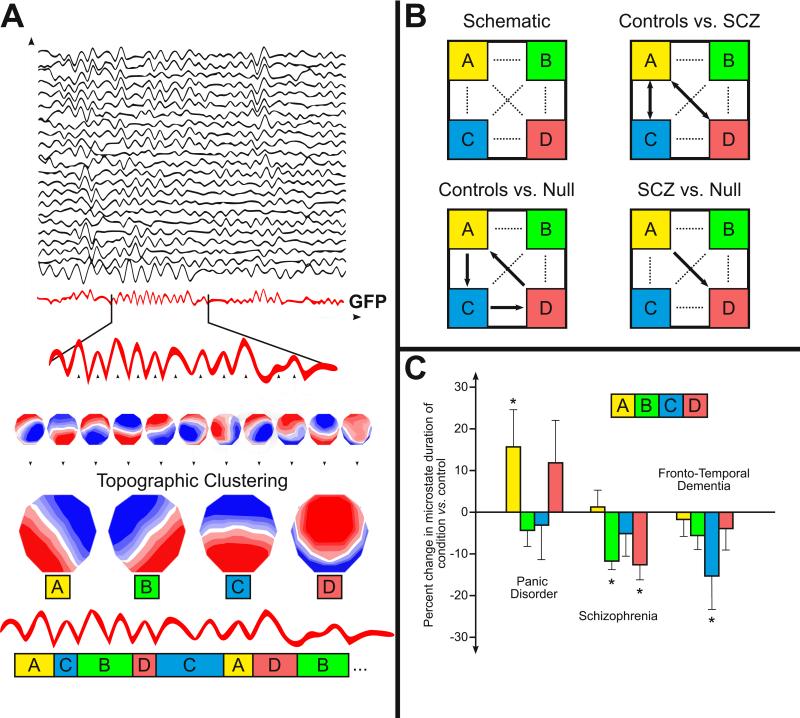
Figure 1.
(A) An illustration of the method of microstate clustering and analysis. Multichannel EEG signal in the frequency band of interest (usually 8-12 Hz, but sometimes wider) is used to calculate the GFP curve (drawn in red), which gives a measure of the instantaneous field strength over time. Peaks of the GFP curve represent instances of highest field strength and largest topographic SNR. The electric potentials of all electrodes at moments of local maxima of the GFP curve are plotted to generate topographic maps of the electrode array. These maps are submitted to a clustering algorithm, which groups the submitted maps into a small set of clusters based on topographic similarity. Most studies of resting-state EEG microstates find the same set of four cluster maps (labeled A-D) that are the “microstate maps.” In the final step, the original maps at maxima of the GFP curve are assigned a label based on the microstate map they best correlate to. A single microstate map remains dominant for 80-120ms before rapidly transitioning to a different map. The multichannel EEG signal is described as a series of alternating microstates. (B) Schematic of transitions between microstates and an example of its impairment in schizophrenia (SCZ). Schematic illustration and data from (Lehmann et al., 2005). The pattern of transitions between microstates is nonrandom and changes significantly in schizophrenia. The dotted paths of the schematic square represent all of the possible transitions between the microstates A-D. When compared against the null hypothesis that transitions among A-D are random, observed transition frequencies are significantly nonrandom at p<0.005 (Lehmann et al., 2005). The arrows in controls vs. null and patients vs. null show the nonrandom transition directionalities. The double-headed arrows in controls vs. patients show significant (p<0.05) differences in transition probabilities between controls and patients with schizophrenia. (C) The average duration of certain microstates are altered in various conditions, including panic disorder (data adapted from Kikuchi et al., 2011), acute schizophrenia (data adapted from Lehmann et al., 2005), and fronto-temporal dementia (data adapted from Nishida et al., 2013). An asterisk indicates significance at p<0.05.
There are two remarkable properties of the EEG recording considered as a series of topographic states in this way. First, although there are a large number of possible maps in multichannel recording, a majority of the signal (usually >70% of total topographic variance) is representable by just a few topographies. Interestingly, most studies that have examined resting-state EEG report the same four archetypal microstates that explain most of the global topographic variance (Lehmann et al., 2009). These four maps have right-frontal left-posterior, left-frontal right-posterior, midline frontal-occipital, and midline frontal topographies, and are labeled A, B, C, and D (Figure 1a). Second, these maps do not gradually morph into one another or overlap in time; instead, a single map remains dominant for about 80-120 ms before abruptly transitioning to another map (Lehmann et al., 1987). These periods of quasi-stability of single maps are called microstates. Thus, when EEG is considered as a topography of electric potentials that evolves over time, the entire signal can be represented by a small set of topographic maps that alternate among themselves at discrete intervals (Wackermann et al., 1993).
The method of microstate analysis has undergone considerable evolution since its first description. Early studies segmented the EEG recording using adaptive segmentation, in which the topography at each successive GFP peak is compared to the one before it, and considered the start of a new microstate if the centroid locations of the positive or negative potentials change by more than a predetermined amount (Lehmann et al., 1987). Investigators could make observations regarding the overall average length and general topographic characteristics of microstates, but early studies using adaptive segmentation rarely grouped microstates into classes. The method of clustering analysis is a more recent development with significant methodological advantages (Pascual-Marqui et al., 1995). In clustering analysis, topographies at all GFP peaks are simultaneously extracted and entered into a clustering algorithm that groups these maps into a small set of classes based on topographic similarity, without regard to the order of their appearance; then, the topography at each GFP peak is labeled as one of these classes, and the EEG signal is re-expressed as a sequence of microstate classes (Figure 1a) (for a technical review of the method of microstate analysis, see Michel et al., 2009). Alternatively, the use of independent component analysis to define microstate classes has been recently proposed (Musso et al., 2010, Yuan et al., 2012).
The microstate time series has a syntax that is rich with parameters of potential neurophysiological relevance. The average duration or lifespan of each microstate is the average length of time a given microstate remains stable whenever it appears (Lehmann et al., 1987). The frequency of occurrence of each microstate is the average number of times per second that the microstate becomes dominant during the recording period (Lehmann et al., 1987). The coverage of a microstate is the fraction of total recording time that the microstate is dominant (Lehmann et al., 1987). The topographical shape of the four microstate maps (A, B, C, and D in Figure 1a) are often compared across groups and behavioral states (for review of methods see Koenig and Melie-García., 2009). The amplitude of each microstate is the average GFP during microstate dominance (Strelets et al., 2003, Nishida et al., 2013). The global explained variance of microstates is the percentage of total variance explained by a given microstate (Brodbeck et al., 2012). The transition probabilities of a microstate to any other are nonrandom, and the sequence of transitions among microstates is potentially significant (Lehmann et al., 2005). In microstate analysis, changes in brain state are described in terms of changes in these parameters.
Functional Interpretation of the Microstate Time Series
Investigating the nature of the neural activities that generate microstates is of potential significance in understanding various behavioral and disease states in humans. The EEG signal at each electrode represents coordinated electrical activity of groups of neurons that make up the source. One possibility is that the signal that defines microstates comes from a small, local group of neurons that happen to become transiently coordinated at certain intervals. However, this seems inconsistent with observed data, which shows a remarkably small number of topographic maps and a well-defined temporal structure, suggesting tighter coordination among neurons across the entire cortical surface. It is far more likely that microstates emerge from coordinated activity of neural assemblies that span large areas of the cortex, giving rise to a global pattern of signal coherence among electrodes over the entire scalp array and generating quasi-stable microstate maps.
Thus, the functional interpretation of microstate analysis of EEG may rest on the notion that different maps are generated by the coordinated activity of different neural assemblies in the brain. A change in the topographical map of recorded potentials represents a change in the distribution or orientation of the underlying active dipoles in the brain that generate the topography (Vaughan, 1982, Lehmann et al., 1987). Therefore, transitions between microstates may be interpreted to represent sequential activation of different neural networks, and the time series of microstates in resting-state EEG gives us a sense of the rapid switching between the activities of neural assemblies of the brain at rest.
In this interpretation, the syntax of the microstate time course holds important information about underlying neural generators (Koenig et al., 2005). For example, the average lifespan of a microstate is interpreted to reflect the stability of its underlying neural assemblies. The frequency of occurrence of a particular microstate may reflect the tendency of its underlying neural generators to become activated. The coverage of a microstate and the global explained variance are interpreted to reflect the relative time coverage of its underlying neural generators compared to others. The amplitude (i.e., average GFP) of a certain microstate may reflect the strength or degree of coordination of the neurons in underlying neural generators. Finally, the nonrandom transition probabilities from each microstate to another are often interpreted to suggest an encoded sequential activation of the neural assemblies that generate microstates. This interpretation becomes particularly interesting when considering the changes in microstates associated with neuropsychiatric illnesses, as discussed below.
Microstates as Indicators of Resting-State Brain Activity
The rich syntax of microstates in resting-state EEG suggests that certain neural assemblies are active at rest. Resting-state neuronal discharges have been recorded from the earliest electrophysiological studies, but more recent evidence suggests that resting-state activity occurs coherently in large neural populations, indicating ongoing activity of entire brain networks at rest (Arieli and Sterkin, 1996, Tsodyks et al., 1999, Leopold et al., 2003). What is the nature of these neural assemblies and the functional role they subserve? One theory suggests that microstates represent discrete mental operations that combine to give rise to conscious mentation, i.e. spontaneous mental activity. Microstates are thus considered “atoms of thought” (Lehmann et al., 1998).
The “atoms of thought” hypothesis postulates that the network(s) that are activated during a particular microstate represent different states of the conscious mind, and that each microstate is associated with a different class of mentation that together make up the conscious state. A number of event-related potential (ERP) studies examining microstates during task-oriented brain activity have discovered associations between the appearance of microstates and particular functions of information processing (Brandeis and Lehmann, 1989, Brandeis et al., 1995, Koenig and Lehmann, 1996, Pizzagalli et al., 2000, Michel et al., 2001). In one study attempting to demonstrate similar associations in resting-state EEG, participants were asked to report their last conscious thought at random intervals during otherwise task-negative recording; thoughts associated with visual imagery were correlated with different microstates than those associated with abstract thought (Lehmann et al., 1998). It has also been repeatedly demonstrated that the microstate at the moment of appearance of an external stimulus influences the processing of this stimulus in the brain (for review, see Britz and Michel, 2011). For example, it has been shown that commission errors in a stroop task are more frequent when the stimulus was presented during a specific microstate configuration (Britz and Michel, 2010), and perceptual awareness of two weak stimuli presented at the threshold of discrimination depends on pre-stimulus microstate (Britz et al., 2014). Spontaneous perceptual reversals of ambiguous visual stimuli such as the Necker cube or during binocular rivalry were shown to depend on the microstate topography at the moment of stimulus presentation (Britz et al., 2009, Britz et al., 2011, Pitts and Britz, 2011). Taken together, these studies suggest that individual microstates may correspond to particular classes of mentation and that these ongoing mental processes influence how incoming information is processed and reacted to.
Microstates and Resting-State Networks
Resting-state EEG microstates reflect neural activity in a task-negative state. Functional MRI has also been used to study the intrinsic activity of the brain at rest by detecting the hemodynamic response to endogenous neural function that is not evoked by stimulus or task – identical to the setting of resting-state EEG recording. These studies have also found a rich complexity of resting-state activity, characterized by temporal correlation of neuronal activity in separate areas that is termed “functional connectivity” (Aertsen et al., 1989). Brain regions exhibiting functional connectivity are thought to be organized into discrete networks associated with distinct functions; among these are a host of so-called resting-state networks (RSNs) that represent functionally connected areas that are active in the task-negative state. One such network is the default-mode network, which is active in the task-negative state but becomes deactivated in a wide array of cognitive tasks (Greicius et al., 2003). RSNs have also been associated with visual, auditory, executive, memory, and other functions (Damoiseaux et al., 2006, Snyder and Raichle, 2012).
A few studies have suggested a link between EEG microstates and RSNs identified by fMRI, suggesting that RSNs of fMRI may be the same ones that give rise to microstates. When the microstate time series is convoluted with the resting-state fMRI BOLD signal, individual microstate maps correlate to the activity of particular RSNs (Britz et al., 2010, Musso et al., 2010, Yuan et al., 2012). Specifically, Britz et al. (2010) showed that microstate A, B, C, and D corresponded to RSNs previously identified as associated with phonological processing, the visual network, the saliency network, and attention, respectively (Mantini et al., 2007, Britz et al., 2010). Similarly, Musso et al. (2010) identified RSNs using fMRI that were significantly temporally associated with the occurrence of specific microstates, although these authors extracted 10 microstates as opposed to four in the study by Britz et al. (2010) and identified RSN-microstate associations individually for each subject, so comparison between these studies is difficult. Yuan et al. (2012) identified 13 microstates and 10 RSNs, and found that individual microstates were temporally associated with the activity of only a few RSNs. Taken together, these studies suggest a relationship between RSN activity and EEG microstates. While exploring how it was possible that the EEG microstate time series could be successfully convoluted to the much slower fMRI hemodynamic response function, Van De Ville and colleagues made the remarkable observation that the microstate time series has scale-free dynamics over six dyadic scales, over the 256 ms to 16 s range, suggesting that resting-state brain activity at the EEG and fMRI timescales reflect the same underlying neurophysiological processes (Van De Ville et al., 2010).
Similarly, one recent report sought to probe the temporal dynamics of RSNs at higher resolution by using magnetoencephalography (MEG) to identify RSNs, rather than the much slower, hemodynamically-driven blood oxygen level-dependent (BOLD) signal of fMRI. After identifying 8 “states” of the MEG signal reflecting individual RSN activity, the authors found that these states each remained stable for approximately 100-200 ms before abruptly transitioning to a new state, and exhibited nonrandom transition patterns among states – both features also characteristic of EEG microstates (Baker et al., 2014). This seems consistent with the notion that EEG microstates and RSNs detected by fMRI and MEG share the same neurophysiological substrate.
The idea that resting-state EEG microstates reflect RSN activity is particularly intriguing in light of the growing number of studies identifying disruptions in the microstate time series associated with some neuropsychiatric disorders. These changes in the microstate time series may correspond to the disrupted functional connectivity of RSNs that have similarly been described in association with these diseases. Thus, resting-state EEG microstates may complement fMRI in the study of RSNs. Future studies may combine these modalities to further elucidate the relationship between RSNs and the neural substrates of EEG microstates. Particularly given its low cost, the use of EEG microstates to examine RSNs would be an important advance in the ability to use RSN disruptions as markers of neuropsychiatric disease in a clinical setting.
Microstates in Neuropsychiatric Disease
A growing body of work has reported that specific parameters of the EEG microstates are significantly changed in certain neuropsychiatric diseases (Table 1).
Table 1.
A selection of key findings in representative studies that find significant changes in microstates and the microstate time series in neuropsychiatric disorders.
| Disorder | Major Findings | References |
|---|---|---|
| Schizophrenia | Increased coverage of microstate B in patients Aberrant spatial configuration of microstate B in patients and to lesser extent in high risk individuals Increased occurrence and coverage of microstate A in high risk individuals |
Andreou et al. (2014) |
| Decreased occurrence of microstate D and increased occurrence of microstate C in adolescents with the 22q11 deletion syndrome (high risk individuals for developing schizophrenia) | Tomescu et al. (2014) | |
| Decrease in average lifespan of microstates B, D | Lehmann et al. (2005), Irisawa et al. (2006), Nishida et al. (2013) | |
| Increase in frequency of microstates A, C | Lehmann et al. (2005) | |
| Altered microstate transition pattern Reduced duration of microstate D Increased occurance of microstate C |
Lehmann et al. (2005), Nishida et al. (2013) | |
| Decrease in average lifespan of microstate C during periods of auditory hallucination | Kindler et al. (2011) | |
| Overall average lifespan of microstates inversely correlated with duration of illness and frequency of psychotic episodes | Stevens et al. (1997) | |
| Shortened microstate D increases average lifespan following treatment with risperidone among patients with symptomatic improvement after treatment | Kikuchi et al. (2007) | |
| Alzheimer's-related dementia | Decreased overall average microstate lifespans (distinguishes cognitive decline/dementia from normal aging) | Stevens and Kircher (1998) |
| Overall average microstate lifespans inversely correlated with degree of cognitive impairment % | Dierks et al. (1997), Strik et al. (1997) | |
| Frontotemporal Dementia | Decrease in average lifespan of microstate C (distinguishes from healthy controls and Alzheimer's Disease) | Nishida et al. (2013) |
| Altered microstate transition pattern | Nishida et al. (2013) | |
| Depression | Decreased overall average microstate lifespans | Strik et al. (1995) |
| Altered microstate topographies | Strik et al. (1995) | |
| Tourette's Syndrome | Increase in frequency of occurrence of microstate A | Stevens et al. (1996) |
| Panic Disorder | Increased average lifespan of microstate A | Kikuchi et al. (2011) |
| Decreased frequency of occurrence of microstate C | Kikuchi et al. (2011) |
Schizophrenia
Several early studies suggested changes in microstate topography or average microstate durations in patients with schizophrenia (Merrin et al., 1990, Koukkou et al., 1994, Stevens et al., 1997, Kinoshita et al., 1998, Koenig et al., 1999, Stevens et al., 1999). In a more recent multicenter study (Lehmann et al., 2005), three distinct changes in the microstate time series were observed in acute, medication-naïve, first-episode patients with schizophrenia compared to healthy control subjects in resting-state eyes-closed EEG: first, when clustered into four microstate maps, two microstates (B and D) had significantly shortened average duration in schizophrenia (Figure 1c), while the other two microstates (A and C) appeared more frequently, subsequently resulting in alteration of total time spent in each microstate; second, the topographic map of microstate B was significantly altered in patients; finally, while controls show a specific microstate transition pattern in the time series (A→C→D→A), a different pattern predominates in schizophrenia (A→D) (Figure 1b). Separate studies have confirmed these findings: shortening of microstate B in patients with schizophrenia has been reported by Irisawa et al. (2006), Nishida et al. (2013), and Strelets et al. (2003), and shortening of microstate D by Kikuchi et al. (2007) and Nishida et al. (2013). Increased occurrence of microstate C in patients with schizophrenia has also been reported by Kikuchi et al. (2007) and Nishida et al. (2013). Tomescu et al. (2014) recently found decreased occurrence of microstate D and increased occurrence of microstate C in adolescents with the 22q11 deletion syndrome, who have a 30 fold increased risk to develop schizophrenia. Interestingly, the increased occurrence of microstate C correlated with positive prodromal symptoms (hallucinations). However, Andreou et al. (2014), who studied subjects in the prodromal phase of schizophrenia, instead reported increased occurrence of microstate A. Taken together, these studies suggest that a reduced presence of microstates B and D and increased presence of C (and perhaps A) may be associated with schizophrenia and its prodromal symptoms.
One study has suggested that individual microstate classes may be associated with specific symptoms of schizophrenia. When patients with schizophrenia were asked to press a button each time they experienced auditory hallucinations, microstate D was found to be significantly shorter during periods of hallucination (Kindler et al., 2011). This indicates that hallucinations may be associated with disruption of the neural and network activities that underlie this specific microstate. An early study also reported that the overall average duration of microstates was negatively correlated with the duration of disease and the frequency of psychotic exacerbations in chronic schizophrenia (Stevens et al., 1997).
If EEG microstates reflect the coordinated activity neural ensembles in the brain, microstate abnormalities in schizophrenia may suggest breakdown of normal network activities that underlies disease pathogenesis. For example, the shortening of certain microstates in schizophrenia may suggest premature termination of their underlying assemblies, and the altered frequency of appearance of some microstates suggests changes in the likelihood of activation of certain neural assemblies. The transition probabilities among microstates are also altered in schizophrenia, suggesting a change in the sequential activation of underlying neural assemblies, i.e. in microstate syntax. Thus, microstate changes in schizophrenia seem to reflect deteriorated connectivity, decreased functional organization, or increased noise in brain processes that have been hypothesized as neurophysiological bases for schizophrenia symptomatology (Lehmann et al., 2005).
Furthermore, there is decreased resting-state functional connectivity in schizophrenia (Venkataraman et al., 2012) that appears consistent with shortened EEG microstates. For example, impairments in the salience network in schizophrenia (Menon, 2011) might underlie the reported increase in occurrence of microstate C in schizophrenia (Kikuchi et al., 2007, Nishida et al., 2013), and microstate C has indeed been proposed to reflect the activity of the saliency network (Britz et al., 2010). If the disruptions in EEG microstates observed in schizophrenia are indeed reflective of RSN alterations, then EEG microstate analysis may represent a powerful alternative method to study RSN abnormalities that underlie the disease.
Dementia
In a series of early studies, the overall average duration of microstates was significantly shorter in both moderate and mild Alzheimer's-related dementia, but not cognitive decline associated with normal aging (Dierks et al., 1997, Strik et al., 1997, Stevens and Kircher, 1998), although at least one study reported longer instead of shorter microstates (Ihl et al., 1993). These early studies used the method of adaptive segmentation, and their findings have yet to be validated with clustering analysis. One recent study reported a significant shortening of microstate C in frontotemporal dementia compared to both healthy control subjects and patients with Alzheimer's disease, as well as a reversal of the predominant microstate transition pattern in frontotemporal dementia (D→C) compared to controls (C→D) (Nishida et al., 2013). These results suggest the possibility of using microstate analysis to both detect the development of dementia and characterize its etiology. In addition, some early studies demonstrated that the degree of change in some parameters correlates with clinical symptoms. For example, two early studies suggested that overall average microstate duration might be inversely correlated with the degree of cognitive dysfunction in Alzheimer's disease (Dierks et al., 1997, Strik et al., 1997).
Similar to the case of schizophrenia, microstate abnormalities associated with dementia may reflect derangements in the activity of underlying neural ensembles, including RSNs, which characterize disease pathogenesis. Indeed, recent evidence from fMRI studies suggest that breakdowns in resting-state functional connectivity can be detected in even preclinical Alzheimer's disease, potentially enabling early detection and intervention (Sheline and Raichle, 2013). EEG microstate analysis may be an alternative method of studying functional connectivity in these patients. Furthermore, EEG is a more cost-effective approach to longitudinal screening for breakdown of functional connectivity in clinical populations.
Other disorders
A few studies have reported changes in microstates in other neuropsychiatric illnesses. In an early study using adaptive segmentation to examine microstates in patients with depression, microstates exhibited abnormal topographies, and the overall average microstate duration was decreased compared to controls (Strik et al., 1995). In another early study of Tourette syndrome, the average microstate centroid locations were shifted laterally (Stevens et al., 1996). In a recent study using clustering analysis of microstates in patients with panic disorder, microstate A was lengthened, and microstate C decreased in frequency of appearance (Kikuchi et al., 2011) (Figure 1c).
Collectively, these findings suggest that the study of resting-state EEG microstates may offer a novel approach for monitoring illness severity through objective neurophysiological biomarkers which, as discussed next, may be further utilized to evaluate treatment efficacy or design targeted treatments.
Microstates and Therapeutic Interventions
There is evidence that neurotropic drugs may modulate EEG microstates in healthy subjects. Piracetam is a modulator of neural membrane ion permeability that affects memory and cognition (Müller et al., 1999). Piracetam is reported to cause clockwise rotation of the topography of fronto-occipital microstates, with increasing rotation as a function of dose (Lehmann et al., 1993). Sulpiride (a D2 and D3 receptor antagonist) and diazepam (a benzodiazepine modulator of the GABAA receptor) prolonged overall average microstate duration and modified the topography of all microstates, respectively (Kinoshita et al., 1995). Perospirone, an antagonist of 5-HT2A and D2 receptors and partial agonist of 5-HT1A receptors, increased the duration of microstate D (that is typically shortened in schizophrenia), while haloperidol, a D2 receptor antagonist increased the mean microstate duration of all microstates (Yoshimura et al., 2007).
The above-mentioned reports in healthy subjects suggest that microstate parameters may be useful indicators of therapeutic efficacy in patients, and preliminary studies seem to support this idea. For example, medicated patients with chronic schizophrenia had microstate parameters that more closely resembled controls as opposed to unmedicated patients with schizophrenia (Stevens et al., 1997). Several microstate parameters were significantly changed in patients with schizophrenia who responded to treatment with risperidone compared to patients who did not respond (Kikuchi et al., 2007). Specifically, responders to risperidone therapy had significantly shorter durations of microstate A, D, and overall microstate duration, as well as more frequent microstate B, C and overall microstate frequency, compared to nonresponders. Following risperidone therapy, responders had significantly prolonged durations of C, D, and overall microstate duration, along with lower frequency of C and overall frequency, compared to nonresponders (Kikuchi et al., 2007). When theta band (4-8 Hz) cortical oscillations are examined in schizophrenia, the number of abnormal microstate topographies were reduced by medication (Merrin et al., 1990). In light of the ability of some neurotropic drugs to change microstates, it is possible that microstates represent promising markers to evaluate the efficacy of various therapeutic interventions on restoring underlying neurophysiological impairments in patients.
Microstates and Brain Developmental and Behavioral States
Changes in brain behavioral state that are not associated with disease have also been associated with specific microstate dynamics. For example, microstates are shorter in drowsiness and REM sleep compared to relaxed wakefulness, and drowsiness is associated with a greater number of unique microstate maps (Cantero et al., 1999). Sleep stage N3 is associated with a dramatic increase in all microstate durations (Brodbeck et al., 2012). Microstates in fatigue show significantly greater amplitude than microstates in alertness (Thuraisingham et al., 2009). The microstate time series in deep hypnosis shows a decrease in the average duration, frequency, and fraction total coverage of two out of four microstates when compared with light hypnosis (Katayama et al., 2007). With increasing age, microstates become shorter and more frequent, and show changes in the distribution of total time covered by each microstate (Koenig et al., 2002). Even personality characteristics, such as strong belief in the paranormal, are associated with microstate changes (Schlegel et al., 2012). Thus, microstates are potentially valuable in examining a wide variety of changes in brain developmental and behavioral states.
Factors Affecting Microstate Parameters
Given the sensitivity of microstates to transient and permanent changes in brain state, it is important to carefully control for various factors when conducting microstate analysis. Specifically, results between cohorts should be controlled for brain developmental and behavioral state (Koenig et al., 2002). For example, subjects should be controlled for age, vigilance state, eyes opened or closed state, and class of mentation (i.e. visual imagery, abstract thought), as all of these affect microstate dynamics. Finally, when studying microstates in disease it is also important to account for disease severity, chronicity (i.e. acute, chronic), and medication.
The degree to which characteristics of the EEG microstate time series correlate with parameters extracted using other analytical methods, such as relative power in various frequency bands, is an open question. There is some evidence that increasing relative power in higher frequency bands correlates with shorter overall microstate lifespan; in the study by Koenig et al (2002), all four microstate classes became shorter with increasing age, which was also accompanied by an increase in relative power in alpha (8-12 Hz) and beta (12-20 Hz) frequencies and a decrease in relative power in delta (2-5 Hz) and theta (5-8 Hz) frequencies (Koenig et al., 2002). However, the correlation between power and microstate lifespan is not specific to any particular microstate – Britz et al. (2010) report that the time course of the occurrence of each microstate map is not correlated to power in any frequency band. Other descriptors of the EEG signal may also relate to microstate characteristics; for example, one study found increased omega complexity, an estimate of the spatial complexity of the signal, in patients with schizophrenia, along with a shortening of microstate B (Irisawa et al., 2006). Further work is required to understand how microstates relate to other descriptors of the EEG signal.
Future Directions
There is ongoing development of the method of microstate analysis that aims to facilitate larger-scale study of microstates in the future. The field would benefit from standardization of the many technical aspects of the method that have been developed over the last two decades (Wackermann et al., 1993, Pascual-Marqui et al., 1995, Tibshirani and Walther, 2005, Britz et al., 2010, Yuan et al., 2012). Early studies reporting changes in microstate dynamics in depression, dementia, panic disorder, and other diseases should be repeated using current methodologies to confirm earlier findings. Larger cohorts are required to make more accurate assessments of the effect of disease on microstates. The test-retest reliability of microstate dynamics within subjects has yet to be established, and is an important prerequisite for study design. Future study is required to determine the relationship between microstate parameters and other descriptors of the EEG signal. These and other continuing developments will help guide future studies.
Recently, a few studies have identified quasi-stable states of the multichannel EEG signal by identifying states of “global functional connectivity,” defined as periods in the recording characterized by a distinct pattern of inter-electrode synchrony (Betzel et al., 2012, Chu et al., 2012). These patterns of inter-electrode time series synchronization are thought to represent coordinated trans-cortical activity – as might be expected, for example, during the activation of a neural network. Remarkably, some of these studies have also recognized that a large proportion of the recording is made up of an alternating sequence of a relatively small set of these patterns, and that these patterns remain stable for tens to hundreds of milliseconds, similar to microstates (Betzel et al., 2012). These findings seem consistent with those of EEG microstate analysis and fMRI studies of RSNs, in that they together support an emerging model of brain function involving a finite number of networks that are sequentially activated to orchestrate brain activity, at least in the resting state.
Whether quasi-stable states of “global functional connectivity” correspond in some way to those defined by electric potential topographies in microstate analysis is an open question. Betzel et al. (2012) reported that functional connectivity states in their study were not associated with topographic EEG microstates. This is perhaps unsurprising, because inter-electrode synchrony in their study was calculated by temporal embedding of the time series at each electrode, whereas EEG microstate topographies effectively represent a spatial embedding of the multichannel EEG signal and so can be expected to generate different depictions of “states” of the underlying signal generator. On the other hand, because spontaneous EEG microstate analysis ignores topographic polarity (i.e. two topographies with exactly opposite polarity are considered the same microstate), and because microstates are stable across multiple periods of the dominant oscillation in any given frequency band, there necessarily must be some degree of temporal synchronicity among electrodes in order to maintain a stable topography with inverting polarity over multiple oscillatory periods (i.e. inversions of the topography). Thus, the relation between EEG microstates and states of functional connectivity defined by inter-electrode temporal synchronicity remains unclear. Further work will be required to clarify how microstates relate to these other “states” of the multichannel EEG signal.
Conclusions
EEG is a relatively inexpensive technique with high temporal resolution that provides snapshots of brain electrical activity and readily complements other neuroimaging (Michel and Murray, 2012). Microstate analysis of the EEG signal has provided enticing early results regarding its potential clinical value; continued development and standardization of the method would enable a more systematic exploration of its utility in detecting and monitoring neuropsychiatric disorders. Microstate analysis also offers a novel way of using the EEG signal to examine fast switching between neural assemblies in the resting brain state. Microstates are relatively easy to compute, and a few freely available software programs exist to help perform the analysis (Pascual-Marqui, 2002, Brunet et al., 2011, Koenig et al., 2011). This method clearly warrants further development to become an important part of how we examine the function of the brain in health and disease.
Highlights.
EEG microstates are quasi-stable periods of electric topography in multichannel EEG
Resting-state EEG is dominated by a small number of alternating microstates
EEG microstates are selectively modified across various neuropsychiatric illnesses
Each EEG microstate may reflect specific fMRI-probed resting-state network(s)
Acknowledgements
CMM is supported by the Swiss National Science Foundation (grant No. 310030-132952). FF received funding from Canadian Institute of Health Research (CIHR - 201102MFE-246635-181538). APL serves on the scientific advisory boards for Nexstim, Neuronix, starlab Neuroscience, Neosync, and Novavision, and is an inventor on patents and patent applications related to noninvasive brain stimulation and real-time integration of TMS with EEG and fMRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements
AK, CMM, FF have no conflict of interest to disclose.
References
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. Journal of Neurophysiology. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Andreou C, Faber PL, Leicht G, Schoettle D, Polomac N, Hanganu-Opatz IL, Lehmann D, Mulert C. Resting-state connectivity in the prodromal phase of schizophrenia: insights from EEG microstates. Schizophr Res. 2014;152:513–520. doi: 10.1016/j.schres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Avila MT, McMahon RP, Elliott AR, Thaker GK. Neurophysiological markers of vulnerability to schizophrenia: Sensitivity and specificity of specific quantitative eye movement measures. Journal of Abnormal Psychology. 2002;111:259–267. [PubMed] [Google Scholar]
- Baker AP, Brookes MJ, Rezek IA, Smith SM, Behrens T, Probert Smith PJ, Woolrich M. Fast transient networks in spontaneous human brain activity. 2014. [DOI] [PMC free article] [PubMed]
- Berger H. Über das Elektrenkephalogramm des Menschen. Archiv f Psychiatrie. 1929;87:527–570. [Google Scholar]
- Betzel RF, Erickson MA, Abell M, O'Donnell BF, Hetrick WP, Sporns O. Synchronization Dynamics and Evidence for a Repertoire of Network States in Resting EEG. Frontiers in Computational Neuroscience. 2012:6. doi: 10.3389/fncom.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis D, Lehmann D. Segments of event-related potential map series reveal landscape changes with visual attention and subjective contours. Electroencephalography and Clinical Neurophysiology. 1989;73:507–519. doi: 10.1016/0013-4694(89)90260-5. [DOI] [PubMed] [Google Scholar]
- Brandeis D, Lehmann D, Michel C, Mingrone W. Mapping event-related brain potential microstates to sentence endings. Brain Topogr. 1995;8:145–159. doi: 10.1007/BF01199778. [DOI] [PubMed] [Google Scholar]
- Britz J, Diaz Hernandez L, Ro T, Michel CM. EEG-microstate dependent emergence of perceptual awareness. Frontiers in behavioral neuroscience. 2014;8:163. doi: 10.3389/fnbeh.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J, Landis T, Michel CM. Right Parietal Brain Activity Precedes Perceptual Alternation of Bistable Stimuli. Cerebral Cortex. 2009;19:55–65. doi: 10.1093/cercor/bhn056. [DOI] [PubMed] [Google Scholar]
- Britz J, Michel CM. Errors can be related to pre-stimulus differences in ERP topography and their concomitant sources. NeuroImage. 2010;49:2774–2782. doi: 10.1016/j.neuroimage.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Britz J, Michel CM. State-dependent visual processing. Frontiers in psychology. 2011;2:370. doi: 10.3389/fpsyg.2011.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J, Pitts MA, Michel CM. Right parietal brain activity precedes perceptual alternation during binocular rivalry. Human Brain Mapping. 2011;32:1432–1442. doi: 10.1002/hbm.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage. 2010;52:1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- Brodbeck V, Kuhn A, von Wegner F, Morzelewski A, Tagliazucchi E, Borisov S, Michel CM, Laufs H. EEG microstates of wakefulness and NREM sleep. NeuroImage. 2012;62:2129–2139. doi: 10.1016/j.neuroimage.2012.05.060. [DOI] [PubMed] [Google Scholar]
- Brunet D, Murray MM, Michel CM. Spatiotemporal analysis of multichannel EEG: CARTOOL. Intell Neuroscience. 2011;2011:1–15. doi: 10.1155/2011/813870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero J, Atienza M, Salas R, Gómez C. Brain Spatial Microstates of Human Spontaneous Alpha Activity in Relaxed Wakefulness, Drowsiness Period, and REM Sleep. Brain Topogr. 1999;11:257–263. doi: 10.1023/a:1022213302688. [DOI] [PubMed] [Google Scholar]
- Carmeli C, Knyazeva MG, Innocenti GM, De Feo O. Assessment of EEG synchronization based on state-space analysis. NeuroImage. 2005;25:339–354. doi: 10.1016/j.neuroimage.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Kramer MA, Pathmanathan J, Bianchi MT, Westover MB, Wizon L, Cash SS. Emergence of stable functional networks in long-term human electroencephalography. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2703–2713. doi: 10.1523/JNEUROSCI.5669-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T, Jelic V, Julin P, Maurer K, Wahlund LO, Almkvist O, Strik WK, Winblad B. EEG-microstates in mild memory impairment and Alzheimer's disease: possible association with disturbed information processing. J Neural Transmission. 1997;104:483–495. doi: 10.1007/BF01277666. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther W, Müller N, Trapp W, Haag C, Putz A, Straube A. Quantitative EEG analysis during motor function and music perception in Tourette's syndrome. European Archives of Psychiatry and Clinical Neurosciences. 1996;246:197–202. doi: 10.1007/BF02188953. [DOI] [PubMed] [Google Scholar]
- Heuvel vd, P M, P H, E H. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Ihl R, Dierks T, Froelich L, Martin E, Maurer K. Segmentation of the Spontaneous EEG in Dementia of the Alzheimer Type. Neuropsychobiology. 1993;27:231–236. doi: 10.1159/000118986. [DOI] [PubMed] [Google Scholar]
- Ingber L, Nunez PL. Neocortical dynamics at multiple scales: EEG standing waves, statistical mechanics, and physical analogs. Mathematical Biosciences. 2011;229:160–173. doi: 10.1016/j.mbs.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Irisawa S, Isotani T, Yagyu T, Morita S, Nishida K, Yamada K, Yoshimura M, Okugawa G, Nobuhara K, Kinoshita T. Increased Omega Complexity and Decreased Microstate Duration in Nonmedicated Schizophrenic Patients. Neuropsychobiology. 2006;54:134–139. doi: 10.1159/000098264. [DOI] [PubMed] [Google Scholar]
- Jelic V, Johansson SE, Almkvist O, Shigeta M, Julin P, Nordberg A, Winblad B, Wahlund LO. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer's disease. Neurobiology of Aging. 2000;21:533–540. doi: 10.1016/s0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Kannathal N, Choo ML, Acharya UR, Sadasivan PK. Entropies for detection of epilepsy in EEG. Computer Methods and Programs in Biomedicine. 2005;80:187–194. doi: 10.1016/j.cmpb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Katayama H, Gianotti LR, Isotani T, Faber P, Sasada K, Kinoshita T, Lehmann D. Classes of Multichannel EEG Microstates in Light and Deep Hypnotic Conditions. Brain Topogr. 2007;20:7–14. doi: 10.1007/s10548-007-0024-3. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Koenig T, Munesue T, Hanaoka A, Strik W, Dierks T, Koshino Y, Minabe Y. EEG Microstate Analysis in Drug-Naive Patients with Panic Disorder. PLoS ONE. 2011;6:e22912. doi: 10.1371/journal.pone.0022912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Koenig T, Wada Y, Higashima M, Koshino Y, Strik W, Dierks T. Native EEG and treatment effects in neuroleptic-naïve schizophrenic patients: Time and frequency domain approaches. Schizophrenia Research. 2007;97:163–172. doi: 10.1016/j.schres.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Kindler J, Hubl D, Strik WK, Dierks T, Koenig T. Resting-state EEG in schizophrenia: Auditory verbal hallucinations are related to shortening of specific microstates. Clinical Neurophysiology. 2011;122:1179–1182. doi: 10.1016/j.clinph.2010.10.042. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Kuginuki T, Yagyu T, Saito N, Hirota T, Saito M. Spatial EEG field configuration in schizophrenics. Psychiatry Research: Neuroimaging. 1998;83:58. [Google Scholar]
- Kinoshita T, Strik WK, Michel CM, Yagyu T, Saito M, Lehmann D. Microstate Segmentation of Spontaneous Multichannel EEG Map Series under Diazepam and Sulpiride. Pharmacopsychiatry. 1995;28:51–55. doi: 10.1055/s-2007-979588. [DOI] [PubMed] [Google Scholar]
- Koenig T, Kottlow M, Stein M, Melie-Garc #237, a L. Ragu: A Free Tool for the Analysis of EEG and MEG Event-Related Scalp Field Data Using Global Randomization Statistics. Computational Intelligence and Neuroscience 2011. 2011. [DOI] [PMC free article] [PubMed]
- Koenig T, Lehmann D. Microstates in language-related brain potential maps show noun-verb differences. Brain and Language. 1996;53:169–182. doi: 10.1006/brln.1996.0043. [DOI] [PubMed] [Google Scholar]
- Koenig T, Lehmann D, Merlo MCG, Kochi K, Hell D, Koukkou M. A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. European Archives of Psychiatry and Clinical Neurosciences. 1999;249:205–211. doi: 10.1007/s004060050088. [DOI] [PubMed] [Google Scholar]
- Koenig T, Melie-García L. Statistical analysis of multichannel scalp field data . In: Michel C, et al., editors. Electrical Neuroimaging. Cambridge University Press; 2009. pp. 169–189. [Google Scholar]
- Koenig T, Prichep L, Lehmann D, Sosa PV, Braeker E, Kleinlogel H, Isenhart R, John ER. Millisecond by Millisecond, Year by Year: Normative EEG Microstates and Developmental Stages. NeuroImage. 2002;16:41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- Koenig T, Studer D, Hubl D, Melie L, Strik WK. Brain connectivity at different time-scales measured with EEG. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:1015–1024. doi: 10.1098/rstb.2005.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukkou M, Lehmann D, Strik W, Merlo MCG. Maps of microstates of spontaneous EEG in never-treated acute schizophrenia. Brain Topogr. 1994;6:251–252. [Google Scholar]
- Lehmann D, Faber PL, Galderisi S, Herrmann WM, Kinoshita T, Koukkou M, Mucci A, Pascual-Marqui RD, Saito N, Wackermann J, Winterer G, Koenig T. EEG microstate duration and syntax in acute, medication-naïve, first-episode schizophrenia: a multi-center study. Psychiatry Research: Neuroimaging. 2005;138:141–156. doi: 10.1016/j.pscychresns.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Ozaki H, Pal I. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalography and Clinical Neurophysiology. 1987;67:271–288. doi: 10.1016/0013-4694(87)90025-3. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Pascual-Marqui RD, Michel C. EEG Microstates. Scholarpedia. 2009;4:7632. [Google Scholar]
- Lehmann D, Pascual-Marqui RD, Strik WK, Koenig T. Core networks for visual-concrete and abstract thought content: A brain electric microstate analysis. NeuroImage. 2010;49:1073–1079. doi: 10.1016/j.neuroimage.2009.07.054. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalography and Clinical Neurophysiology. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Strik WK, Henggeler B, Koenig T, Koukkou M. Brain electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. International Journal of Psychophysiology. 1998;29:1–11. doi: 10.1016/s0167-8760(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Wackermann J, Michel CM, Koenig T. Space-oriented EEG segmentation reveals changes in brain electric field maps under the influence of a nootropic drug. Psychiatry Research: Neuroimaging. 1993;50:275–282. doi: 10.1016/0925-4927(93)90005-3. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very Slow Activity Fluctuations in Monkey Visual Cortex: Implications for Functional Brain Imaging. Cerebral Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Merrin EL, Meek P, Floyd TC, Callaway E., Iii Topographic segmentation of waking EEG in medication-free schizophrenic patients. International Journal of Psychophysiology. 1990;9:231–236. doi: 10.1016/0167-8760(90)90055-i. [DOI] [PubMed] [Google Scholar]
- Michel C, Koenig T, Brandeis D. Electrical neuroimaging in the time domain. Electrical neuroimaging. 2009:111–144. [Google Scholar]
- Michel CM, Murray MM. Towards the utilization of EEG as a brain imaging tool. NeuroImage. 2012;61:371–385. doi: 10.1016/j.neuroimage.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Michel CM, Thut G, Morand S, Khateb A, Pegna AJ, Grave de Peralta R, Gonzalez S, Seeck M, Landis T. Electric source imaging of human brain functions. Brain Research Reviews. 2001;36:108–118. doi: 10.1016/s0165-0173(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Müller W, Eckert G, Eckert A. Piracetam: Novelty in a unique mode of action. Pharmacopsychiatry. 1999 doi: 10.1055/s-2007-979230. [DOI] [PubMed] [Google Scholar]
- Musso F, Brinkmeyer J, Mobascher A, Warbrick T, Winterer G. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. NeuroImage. 2010;52:1149–1161. doi: 10.1016/j.neuroimage.2010.01.093. [DOI] [PubMed] [Google Scholar]
- Nishida K, Morishima Y, Yoshimura M, Isotani T, Irisawa S, Jann K, Dierks T, Strik W, Kinoshita T, Koenig T. EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer's disease. Clinical Neurophysiology. 2013;124:1106–1114. doi: 10.1016/j.clinph.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Ott CG, Langer N, Oechslin MS, Meyer M, Jancke L. Processing of voiced and unvoiced acoustic stimuli in musicians. Frontiers in psychology. 2011;2:195. doi: 10.3389/fpsyg.2011.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;25:5–12. [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Segmentation of brain electrical activity into microstates: model estimation and validation. Biomedical Engineering, IEEE Transactions on. 1995;42:658–665. doi: 10.1109/10.391164. [DOI] [PubMed] [Google Scholar]
- Pitts MA, Britz J. Insights from intermittent binocular rivalry and EEG. Frontiers in Human Neuroscience. 2011:5. doi: 10.3389/fnhum.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D, Lehmann D, Koenig T, Regard M, Pascual-Marqui RD. Face-elicited ERPs and affective attitude: brain electric microstate and tomography analyses. Clinical Neurophysiology. 2000;111:521–531. doi: 10.1016/s1388-2457(99)00252-7. [DOI] [PubMed] [Google Scholar]
- Ponomareva NV, Fokin VF, Selesneva ND, Voskresenskaia NI. Possible Neurophysiological Markers of Genetic Predisposition to Alzheimer's Disease. Dementia and Geriatric Cognitive Disorders. 1998;9:267–273. doi: 10.1159/000017071. [DOI] [PubMed] [Google Scholar]
- Schlegel F, Lehmann D, Faber P, Milz P, Gianotti LR. EEG Microstates During Resting Represent Personality Differences. Brain Topogr. 2012;25:20–26. doi: 10.1007/s10548-011-0189-7. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME. Resting State Functional Connectivity in Preclinical Alzheimer's Disease. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AZ, Raichle ME. A brief history of the resting state: The Washington University perspective. NeuroImage. 2012;62:902–910. doi: 10.1016/j.neuroimage.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Günther W, Lutzenberger W, Bartels M, Müller N. Abnormal topography of EEG microstates in Gilles de la Tourette syndrome. European Archives of Psychiatry and Clinical Neurosciences. 1996;246:310–316. doi: 10.1007/BF02189024. [DOI] [PubMed] [Google Scholar]
- Stevens A, Kircher T. Cognitive decline unlike normal aging is associated with alterations of EEG temporo-spatial characteristics. European Archives of Psychiatry and Clinical Neurosciences. 1998;248:259–266. doi: 10.1007/s004060050047. [DOI] [PubMed] [Google Scholar]
- Stevens A, Lutzenberger W, Bartels DM, Strik W, Lindner K. Increased duration and altered topography of EEG microstates during cognitive tasks in chronic schizophrenia. Psychiatry Research. 1997;66:45–57. doi: 10.1016/s0165-1781(96)02938-1. [DOI] [PubMed] [Google Scholar]
- Stevens A, Mattes R, Günther W, Müller N, Trapp W. First-episode schizophrenics show normal duration and topography of quasistationary EEG segments as compared to controls, during rest as well as during active tasks. Psychiatry Research: Neuroimaging. 1999;91:111–120. doi: 10.1016/s0925-4927(99)00022-0. [DOI] [PubMed] [Google Scholar]
- Strelets V, Faber PL, Golikova J, Novototsky-Vlasov V, Koenig T, Gianotti LRR, Gruzelier JH, Lehmann D. Chronic schizophrenics with positive symptomatology have shortened EEG microstate durations. Clinical Neurophysiology. 2003;114:2043–2051. doi: 10.1016/s1388-2457(03)00211-6. [DOI] [PubMed] [Google Scholar]
- Strik WK, Chiaramonti R, Muscas GC, Paganini M, Mueller TJ, Fallgatter AJ, Versari A, Zappoli R. Decreased EEG microstate duration and anteriorisation of the brain electrical fields in mild and moderate dementia of the Alzheimer type. Psychiatry Research: Neuroimaging. 1997;75:183–191. doi: 10.1016/s0925-4927(97)00054-1. [DOI] [PubMed] [Google Scholar]
- Strik WK, Dierks T, Becker T, Lehmann D. Larger topographical variance and decreased duration of brain electric microstates in depression. Journal of Neural Transmission / General Section JNT. 1995;99:213–222. doi: 10.1007/BF01271480. [DOI] [PubMed] [Google Scholar]
- Thuraisingham RA, Tran Y, Craig A, Wijesuriya N, Nguyen H. Using microstate intensity for the analysis of spontaneous EEG: Tracking changes from alert to the fatigue state.. Engineering in Medicine and Biology Society, 2009 EMBC 2009 Annual International Conference of the IEEE; 2009. pp. 4982–4985. [DOI] [PubMed] [Google Scholar]
- Tibshirani R, Walther G. Cluster Validation by Prediction Strength. Journal of Computational & Graphical Statistics. 2005;14:511–528. [Google Scholar]
- Tomescu MI, Rihs TA, Becker R, Britz J, Custo A, Grouiller F, Schneider M, Debbané M, Eliez S, Michel CM. Deviant dynamics of EEG resting state pattern in 22q11.2 deletion syndrome adolescents: A vulnerability marker of schizophrenia? Schizophrenia Research. 2014;157:175–181. doi: 10.1016/j.schres.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking Spontaneous Activity of Single Cortical Neurons and the Underlying Functional Architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- Van De Ville D, Britz J, Michel CM. EEG microstate sequences in healthy humans at rest reveal scale-free dynamics. Proceedings of the National Academy of Sciences. 2010;107:18179–18184. doi: 10.1073/pnas.1007841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan HG. The Neural Origins of Human Event-Related Potentials. Annals of the New York Academy of Sciences. 1982;388:125–138. doi: 10.1111/j.1749-6632.1982.tb50788.x. [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Whitford TJ, Westin C-F, Golland P, Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophrenia Research. 2012;139:7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackermann J, Lehmann D, Michel CM, Strik WK. Adaptive segmentation of spontaneous EEG map series into spatially defined microstates. International Journal of Psychophysiology. 1993;14:269–283. doi: 10.1016/0167-8760(93)90041-m. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Koenig T, Irisawa S, Isotani T, Yamada K, Kikuchi M, Okugawa G, Yagyu T, Kinoshita T, Strik W, Dierks T. A pharmaco-EEG study on antipsychotic drugs in healthy volunteers. Psychopharmacology. 2007;191:995–1004. doi: 10.1007/s00213-007-0737-8. [DOI] [PubMed] [Google Scholar]
- Yuan H, Zotev V, Phillips R, Drevets WC, Bodurka J. Spatiotemporal dynamics of the brain at rest — Exploring EEG microstates as electrophysiological signatures of BOLD resting state networks. NeuroImage. 2012;60:2062–2072. doi: 10.1016/j.neuroimage.2012.02.031. [DOI] [PubMed] [Google Scholar]