Abstract
The purpose of this study was to determine whether the tendency to sign-track to a food cue was predictive of rats’ choice of cocaine over food. First, rats were trained on a procedure where insertion of a retractable lever was paired with food. A sub-group of rats – sign-trackers – primarily approached and contacted the lever, while another subgroup – goal-trackers – approached the site of food delivery. Rats were then trained on a choice task where they could choose between an infusion of cocaine (1.0 mg/kg) and a food pellet (45 mg). Sign-trackers chose cocaine over food significantly more often than did goal-trackers. These results support the incentive-salience theory of addiction and add to a growing number of studies which suggest that sign-trackers may model an addiction-prone phenotype.
Keywords: Addiction, Choice, Cocaine self-administration, Sign-tracking, Goal-tracking
1. Introduction
Sign-tracking – also called autoshaping or Pavlovian conditioned approach – describes animals’ approach and contact behavior directed towards a conditioned stimulus (CS) that has been paired with an appetitive unconditioned stimulus (US; for reviews, see Hearst & Jenkins, 1974; Tomie, Brooks & Zito, 1989). For example, when insertion of a retractable lever precedes delivery of a food pellet, rats often come to bite, gnaw, and touch the lever (e.g., Davey, Oakley & Cleland, 1981; Kearns & Weiss, 2004). Importantly, these lever-directed behaviors occur even though delivery of food is not dependent on them – the conditioning procedure is a purely Pavlovian one. Rats will even touch the lever when doing so results in the omission of food (Stiers & Silberberg, 1974; Kearns et al., 2006). That sign-tracking occurs despite being unnecessary for the receipt of food has led to the suggestion that it is a form of maladaptive cue-focused behavior (Tomie, Grimes & Pohorecky, 2008). Given the central role that drug cues play in addiction, it has been hypothesized that sign-tracking importantly contributes to the disorder (Tomie, 1995, 1996, 2001; Tomie, Grimes & Pohorecky, 2008; Tomie & Sharma, 2013).
There are large individual differences in the extent to which subjects engage in sign-tracking (Flagel et al., 2007, 2008; Meyer, Ma & Robinson, 2012; Tomie et al., 1998, 2000; Robinson et al., 2014). Some rats approach the location of reward delivery (e.g., the food receptacle) during presentation of the CS rather than approaching and contacting the CS itself. These rats are called goal-trackers (GTs) instead of sign-trackers (STs). It has been argued that STs approach the cue predictive of reward because they attribute incentive salience to the cue itself, while GTs do not (Flagel et al., 2007; Robinson et al., 2014). According to the incentive-salience theory of addiction (Robinson & Berridge, 1993, 2000, 2001), an increased tendency to attribute incentive salience to cues is a characteristic of individuals prone to addiction. Thus, in animal models, STs should show greater addiction-like behavior than GTs.
Recent experiments have shown that STs do in fact engage in many addiction-like behaviors to a greater extent than do GTs. For example, STs work harder than GTs for cocaine on a progressive ratio schedule (Saunders & Robinson, 2011). STs also display more cocaine seeking than GTs despite electric footshock punishment, when “goaded” by a cocaine cue (Saunders, Yager & Robinson, 2013). STs display more cue- and cocaine-induced reinstatement than GTs (Saunders & Robinson, 2010, 2011; Yager & Robinson, 2013). Cocaine cues elicit more approach behavior in STs than in GTs and also serve as more effective conditioned reinforcers (Meyer, Ma & Robinson, 2012; Yager & Robinson, 2013). Tomie et al. (2003) have also found that sign-tracking is associated with increased alcohol drinking. Contrasting this trend, Saunders et al. (2014) recently found that GTs demonstrate greater contextual renewal of cocaine seeking than STs. With the exception of their response to contextual cues, these studies show that STs generally display more addiction-like behaviors than GTs on a variety of measures.
It has been argued that a critical symptom of addiction is the choice of the drug over non-drug alternatives (Ahmed, 2010; Vanderschuren & Ahmed, 2013). A growing number of rat studies have recently appeared that have modeled this behavior by having rats make mutually exclusive choices between drug and a non-drug alternative reinforcer (e.g., Augier, Vouillac & Ahmed, 2012; Cantin et al., 2010; Kerstetter et al., 2012; Lenoir et al., 2013; Madsen & Ahmed, 2014; Perry, Westenbroek & Becker 2013; Tunstall & Kearns, 2013; Tunstall, Riley & Kearns, 2014). To date, there are no known behavioral predictors of increased choice of the drug over the non-drug alternative. The goal of the present experiment was to determine whether a predisposition to sign-track to a food cue would predict increased choice of the drug. That is, would STs be more inclined than GTs to choose cocaine over food? Such an outcome would further cement the case for STs being a model of an addiction-prone phenotype.
2. Materials and methods
2.1. Subjects
Twenty one adult male Long-Evans rats were initially screened for ST vs. GT behavior. Five rats eventually dropped out of the experiment due to non-patent catheters. Rats were individually housed in plastic cages with wood-chip bedding and metal wire tops. They were maintained at 85% of their free-feeding weights (approximately 300–400 g) throughout the experiment by feeding them approximately 15–20 g of rat chow following training sessions. Rats had unlimited access to water in their home cages. The colony room where the rats were housed had a 12-h light:dark cycle with lights on at 08:00 h. Training sessions were conducted 5–7 days per week during the light phase of the light:dark cycle. Throughout the experiment, rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 2011) and all procedures were approved by American University’s Institutional Animal Care and Use Committee (IACUC).
2.2. Apparatus
Training took place in 10 Med-Associates (St. Albans, VT) or Coulbourn Instruments (Whitehall Township, PA) modular test chambers (30.5 × 24 × 29 cm and 30 × 25.5 × 29 cm, respectively) enclosed in sound attenuation chests. Each chamber had aluminum front and rear walls, a grid floor, and two clear plexiglass side walls. Two Med-Associates retractable levers (model ENV-112CM) were positioned 5 cm from the floor and located on the front wall of the chamber, equidistant from the center where a food trough was located. A photobeam horizontally spanned the food trough opening and would record a nosepoke if the rat inserted its nose 1.0 cm into the trough. Tone (4000 Hz and 70 dB) and white noise (65 dB) stimuli were delivered through a speaker mounted on top of the chamber. A shielded 100-mA houselight mounted to the ceiling at the front of the chamber was used to signal the start and end of sessions. Two 100-mA cue lights were also mounted to the front wall, located approximately 10 cm above the floor and directly above each lever. Experimental events were controlled by a Med-Associates computer system located in an adjacent room.
Cocaine (National Institute on Drug Abuse) in a saline solution at a concentration of 2.56 mg/ml was infused at a rate of 3.19 ml/min by 10-ml syringes driven by Med-Associates syringe pumps located outside of the sound attenuation chests. Tygon tubing extended from the 10-ml syringes to a 22-gauge rodent single-channel fluid swivel and tether apparatus (Alice King Chatham Medical Arts, Hawthorne, CA) that descended through the ceiling of the chamber. Cocaine was delivered to the subject through Tygon tubing that passed through the metal spring of the tether apparatus. This metal spring was attached to a plastic screw cemented to the rat’s head to reduce tension on the catheter.
2.3. Procedure
2.3.1. Phase 1: Screening for ST vs. GT Behavior
Rats were screened for ST vs. GT behavior using an autoshaping procedure previously developed by others (Saunders & Robinson, 2011). Each training session in this phase began with the illumination of the houselight. Rats were first habituated to pellet delivery (45-mg dustless precision grain pellet, Bio-serv, New Brunswick, NJ) for two sessions, in the absence of predictive stimuli. During these sessions, 50 pellets were delivered according to a variable-time (VT) 90-s schedule (sessions lasted approximately 75 min). Next, autoshaping training began. The left lever was used as a CS, with lever insertion signaling impending delivery of the food pellet US. Each autoshaping trial consisted of insertion of the lever CS for 8 seconds, then simultaneous lever retraction and pellet delivery. Trials were separated by inter-trial intervals lasting 60 s on average (range: 30 to 90 s). There were 25 trials per each session (sessions lasted approximately 25 min), with 5 such sessions making up the screening phase (Saunders & Robinson, 2011). The behavior of interest during the 8-s CS periods were lever deflections, used as a measure of sign-tracking (i.e., CS contact). Nosepoking in the food trough was taken as a measure of goal-tracking (i.e., contacting the site of US delivery). The Pavlovian Conditioned Approach (PCA) index (Saunders & Robinson, 2011) was used to assess the degree to which a rat engaged in ST vs. GT behavior. The PCA index is the average of three ratios: 1) the probability of a lever contact vs. nosepoke being made on a trial, 2) the ratio of lever contacts vs. nosepokes made in a session, 3) the ratio of average latency to lever contact vs. average latency to nosepoke in a session. As each of these ratios yields a value between −1.0 (exclusively GT behavior) and +1.0 (exclusively ST behavior), the average of these ratios yielded for each rat on each session a value between −1.0 (all GT behavior, every trial) and +1.0 (all ST behavior, every trial). The average of the last two sessions was used as the final PCA score for each rat. Rats that predominantly sign-tracked (i.e., positive PCA scores) were defined as STs, while rats that predominantly goal-tracked (i.e., negative PCA scores) were defined as GTs.
2.3.2. Surgery
Following ST vs. GT screening, all rats were surgically prepared with chronic indwelling jugular vein catheters, using a modification of the procedure originally developed by Weeks (1962) and described in detail elsewhere (Tunstall & Kearns, 2013). Rats were given 5–7 days to recovery from surgery. Catheters were flushed daily with 0.1 ml of a saline solution containing 1.25 µg/ml heparin and 0.08 mg/ml gentamycin.
2.3.3. Phase 2: Operant Response Acquisition
For half of the rats, the left lever was the cocaine lever and the right lever was the food lever. For the other half, this arrangement was reversed. This was done to ensure that previous autoshaping experience did not have carry-over effects which systematically enhanced response acquisition with either reinforcer. To further ensure that both responses would be similarly acquired, only one lever was inserted into the chamber per session, with the lever inserted alternating over sessions (i.e., cocaine or food lever). The start of each session was signaled by illumination of the houselight and insertion of the designated lever. A response on the food lever was reinforced with a food pellet (45-mg grain pellet, see Phase 1) and a response on the cocaine lever was reinforced with a 1.0 mg/kg cocaine infusion. The selected cocaine dose was based on previous studies from this lab (Tunstall & Kearns 2013; Tunstall, Riley & Kearns, 2014). A press on either lever also initiated a 10-s timeout (TO) period signaled by a distinct audiovisual cue. Each cue consisted of an auditory component (tone or white noise, counterbalanced over reinforcer type) and a visual component (illumination of the cue light located above the pressed lever). Lever presses during the TO were recorded, but had no consequences. During this phase, rats could earn up to 30 reinforcers in a session (i.e., cocaine or food, depending on training day). If rats did not reach this criterion within 2 hours, the session was terminated. Rats continued training with the each lever alternating over sessions, until they earned a cumulative total of at least 150 reinforcers of each type. Once they reached this criterion, they continued to receive training sessions with the other lever until they earned at least 150 deliveries of that reinforcer. This ensured all rats had an approximately equal amount of lever-press training with each lever and its associated reinforcer prior to advancing to the choice phase.
2.3.4. Phase 3: Choice Between Cocaine and Food
During this phase, rats could choose to respond on either the cocaine lever or the food lever. Following an established procedure (Cantin et al., 2010; Lenoir et al., 2013; Tunstall & Kearns, 2013; Tunstall, Riley & Kearns, 2014), each choice session began with four forced-choice trials, in which either the right or left lever was inserted. There were two trials of each type, with the order of presentation randomized within blocks of two. This ensured that rats sampled each response-outcome contingency twice at the beginning of each choice session. A lever press would result in delivery of the designated reinforcer (i.e., a cocaine infusion or a food pellet), presentation of the TO cue, and retraction of the lever. Following the completion of the forced-choice trials, there were 14 free-choice trials. Each free-choice trial began with the simultaneous insertion of both levers. A lever press on either lever resulted in the delivery of the designated reinforcer and TO cue, with the immediate retraction of both levers. All trials in these sessions were separated by a 10-min ITI. This relatively long ITI length was chosen to minimize the potential influence of accumulating cocaine doses on choice behavior (Cantin et al., 2010; Perry, Westenbroek & Becker, 2013). In total, choice sessions lasted approximately 3 h and there were 5 such sessions.
2.4. Data Analyses
For all statistical tests, significance was set at α = 0.05. The primary dependent measure from the autoshaping phase was the PCA index. Numbers of lever contacts and nosepokes were also analyzed individually. The primary dependent measure from the choice phase was the percentage of trials on which cocaine was chosen. Repeated measures ANOVA and/or independent-samples t-tests were performed on these measures. The Benjamani-Hochberg procedure was used to keep α ≤ 0.05 for collections of t-tests. In cases where the assumption of equal variance was not met (i.e., Levene’s test was significant), Welch’s t-test was used instead of Student’s t-test.
3.1 Results
3.1.1. Phase 1: Screening for ST vs. GT Behavior
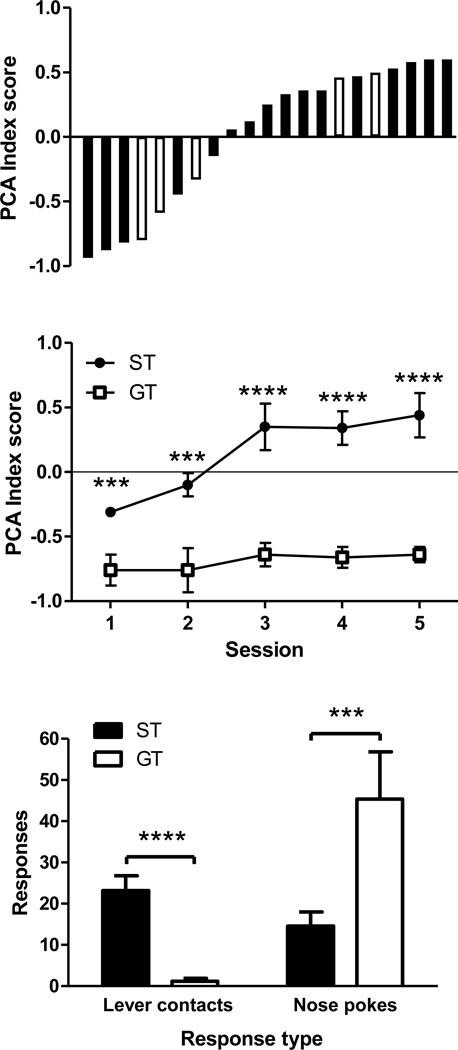
Fig. 1a shows individual subjects’ final PCA scores averaged over the final two sessions of Phase 1. There was much individual-subject variability, with scores ranging from −0.94 to +0.6. The white bars in Fig. 1a represent rats that dropped out of the experiment due to catheter non-patency (their data was not used in any analyses). Of the remaining rats, there were 11 STs that had positive PCA scores and 5 GTs that had negative PCA scores.
Figure 1. Screening for ST vs. GT.
a) Pavlovian Conditioned Approach index scores (averaged over last two days of PCA training) are shown for each animal in the experiment. White bars represent animals which dropped out of the experiment. These animals did not contribute data to any statistical analyses. b) Mean (±SEM) PCA scores of ST and GT rats (that completed the experiment) across PCA training. c) Mean (±SEM) lever contacts and nose pokes made during CS periods by STs and GTs, averaged over all sessions of PCA training. *** p ≤ 0.005, **** p < 0.001.
To confirm that the method for classifying rats as STs and GTs produced distinct subsets of rats, we retroactively plotted PCA acquisition for the two groups. Fig. 1b presents group mean (±SEM) PCA scores of STs and GTs on each of the 5 autoshaping sessions. As shown, STs and GTs differed in ST vs. GT behavior from the first session and this difference widened over subsequent sessions. A 2 × 5 (Group by Session) repeated measures ANOVA detected a significant main effect of Group (F[1, 14]= 15.07, p < 0.001), Session (F[1, 14] = 14.53, p < 0.005) and a significant Group-by-Session interaction (F[1, 14] = 7.23, p < 0.05). Independent-samples t-tests confirmed that a significant difference existed between ST and GT rats on the first session and this difference persisted through all sessions (t’s ≥ 3.36, p’s ≤ 0.005). To further confirm the validity of classifications, STs and GTs were compared in terms of CS contacts (i.e., the defining feature of sign-tracking) and US contacts (i.e., the defining feature of goal-tracking). Fig. 1c shows the raw lever-contact and nose-poke responses, which the PCA index is based upon, averaged over the 5 PCA sessions. This illustrates a stark difference between STs and GTs. STs made almost 25 lever contacts per session, while GTs made almost no lever contacts (t[10.7] = 5.99, p < 0.001). GTs, however, made many more nose-poke responses in the food receptacle than STs (t[14] = 3.42, p < 0.005).
3.2. Phase 2: Operant Response Acquisition
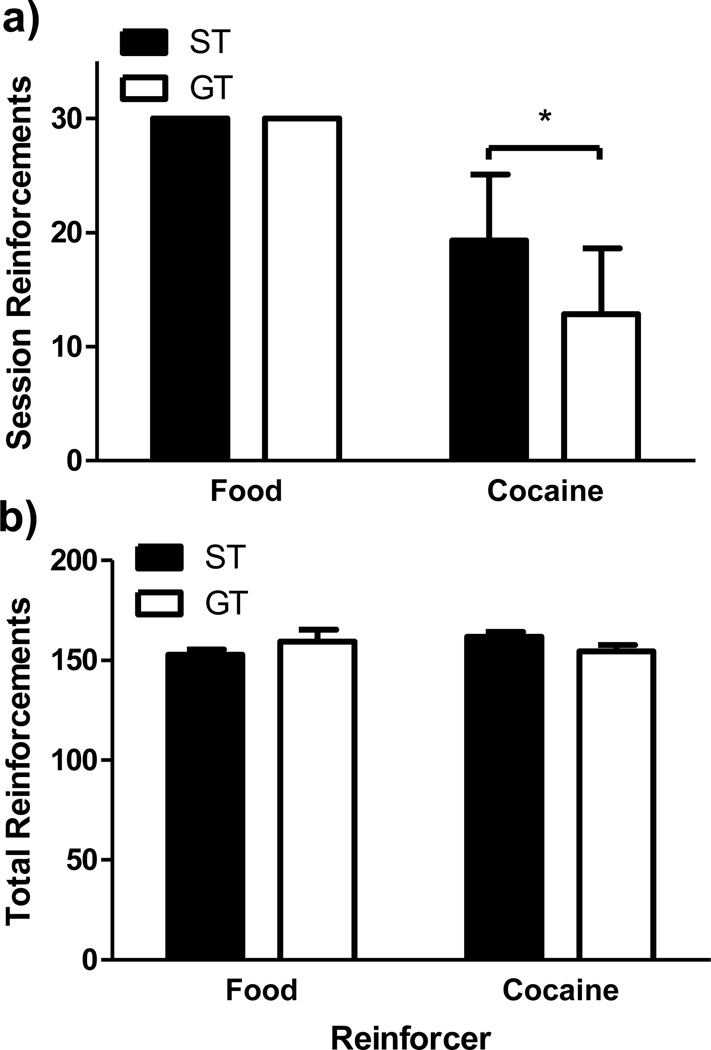
Fig. 2a shows for all STs and GTs, the average number of reinforcers earned per session over the last 5 food- and cocaine-lever training sessions. (Five was the fewest number of sessions in which a rat completed acquisition training for both food and cocaine). While all rats (both STs and GTs) earned the maximum 30 reinforcers per session on each of the last 5 food-training sessions, STs self-administered significantly more cocaine infusions than GTs over the last 5 cocaine-training sessions (t[14] = −2.25, p < 0.05). However, as can be seen in Fig. 2b, the 150-cumulative-reinforcements criterion ensured that by the end of training, STs and GTs received a comparable number of reinforced lever presses with food (t[14] = 1.18, p = 0.26) and cocaine (t[14] = −1.69, p = 0.11). The lower rate of cocaine self-administration in GTs resulted in them needing more sessions on average (M = 20, SEM = 3.9) than STs (M = 12.4, SEM = 1.3) to reach criterion, although this difference did not reach significance (t[4] = 1.83, p = 0.14). There was no significant difference in the number of food training sessions required either (STs: M = 5, SEM = 0; GTs: M = 7.8, SEM = 1.53; t[4.9] = 1.85, p = 0.126).
Figure 2. Operant Lever-Press Acquisition.
a) Mean reinforcers (±SEM) per session earned by STs and GTs during acquisition of food- and cocaine-maintained lever pressing (averaged over the last 5 sessions of each type). b) Mean total (±SEM) food and cocaine reinforcers earned by STs and GTs upon meeting the response acquisition criterion for each response/reinforcement alternative. * p < 0.05.
3.3. Phase 3: Choice Between Cocaine and Food
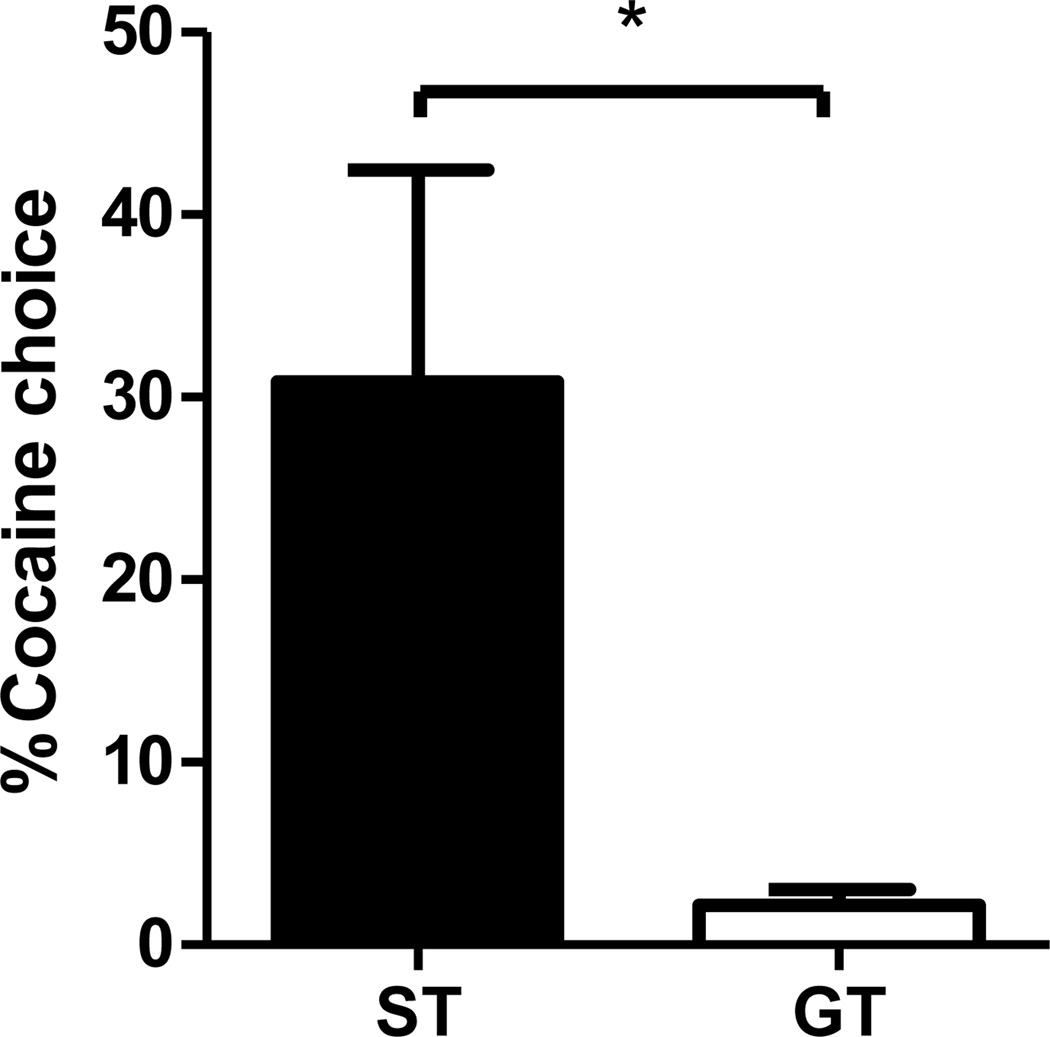
The average number of choices for cocaine averaged over the last two choice sessions was taken as a measure of rats’ degree of preference. As shown in Fig. 3, rats identified as STs had a significantly higher cocaine preference (M = 30.6%, SEM = 11.6) as compared to GTs (M = 2.1%, SEM = 0.9), and this was confirmed by a t-test (t[10.1] = −2.47, p < 0.05). Among all rats, only a small subset (3 of the 16 rats, or 18.8%) chose cocaine more frequently than food. Importantly, all of these rats were STs. In contrast, no GT chose cocaine more than once on the 28 free-choice trials making up the final two choice sessions (i.e., all GTs’ average cocaine preferences were ≤ 3.6%).
Figure 3. Cocaine vs. Food Choice.
Mean percentage (±SEM) of free choice trials (14 per session) on which STs and GTs chose drug in preference to food, averaged over the last two choice sessions. * p < 0.05.
The choice behavior of individual subjects was stable by the end of the choice phase. The average shift in preference from the 3rd to 4th (M = 4.9%, SEM = 1.6) and 4th to 5th choice session (M = 8.9%, SEM = 3.2) was approximately a one-choice difference (i.e., 7.1% change). During the choice phase, all rats completed all forced choice trials at the beginning of each of the 5 choice sessions (i.e., 2 drug and 2 food) as well as all 14 free-choice trials available in each of the 5 choice sessions. STs and GTs were compared in terms of their response latencies on the cocaine and food forced-choice trials that began the last two sessions of the choice phase (the sessions used to determine individuals’ preferences). On cocaine forced-choice trials, STs waited a mean of 7.9 s (SEM = 2.8) to press the lever, while GTs waited a mean of 179.9 s (SEM = 103.7). This difference was not significant, due to the large variability in the STs (t[4] = 1.7, p = 0.17). On the food forced-choice trials, STs and GTs had comparable latencies (M = 8.8, SEM = 4.7 vs. M = 4.9, SEM = 1.5, respectively; t[14] = 1, p = 0.32).
4. Discussion
In the present study, rats that sign-tracked to a stimulus predictive of food delivery subsequently chose cocaine over food more frequently than rats that goal-tracked in response to the same stimulus. The finding that STs chose cocaine over food significantly more than GTs adds to previous studies showing that STs generally engage in addiction-like behaviors to a greater extent than GTs. That STs (as compared to GTs) reach higher breakpoints in responding for cocaine on a progressive ratio schedule (Saunders & Robinson, 2011), show greater cocaine- and cue-induced reinstatement (Saunders & Robinson, 2010, 2011; Yager & Robinson, 2013), display greater cocaine seeking despite punishment (Saunders, Yager & Robinson, 2013), show more conditioned approach towards a cocaine-paired cue (Meyer, Ma & Robinson, 2012; Yager & Robinson, 2013), display greater cocaine-induced psychomotor sensitization (Flagel et al., 2008), and also more frequently choose cocaine over a non-drug alternative (present study) all suggest that sign-tracking is a biobehavioral marker for addiction-like behavior.
Robinson et al. (2014) hypothesize that the tendency to sign-track to a food cue is reflective of an underlying trait to generally attribute salience to incentive stimuli, including interoceptive stimuli. They suggest that STs work harder than GTs to obtain cocaine on a progressive ratio schedule because STs attribute more incentive salience to the internal cue produced by cocaine (Saunders & Robinson, 2011). Increased attribution of incentive salience to the internal cocaine cue may also explain why STs in the present experiment chose cocaine more frequently than GTs. But, if attribution of incentive salience is a general trait, then STs should have also attributed more incentive salience than GTs to interoceptive cues associated with receipt of the food alternative. If so, they would not necessarily be expected to choose cocaine more often than GTs. A higher cocaine preference in STs compared to GTs would only be expected if the heightened attribution of incentive salience in STs were at least somewhat selective to the internal cocaine cue. Such selectivity may occur as a result of cocaine producing a more readily discriminable interoceptive cue than food. Results from a recent experiment that compared cocaine and food as primers for reinstatement suggest that cocaine may indeed produce a more effective interoceptive cue (Tunstall & Kearns, 2013).
A strength of the choice procedure used in the present study is that the cocaine lever was pitted directly against the food lever. This allows for the ruling out of potential alternative explanations for increased cocaine taking by STs. For example, if STs compared to GTs simply had higher levels of non-specific motor activity or arousal, they might be more inclined to press levers generally, regardless of the reinforcer received. In the mutually exclusive choice procedure used here, however, choosing one alternative meant forgoing the other. This means that the greater cocaine taking in STs was not a function of heightened non-specific locomotor activity or arousal, but that the STs preferred pressing the cocaine lever to a greater extent than GTs. It is worth noting that lever presses in the present procedure resulted in both the primary reinforcer and the conditioned cues associated with that reinforcer. Future experiments will be necessary to determine the extent to which the choices of STs and GTs are controlled by primary vs. conditioned reinforcers in designs like that used here.
Underlying neurobiological differences may help to explain why STs differ from GTs with respect to cocaine-reinforced behaviors. Several studies have identified dopamine as the critical neurotransmitter involved in sign-tracking. Dopamine antagonism or depletion in the nucleus accumbens blocks both the acquisition and the performance of the sign-tracking response (Di Ciano et al., 2001; Flagel et al., 2011; Parkinson et al., 2002; Saunders & Robinson, 2012). Goal-tracking evoked by a lever CS is not affected by dopamine antagonism, indicating that sign- and goal-tracking (at least, with a lever CS; see Meyer, Cogan & Robinson, 2014) are mediated by dissociable neural mechanisms (Flagel et al., 2011; Saunders & Robinson, 2012). High levels of sign-tracking behavior have been associated with increased dopamine neurotransmission (Tomie et al., 2000). Further, STs differ from GTs in the level of D1 receptor mRNA in the nucleus accumbens after a single session of PCA training, and after five sessions, differ in the level of the D2 receptor mRNA in the nucleus accumbens as well as in levels of tyrosine hydroxylase and the dopamine transporter (Flagel et al., 2007).
The dopamine system is critically involved in cocaine-seeking and -taking behaviors. For example, manipulations of dopamine functioning have been shown to affect a number of the cocaine-addiction-like behaviors on which STs and GTs differ, including responding for cocaine on a progressive ratio schedule (e.g., Bari & Pierce, 2005; Hubner & Moreton, 1991; Nicola & Deadwyler, 2000; Thomsen et al., 2009; Xi et al., 2005), cocaine- and cue-induced reinstatement of cocaine seeking (e.g., Anderson, Schmidt & Pierce, 2006; Cervo et al., 2007; Crombag, Grimm & Shaham, 2002; Khroyan et al., 2000; Schmidt & Pierce, 2006; See, Kruzich & Grimm, 2001; Self et al., 1996; Pilla et al., 1999), and choice between cocaine and a non-drug alternative (e.g., Gasior, Paronis & Bergman et al., 2004; Thomsen et al., 2008; Thomsen et al., 2013). Thus, it seems likely that differences in dopamine functioning between STs and GTs play a key role in mediating the observed differences in cocaine-related behavior. Future experiments that directly manipulate the dopamine system in STs and GTs and then tests them on measures of addiction-like behavior will be needed to confirm this hypothesis.
A potential concern in the present study is that learning during the Pavlovian ST/GT phase influenced behavior during the subsequent operant choice phase. A primary concern is that STs learned to approach and contact the lever CS in the Pavlovian phase, and that this might have led them to prefer that lever during the choice phase. But there are reasons why it is unlikely that such carryover effects contributed to the main conclusion that STs choose cocaine over food more often than GTs. First, to prevent systematic bias in response/reinforcer preferences, the reinforcer designation (food vs. cocaine) across levers was counterbalanced during the choice phase, such that the lever serving as the CS during the Pavlovian phase became the cocaine response alternative for approximately half the rats while it became the food response alternative for the rest of the rats. Second, sign-tracking behavior could not have generated a bias in the amount of training received with each response/reinforcer, as the acquisition criteria ensured that rats had made approximately equal numbers of reinforced lever presses on each lever prior to entering the choice phase. Third, while rats engage in vigorous approach and contact behavior towards a lever paired with food or saccharin, they generally do not contact but only approach a lever paired with i.v. cocaine infusions (Kearns & Weiss, 2004; Madsen & Ahmed, 2014; Uslaner et al., 2006). Thus, if anything, sign-tracking during the choice phase should have contributed to increased preference for the food lever in STs (but not GTs), potentially making the conclusion that STs choose cocaine over food more than GTs a conservative one. But it is most likely that sign-tracking responses during the choice procedure contributed little, if anything, to rats’ observed preferences. In a recent study that investigated the possible influence of such sign-tracking responses in a two-lever choice procedure very similar to that used here, Madsen and Ahmed (2014) found no evidence that sign-tracking behavior contributed to rats’ choice between cocaine and saccharin.
In the present study, STs self-administered more cocaine than GTs during self-administration acquisition. Previous studies have reported either no difference in acquisition (Saunders & Robinson, 2010, 2011), or that STs self-administer more than GTs at low doses, but not high doses (Beckmann et al., 2011). Procedural differences across studies likely explain these discrepancies. Saunders and Robinson (2011) noted that their procedure was specifically designed to minimize group differences in acquisition. They used an infusion criterion that initially limited the number of infusions to 10 per session and then gradually raised this criterion as rats acquired the response. In contrast, rats in the present experiment and in Beckmann et al. (2011) were less restricted in terms of the number of infusions they could self-administer during early acquisition. In a 2013 study, Saunders et al. found no overall difference between STs and GTs across acquisition sessions, but did find a significant interaction where STs self-administered approximately the same number of infusions when the limit per session was 10 or 20, but self-administered more infusions than GTs when the infusion limit was raised to 40. It appears that differences in cocaine-taking behavior between STs and GTs may emerge as early as acquisition under conditions that allow for a sufficient number of infusions per session.
In summary, the present study adds to a growing body of research showing that the tendency to sign-track to food cues predicts addiction-like behavior in rats. These results lend further support to the incentive salience theory of addiction (Robinson & Berridge, 1993, 2000, 2001; Robinson et al., 2014). The central premise of this theory is that over-attribution of incentive salience to stimuli is the reason why individuals become addicted to drugs. Future research that identifies why certain individuals tend to attribute excessive incentive salience to stimuli, and others do not, could help to explain individual differences in the propensity to develop drug addiction. Future research that determines how excessive incentive salience attribution can be corrected may lead to the development of novel addiction treatments, or more proactively, a means for preventative intervention. Use of the ST/GT rat model will be important in accomplishing both of these goals.
Highlights.
-
-
Sign-trackers compared to goal-trackers had a higher preference for cocaine over food.
-
-
Sign-tracking is the first known behavioral predictor of increased cocaine choice in rats.
-
-
Results provide further evidence sign-tracking is a biobehavioral marker for addiction proneness.
Acknowledgements
This research was supported by Award Number R01DA008651 from the National Institute on Drug Abuse. The National Institute on Drug Abuse had no role other than financial support and as such the content is solely the responsibility of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Augier E, Vouillac C, Ahmed SH. Diazepam promotes choice of abstinence in cocaine self-administering rats. Addict Biol. 2012;17:378–391. doi: 10.1111/j.1369-1600.2011.00368.x. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PloS one. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacology. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Davey GCL, Oakley D, Cleland GC. Autoshaping in the rat: Effects of omission on the form of the response. J Exp Anal Behav. 1981;36:75–91. doi: 10.1901/jeab.1981.36-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Paronis CA, Bergman J. Modification by dopaminergic drugs of choice behavior under concurrent schedules of intravenous saline and food delivery in monkeys. J Pharm Exp Therapeutics. 2004;308:249–259. doi: 10.1124/jpet.103.052795. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign-tracking: The stimulus-reinforcer relation and directed action. Austin, TX: Psychonomic Society; 1974. [Google Scholar]
- Hubner CB, Moreton JE. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology. 1991;105:151–156. doi: 10.1007/BF02244301. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. Sign-tracking (autoshaping) in rats: a comparison of cocaine and food as unconditioned stimuli. Learn Behav. 2004;32:463–476. doi: 10.3758/bf03196042. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Gomez-Serrano MA, Weiss SJ, Riley AL. A comparison of Lewis and Fischer rat strains on autoshaping (sign-tracking), discrimination reversal learning and negative automaintenance. Behav Brain Res. 2006;169:193–200. doi: 10.1016/j.bbr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37:2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1-and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharm Exp Therapeutics. 2000;294:680–687. [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. 2014 doi: 10.1111/adb.12134. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PloS one. 2014;9:e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012;219:999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 2011. [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PloS one. 2013;8:e79465. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O'Donnell EG, Aurbach EL, Robinson TE. A Cocaine Context Renews Drug Seeking Preferentially in a Subset of Individuals. Neuropsychopharmacology. 2014;39:2816–2823. doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some, but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. The Journal of Neuroscience. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology. 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1-and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Stiers M, Silberberg A. Lever-contact responses in rats: Automaintenance with and without a negative response–reinforcer dependency. J Exp Anal Behav. 1974;22:497–506. doi: 10.1901/jeab.1974.22-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DP, Wörtwein G, Sager TN, Holm R, Caine SB. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology. 2008;201:43–53. doi: 10.1007/s00213-008-1245-1. (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. The Journal of Neuroscience. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–233. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology. 1998;139:376–382. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65:509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Tomie A, Brooks W, Zito B. Sign-tracking: the search for reward. In: Klein S, Mowrer R, editors. Contemporary Learning Theories: Pavlovian Conditioning and the Status of Traditional Learning Theory. Hillsdale, NJ: Lawrence Erlbaum Associates; 1989. pp. 191–223. [Google Scholar]
- Tomie A, Festa ED, Sparta DR, Pohorecky LA. Lever conditioned stimulus-directed autoshaping induced by saccharin-ethanol unconditioned stimulus solution: effects of ethanol concentration and trial spacing. Alcohol. 2003;30:35–44. doi: 10.1016/s0741-8329(03)00069-7. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A. Autoshaping and drug-taking. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Lawrence Erlbaum; 2001. pp. 409–438. [Google Scholar]
- Tomie A. Cam: An animal learning model of excessive and compulsive implement assisted drug-taking in humans. Clin Psychol Rev. 1995;15:145–167. [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neurosci Biobehav Rev. 1996;20:505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Tomie A, Sharma N. Pavlovian sign-tracking model of alcohol abuse. Curr Drug Abuse Rev. 2013;6:201–219. doi: 10.2174/18744737113069990023. [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Kearns DN. Reinstatement in a cocaine vs. food choice situation: reversal of preference between drug and non-drug rewards. Addict Biol. 2013;19:838–848. doi: 10.1111/adb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Riley AL, Kearns DN. Drug specificity in drug versus food choice in male rats. Exp Clin Psychopharmacology. 2014 doi: 10.1037/a0037019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Ahmed SH. Animal studies of addictive behavior. Cold Spring Harb Perspect Med. 2013;3:a011932. doi: 10.1101/cshperspect.a011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2005;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2013;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]