Abstract
Stress during pregnancy has a wide variety of negative effects in both human [1] and animal offspring [2]. These effects are especially apparent in various forms of learning and memory such as object recognition [3] and spatial memory [4]. The cognitive effects of prenatal stress (PNS) may be mediated through epigenetic changes such as histone acetylation and DNA methylation [5]. As such, the present study investigated the effects of chronic unpredictable PNS on memory and epigenetic measures in adult offspring. Mice that underwent PNS exhibited impaired spatial memory in the Morris water maze, as well as sex-specific changes in levels of DNA methyltransferase (DNMT) 1 protein, and acetylated histone H3 (AcH3) in the hippocampus, and serum corticosterone. Male mice exposed to PNS exhibited decreased hippocampal AcH3, whereas female PNS mice displayed a further reduction in AcH3, as well as heightened hippocampal DNMT1 protein levels and corticosterone levels. These data suggest that PNS may epigenetically reduce transcription in the hippocampus, particularly in females in whom this effect may be related to increased baseline stress hormone levels, and which may underlie the sexual dimorphism in rates of mental illness in humans.
Keywords: Chronic unpredictable stress, Sex differences, Mouse, Morris water maze, Histone acetylation, DNA methyltransferase
Stress has a variety of detrimental effects on both health and cognition in adult animals and humans [1,2]. Perhaps less well known is that chronic stress in pregnant mothers can substantially impact the well being of their children [1]. In rodents, experimentally-induced prenatal stress (PNS) can lead to reduced birth weight [3], masculinization of female behavior and vice versa [6], reduced immune function [7], retarded motor development and motor deficits [3,8], changes in the length of telomeric DNA [9], reduced exploratory behavior [10], and increased anxiety [3]. Furthermore, rodents exposed to PNS are impaired in a variety of cognitive tasks, including those mediated by the hippocampus. For example, prenatally stressed rodents exhibit impaired object recognition [3], active avoidance learning [11], and spatial learning in the Morris water maze [4,12].
Interestingly, the effects of PNS on behavior appear dependent on sex, although relatively few studies have examined sex differences in response to PNS. One study by Bowman et al. [6] indicated that PNS significantly increased anxiety in an open field in females relative to same sex controls, but not in males. This increased anxiety in PNS females may result from the masculinization of the female stress response, as corticosteroid levels in PNS females were similar to those of control and PNS males after a restraint stress challenge. This conclusion is consistent with the fact that corticosteroid release in all three of these groups became attenuated over the 2-h monitoring period in stark contrast to the sustained high corticosteroid levels measured in control females [6]. Other work shows that sex-differences in neuronal gene expression are reduced in rats exposed to PNS, further supporting an overall feminization of male animals and/or masculinization of females [13]. Interestingly, the study by Bowman et al. found that PNS had no effect on object recognition memory, but instead eliminated the observed male advantage in spatial working memory tested in the radial arm maze [6]. However, other work has shown that PNS impairs object recognition and extinction of cued fear conditioning in male, but not female rats [12]. Other studies using the Morris water maze have shown that PNS impairs spatial memory in males relative to females only when the task is conducted using cold (10 °C) water [14]. PNS has been reported to impair passive avoidance learning in females [15], but improves spatial memory in females [16], highlighting how substantially sex-differences in the effects of PNS depend on task and testing conditions.
Although much of the literature assumes that the mnemonic effects of PNS are due to activation of corticosteroid receptors [1,8,17], the means by which this might occur is unclear. Indeed, the relationship between cognitive function and corticosterone levels in PNS rats can be counterintuitive. For example, high levels of corticosterone are typically associated with impaired memory [18]. Yet at least one study of PNS rats tested in the Morris water maze found that the stress response was highest in mnemonically unimpaired PNS females, and minimal in mnemonically impaired PNS females [14]. As such, other neurobiological alterations may contribute to the effects of PNS on memory in males and females. For example, PNS also appears to influence neurotransmitter function, synaptic plasticity and gene expression in a sex-dependent manner. In rats and mice, PNS reduces dopamine levels in the hippocampus and prefrontal cortex of males [6] and NMDA receptors (NMDARs) in the hippocampus of both sexes [19]. The latter effect leads to reduced NMDA excitatory post-synaptic potentials (EPSPs) and decreased hippocampal long-term potentiation (LTP) [19]. This diminished NMDA activity was more substantial for female rats, which may be related to their heightened corticosteroid response to PNS [20]. However, the effects of PNS on the hippocampus are not limited to NMDARs and LTP, as neurogenesis over the lifespan decreases and age-related granule cell loss is accelerated in PNS rats of both sexes [21]. These data suggest that PNS may induce a cascade of neural events that lead to a maladaptive and dysregulated stress response, as well as impaired learning and memory, particularly in females.
Recently, epigenetic alterations have been shown to substantially regulate hippocampal memory [22–26], yet the role of epigenetic processes in mediating the effects of PNS on memory is not well understood. The most well characterized epigenetic alterations that affect hippocampal learning and memory are histone acetylation and DNA methylation [27]. The most basic unit of chromatin above the level of DNA, the nucleosome, is a segment of DNA coiled around an octamer of proteins called histones. This octamer consists of two each of the histones H2A, H2B, H3 and H4. The N-tails of these proteins protrude from the nucleosome complex and are, thus, accessible to various enzymes in the nucleus. The addition and subtraction of chemical groups on the N-tails of DNA histones plays a major role in gene regulation, particularly as it relates to vertebrate learning and memory [24,28]. This regulation at the level of the histone is referred to as the histone code [23]. The amino acid residues on histone tails can be altered by numerous post-translational modifications including acetylation, methylation, phosphorylation, ubiquitination and sumoylation [24,29]. In particular, histone acetylation is necessary for many forms of hippocampal-dependent memory in both sexes, including spatial memory, object recognition, and contextual fear conditioning [22,30–34]. Acetyl groups are added by histone acetyltransferases (HATs) and removed by histone deacetylases (HDACs). Lysine-14 acetylation on histone H3 leads to overall transcriptional activation [35], and increases expression of genes necessary for hippocampal synaptic plasticity [26]. As such, one of the goals of the present study was to determine the effects of PNS on H3 (Lysine-14) acetylation in the hippocampus.
Emerging evidence links histone acetylation with DNA methylation. DNA methylation involves the addition of a methyl group to a cytosine adjacent to a guanine in so-called CpG islands. The molecule MeCP2, which binds to methylated CpG regions on DNA and silences them, can bind HDAC1 and HDAC2 to induce histone deacetylation [36]. Although DNA methylation typically leads to transcriptional repression, this process is critical for development [37], imprinting [38], and genome stability [39], as well as many other important processes in vertebrates. DNA methylation is catalyzed by three DNA methyltransferase (DNMT) enzymes: DNMT 1, a maintenance methyltransferase, and DNMT 3a and 3b, which are de novo methyltransferases [25,42]. In particular, DNMT1 is a large enzyme (193.5 kDa) composed of a C-terminal catalytic domain with a large N-terminal regulatory domain possessing several functions [41]. Because DNMT1 has the highest expression of the three DNMTs in the brain, and directly binds to HDAC1 to suppress gene expression [43], levels of this DNMT are likely to reflect the overall amount of methylation in the genome. Relevant to the present study, DNA methylation is required for hippocampal function, as illustrated by data showing that intrahippocampal infusion of DNMT inhibitors blocks induction of hippocampal LTP, memory consolidation, and acquisition of a conditioned fear response [25,40,41]. Therefore, another goal of the present study was to examine the effects of PNS on hippocampal DNMT1 levels in males and females.
The overall goal of the present study was to determine the effects of chronic unpredictable prenatal stress on spatial memory, histone H3 acetylation, DNMT1 levels, and serum corticosterone levels. In contrast to chronic immobilization stress (CIS), where the animal is closely confined in a tube on a daily basis, chronic unpredictable stress (CUS) generally uses milder daily stressors such as light cycle disruption and overnight food deprivation given in a random order and at random times throughout the day and night [3]. There are two advantages to CUS. First, by employing a series of variable random stressors, CUS more closely resembles stressors encountered in the everyday lives of humans than restraint or footshock stressors. Second, CUS more effectively maintains an elevated stress response than CIS because it prevents habituation to the stressor [3]. As such, CUS will be used here to examine the effects of prenatal stress on memory and epigenetic mechanisms. Pregnant mouse dams were treated with CUS for 4 weeks prior to parturition. Spatial memory and levels of acetylated histone H3, DNMT1, and serum corticosterone levels were then measured in the resultant adult offspring to investigate the effects of PNS on memory, epigenetic processes, and hypothalamic-pituitary-adrenal (HPA) axis activation.
1. Methods
1.1. Subjects
Subjects were 22 female and 22 male C57BL/6 mice bred in the laboratory from dams obtained from Taconic Farms (Germantown, NY). Of these mice, 21 were born of non-stressed mothers and 23 from mothers who underwent CUS. This resulted in four groups: female control (n = 8), male control (n = 13), female prenatal stress (n = 14) and male prenatal stress (n = 9). These animals began testing at approximately 8 weeks of age and were sacrificed following testing at approximately 12 weeks. Mice were housed with littermates of the same sex in standard shoebox cages with up to five animals in a cage in a room with a 12/12 h light/dark cycle (lights on at 7:00 am). All behavioral testing was performed during the light phase of the cycle. Food and water were provided ad libitum. Dams (non-stressed, n = 4; stressed, n = 3) were housed under the same conditions, except that they were group housed in large cages with up to 10 males and females per cage. The dams gave birth in these housing conditions at approximately 12–14 weeks of age. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
1.2. Chronic unpredictable stress
CUS was administered on a daily basis for 4 weeks before and during gestation, with types and number of stressors per day varying randomly. Two to four stressors were given per day, which were chosen out a list of 18 stressors and administered in various combinations. Stressors included 15 min of aversive sound (cat meowing), 3 h of a sawdust-free cage, 1 h confinement in tube, 8 h of social isolation, 1 h of movement on an orbital shaker, 15 min of ambulation on a cart, food deprivation overnight, social instability (three bedding changes in 3 h), cage tilt of 45° overnight, lights on overnight and stroboscope lighting overnight. All stressors, except overnight stressors, were performed during the light cycle.
1.3. Morris water maze
The water maze consisted of a white circular tank (103 cm in diameter) filled with water colored white with tempera paint and maintained at 24 ± 2 °C during testing. Data were recorded using an automated tracking system (HVS Image, Hampton, U.K.). Prior to testing, the tank was divided using the tracking software into four quadrants and the start positions of the mice were located at the intersection of the quadrants 4–5 cm away from the edge of the tank. To train the mice to climb onto the platform and habituate them to the pool, mice were run in a four-trial shaping procedure in a small ring (55 cm) to limit the area of the pool. Each mouse was first placed on a visible lucite platform (10 × 10 cm) for 15 s, and then placed successively in the water with paws touching the platform so that they could climb up, halfway between the platform and the edge of the tank, and at the edge of the tank. Mice remained on the platform for 15 s after each trial. Following shaping, mice underwent spatial and cued testing.
1.3.1. Spatial testing
Spatial reference memory in the Morris water maze is dependent on the integrity of the hippocampus [44,45]. Spatial Morris water maze testing was conducted for 5 consecutive days, with each testing day consisting of six trials. The first five trials were called platform trials because a hidden escape platform was available for escape. During these trials, mice were placed at one of four starting locations and given 120 s to find an escape platform (10 cm × 10 cm), which was hidden just underneath the surface of the tempera-colored water. If the mouse failed to find the platform within 120 s, then it was manually guided there by the experimenter. Mice remained on the platform for 15 s. During these trials, swim time (s), swim distance (cm), and swim speed (cm/s) were recorded using the automated tracking system. After each trial, the mouse was dried with towels and placed in a heated holding cage until the next trial. The inter-trial interval was approximately 15 min. Of particular interest was memory during daily probe trials, because such trials assess learning of the platform location in the absence of the platform itself. Therefore, the sixth trial of each day was a 60-s variable interval probe trial in which the platform was collapsed below the surface of the water so the mice could not escape onto it for a period of time (20, 30 or 40 s) that varied randomly across days. During this period, platform crossings were manually recorded by the experimenter and normalized across intervals to yield a final measure of platform crossings/10 s interval. After the interval had elapsed, the platform was raised and the mouse given the remainder of the 60 s period to find the platform. This protocol, as described previously in [46], allows assessment of memory for the platform location without the risk of extinction, as searching behavior is reinforced by the eventual appearance of the platform.
1.3.2. Cued testing
Cued water maze learning is not dependent on the hippocampus [44] and, therefore, allows for examination of non-mnemonic aspects of task performance. Cued testing was similar to spatial testing except that the platform was covered in red and yellow tape and raised above the surface of the water so it became visible. A circular flag was also attached to the platform that extended 5 cm above the water and increased the salience of the platform. This visible platform was moved to a different quadrant for each trial. As in spatial testing, cued testing consisted of six trials/day for 5 consecutive days. However, no variable interval probe trials were conducted, so all six trials were the same. Swim time (s), swim distance (cm) and swim speed (cm/s) were recorded using the automated tracking system.
1.4. Tissue processing and Western blotting
The day after the completion of cued testing, mice were transported from the colony room to the laboratory, where they remained in their home cages for >3 min prior to cervical dislocation and decapitation. Once removed from the home cage, mice were immediately sacrificed; the interval between removal from the cage and sacrifice was the same for all mice. After collecting trunk blood for measurement of baseline corticosterone levels, the dorsal hippocampus was rapidly dissected on wet ice, and immediately frozen on dry ice in a buffer solution and stored at −80 °C until use. The dorsal hippocampus was chosen for analysis because of its particular involvement in spatial memory [47]. Tissue was homogenized by sonication. The homogenized samples were diluted 1:1 with Laemmli buffer and boiled for 5 min. SDS–PAGE was then performed using 18% (for acetylated histone H3) or 7.5% (for DNMT1) polyacrylamide gels, which were then transferred to a polyvinylidene fluoride polymer (PVDF) membrane at a constant voltage of 100 V for 70 min. The PVDF membrane was then transferred to Tris-buffered saline (TBS, 0.9% NaCl, 10 mM Tris–HCl, pH 7.5) containing 0.05% Tween 20 (TTBS) before being washed for 5 min in TBS and incubated for 1 h at room temperature in TTBS containing 5% milk. After blocking, the membrane was washed in TTBS for 20 min.
To measure levels of DNMT1 protein, membranes were incubated in a 1:500 dilution of mouse anti-DNMT1 antibody (Abcam, cat # 13537) in 1% dry milk-TTBS overnight at 4 °C on an orbital shaker. The following day, the membrane was incubated in 1:2000 anti-mouse horseradish peroxidise in 1% dry milk-TTBS for 1 h at room temperature. Bands were visualized using enhanced chemiluminescence (Perkin-Elmer, cat # NEL104001EA) and imaged on a Kodak Image Station 440CF. Kodak ID 3.6 software was used to quantify the density of each band. To normalize DNMT1 levels to total protein content, blots were subsequently stripped and reprobed for monoclonal β-Actin (1:5000; Sigma). Data were then normalized as a percentage of density relative to density of β-Actin. Effects were normalized and measured within single gels.
For levels of acetylated H3 at lysine-14 (AcH3Lys14), membranes were incubated in a 1:2000 dilution of rabbit anti-AcH3Lys14 antibody (Upstate Biologicals, cat # 06-911) in 100% TBS overnight at 4 °C on an orbital shaker. The following day, blots were rinsed with TTBS and then incubated with anti-rabbit horseradish peroxidise-conjugated IgG (Cell Signaling) in 5% milk-TTBS for 1 h at room temperature. Membranes were subsequently rinsed, incubated, and imaged as above. To normalize AcH3Lys14 levels to total H3Lys14 levels, blots were then stripped as above and reprobed with a 1:5000 dilution of rabbit anti-histone H3 antibody (Millipore, cat # 05-928) in 5% bovine serum albumin-TTBS overnight at 4 °C on an orbital shaker. The following day, the blot was incubated with anti-rabbit-HRP secondary at a 1:10,000 dilution and imaged as described above.
1.5. Corticosterone levels
Serum was extracted from trunk blood using Greiner Bio-One Minicollect tubes with centrifugation at 2000 × g for 10 min. Corticosterone levels were measured using an ELISA kit (Enzo Life Sciences, sensitivity = 26.99 pg/ml) according to the manufacturer’s instructions. This luminescence assay provided raw intensity scores from which corticosterone concentrations were calculated in reference to the equation of the standard curve as per the kit manufacturer’s instructions. These concentrations were then used in subsequent ANOVA and post-hoc tests.
1.6. Data analysis
For the spatial Morris water maze, separate 2 × 2 × 5 (stress condition × sex × day as the repeated measure, respectively) two-way repeated measures ANOVAs were conducted for the swim time, swim distance, swim speed, platform crossings, and quadrant time measures. For the cued water maze, separate 2 × 2 × 3 (stress condition × sex × day as the repeated measure, respectively) two-way repeated measures ANOVAs were conducted for the swim time, swim distance, and swim speed measures. For both Western blot and ELISA data, separate 2 × 2 ANOVAs (stress × sex) were conducted, followed by Tukey post-hoc tests to compare between groups. Finally, to examine potential correlations among platform crossing scores, corticosterone levels, DNMT1 levels, and AcH3Lys14 levels, Pearson correlation coefficients and p values were calculated for these variables. All statistical tests were performed using SPSS 17.0 software (SPSS Inc.).
2. Results
2.1. Spatial water maze
The main effect of sex was significant for swim time (F(1,40) = 6.87, p = 0.012), and swim speed (F(1,40) = 6.75, p = 0.013) with males swimming slower and taking longer to find the platform than females. The main effect of day was significant for swim time (F(4,160) = 12.65, p < 0.0001) and swim distance (F(4,160) = 26.85, p < 0.0001), reflecting a reduction in both measures over the course of testing. The main effect of PNS was not significant for any platform trial measure, nor were any interactions. See Table 1 for group means for all spatial water maze measures.
Table 1.
Group means for Morris water maze measures averaged over all spatial or cued testing trials.
| Task | Measure | Male control | Female control | Male PNS | Female PNS |
|---|---|---|---|---|---|
| Spatial | Swim time (s) | 13.5 ± 5.2 | 11.7 ± 6.6 | 28.1 ± 6.3*,+ | 20.0 ± 5.0+ |
| Swim distance (cm) | 188.2 ± 53.9 | 153.6 ± 68.8 | 228.2 ± 64.8 | 327.1 ± 52.0 | |
| Swim speed (cm/s) | 13.1 ± 1.0 | 13.0 ± 1.3 | 11.9 ± 1.2*,+,# | 13.8 ± 1.0 | |
| Platform crossings | 0.52 ± 0.06 | 0.49 ± 0.07 | 0.25 ± 0.06 | 0.43 ± 0.05 | |
| Cued | Swim time (s) | 3.7 ± 0.2 | 4.1 ± 0.3 | 4.8 ± 0.3 | 3.8 ± 0.2 |
| Swim distance (cm) | 61.5 ± 5.1 | 56.4 ± 6.5 | 85.3 ± 6.1*,+ | 76.7 ± 4.9*,+ | |
| Swim speed (cm/s) | 16.6 ± 0.8 | 14.6 ± 1.0* | 17.8 ± 1.0+ | 19.0 ± 0.8*,+ |
Note: Groups consisted of male control (n = 13), female control (n = 8), male prenatal stress (n = 9), and female prenatal stress (PNS, n = 14). Values represent the group mean ± SEM for 5 days of spatial testing.
Indicates a significant difference from male control.
Indicates a significant difference from female control.
Indicates a significant difference from female prenatal stress (p < 0.05).
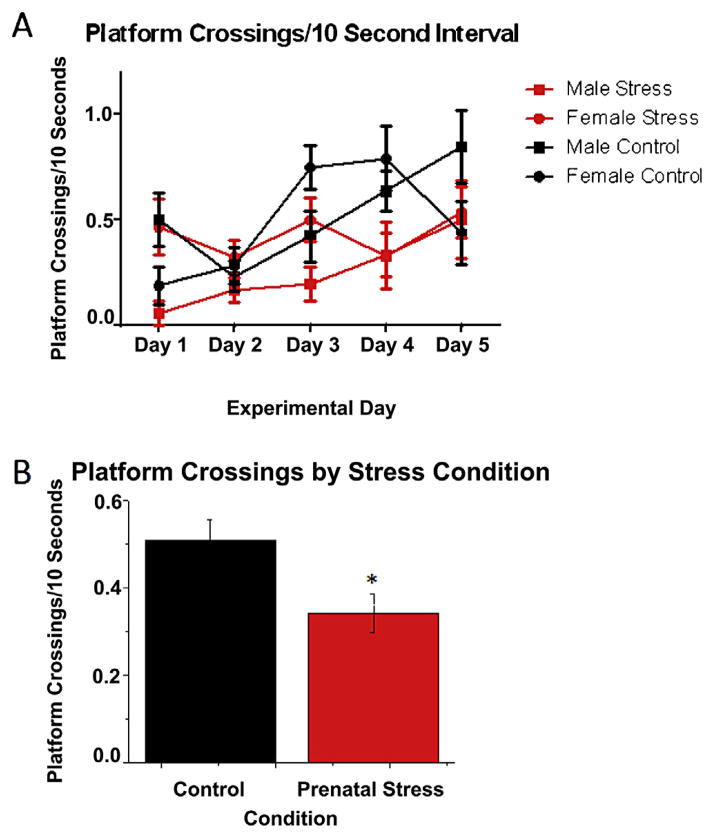
Despite the lack of effect of PNS in the platform trials, PNS affected memory in the probe trials. Platform crossings made by each group during the 5 days of testing are illustrated in Fig. 1A. Although the sex × stress × day interaction was not significant for platform crossings, the main effect of stress was significant (F(1,40) = 6.54, p = 0.014; Fig. 1B), such that the PNS mice (x̄ = 0.36) made fewer platform crossings/10 s than controls (x̄ = 0.52). The main effect of day was also significant for platform crossings (F(4,160) = 5.78, p = 0.0002; Fig. 1A) reflecting an overall increase in platform crossings made as testing progressed. The main effect of sex was not significant, nor were any other interactions.
Fig. 1.
A) Number of platform crossings per 10-s interval in the probe trial of each day of spatial Morris water maze testing. PNS mice made significantly fewer platform crossings than controls, but no other main effects or interactions were significant. B) Main effect of PNS on platform crossings, collapsed across days and sexes. Control mice made significantly more platform crossings than prenatally stressed mice (p < 0.05). Each symbol in (A) and bar in (B) represents the mean ± standard error of the mean (SEM).
2.2. Cued water maze
The main effect of PNS was significant for swim distance (F(1,40) = 8.06, p = 0.0071) and swim speed (F(1,40) = 8.96, p = 0.005), reflecting the fact that PNS mice swam shorter distances to the platform faster than controls (Table 1). The main effect of day was significant for swim time (F(2,80) = 44.55, p < 0.0001) and swim distance (F(2,80) = 11.14, p < 0.0001), resulting from a decrease in both measures across testing. The main effect of sex was not significant for any measure, nor were any interactions significant. See Table 1 for group means for all cued water maze measures.
2.3. Western blots
2.3.1. AcH3Lys14
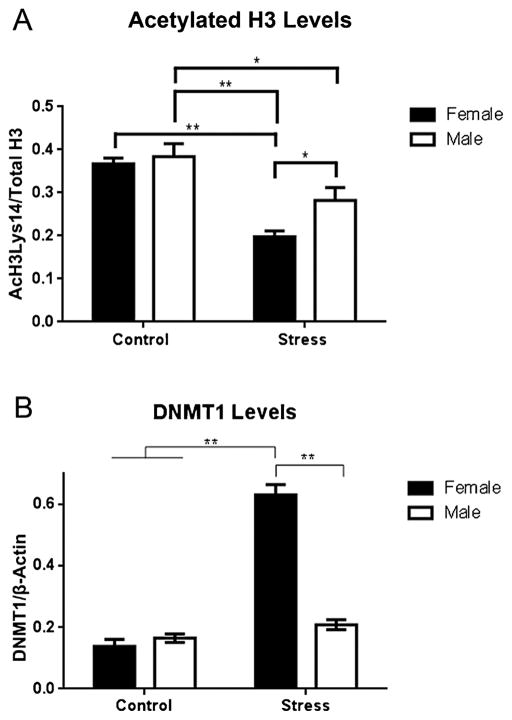
The ANOVA for AcH3Lys14 levels revealed a significant main effect of sex (F(1,35) = 5.1, p = 0.03) and stress (F(1,35) = 37.01, p < 0.0001), but not a significant interaction between the two. As shown in Fig. 2A, male controls exhibited significantly higher AcH3Lys14 levels than male PNS (p = 0.02) and female PNS (p < 0.0001) groups, but not female controls. Among PNS mice, AcH3Lys14 levels were higher in males than females (p = 0.03). Female controls also displayed significantly higher AcH3 levels than female PNS animals (p < 0.0001).
Fig. 2.
A) AcH3Lys14 levels were decreased by PNS in both sexes. AcH3Lys14 levels were significantly higher in male controls than in male and female PNS groups. The female PNS group also had lower AcH3Lys14 levels than the male PNS and female control groups. B) DNMT1 levels were significantly increased by PNS in females. The female PNS group had significantly higher DNMT1 levels than all other groups. PNS did not significantly affect DNMT1 levels in males * indicates p < 0.05, ** indicates p < 0.0001. Each bar represents the mean ± SEM.
2.3.2. DNMT1
The main effects of sex (F(1,33) = 43.65, p < 0.0001) and stress (F(1,33) = 80.04, p < 0.0001) were significant, as was the interaction (F(1,33) = 56.25, p < 0.0001) between the two. As shown in Fig. 2B, these effects were driven by the fact that DNMT1 levels were significantly higher in female PNS mice than all other groups (Fig. 3).
Fig. 3.
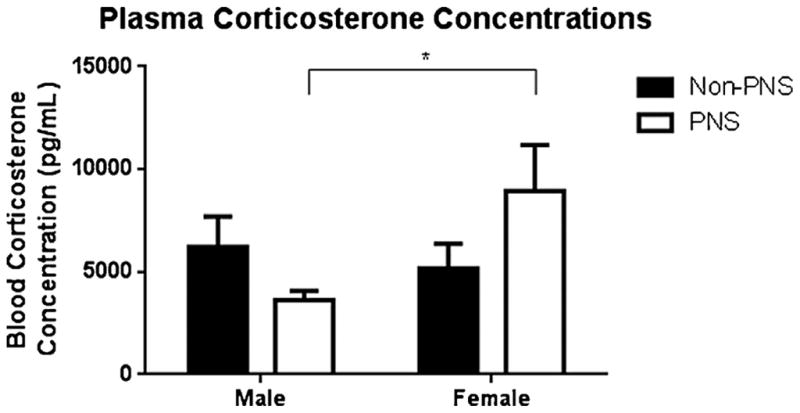
Serum corticosterone concentrations in each group. Corticosterone concentrations were higher in female PNS mice relative to male PNS mice. * indicates p < 0.05 (t-test). Each bar represents the mean ± SEM.
2.4. Corticosterone levels
Corticosterone levels were not significantly affected by sex or stress. However, the interaction approached significance (F(1,39) = 3.0, p = 0.09). An a priori t-test revealed a significant difference between female PNS (x̄ = 8958.31 pg/ml) and male PNS (x̄ = 3617.25 pg/ml) groups (p = 0.034; Fig. 4).
2.5. Correlations
Finally, we conducted a Pearson correlation analysis to examine potential relationships among all of the biochemical measures and the platform crossings data for day 5 of the spatial Morris water maze training (Table 2). None of the measures were significantly correlated except levels of H3 acetylation and DNMT1 protein, which were inversely correlated (r = −0.693, p < 0.0001). These data indicate that increased H3 acetylation was associated with decreased levels of the maintenance methyltransferase.
Table 2.
Correlation matrix for select biological and behavioral measures.
| Corticosterone level | AcH3 level | DNMT1 level | Platform crossings – Day 5 | |
|---|---|---|---|---|
| Corticosterone level | r = −0.12 | r = 0.15 | r = 0.19 | |
| p = 0.48 | p = 0.4 | p = 0.22 | ||
| AcH3 level | r = −0.12 | r = −0.693 | r = −0.01 | |
| p = 0.48 | p = 0.000003 | p = 0.94 | ||
| DNMT1 level | r = 0.15 | r = −0.69 | r = −0.08 | |
| p = 0.4 | p = 0.000003 | p = 0.64 | ||
| Platform crossings – Day 5 | r = 0.19 | r = −0.01 | r = −0.08 | |
| p = 0.22 | p = 0.94 | p = 0.64 |
3. Discussion
The effects of prenatal stress on epigenetic and behavioral measures were complex and, in the case of epigenetic measurements, sex-dependent. Our finding that prenatal chronic unpredictable stress generally impaired spatial memory in the Morris water maze is in agreement with previous work using chronic immobilization stress [12,14,21] and footshock stress [48]. However, other studies report PNS-induced spatial water maze impairments in male rats that were dependent on water temperature and phase of testing [13,14], and yet other findings indicate that PNS impairs spatial water maze performance in females only [49]. Another study testing spatial memory among mice in an object location task found a male-only deficit and a female-specific enhancement [50]. The discrepant findings produced by these studies are likely due to widely varying experimental designs that employ different types of stressors applied at different gestational days in different species and strains tested using varying Morris water maze protocols. To our knowledge, our study is the first to examine the effects of prenatal chronic unpredictable stress on spatial memory in the Morris water maze. This is important, given that CUS is likely to be a more translational animal model of PNS than restraint or footshock. Here, we found that the only measure affected by PNS was platform crossings during the spatial probe trials. The fact that the more common platform trial measures of spatial or cued learning (swim time, swim distance, and swim speed) were not affected by stress or sex suggests that the effects of prenatal CUS on spatial learning and memory are somewhat subtle. This conclusion is underscored by the fact that the only water maze measure affected by prenatal CUS was platform crossings during the probe trial, which was impaired in both males and females. Platform crossing is the most sensitive measure of memory for the platform location, as animals must be able to determine the platform location based on the spatial cues in the room without the platform itself being present. As such, this finding suggests that the effects of prenatal CUS on spatial memory in the Morris water maze are only apparent in the most challenging aspects of this task. Interestingly, the effects of PNS on platform crossings did not differ between males and females, suggesting no interaction between sex steroid hormones and the stress response in development or adulthood. However, it is important to note when interpreting these data that litter was not controlled as a variable due to the group housing environment in which our dams gave birth. Therefore, it is possible that maternal behavior and/or litter of origin could have mediated the observed effects of PNS on spatial memory. As such, these variables should be more rigorously examined in future studies.
Acetylated histone H3 (Lys 14) levels were reduced in the dorsal hippocampus by PNS in both sexes, but significantly more so for the female PNS group. This effect is notable in that previous studies have not examined the effects of PNS on histone acetylation in the brains of adult offspring. However, these data are consistent with one study showing that PNS increased histone H3 acetylation in a specific Bdnf promoter in the spinal cord dorsal horn of female, but not male, rat offspring [51]. The larger decrease in histone acetylation in PNS females is consistent with our other finding that levels of the maintenance methyltransferase DNMT1 are increased by PNS in females only. Unfortunately, the few studies examining PNS on DNMT1 levels do not specify the sex of the offspring or examine just males. In one study where offspring sex was not specified, prenatal restraint stress in rats increased DNMT1 mRNA in the cerebral cortex of adult offspring [52]. In other studies, prenatal restraint stress in male Swiss-albino mice increased DNMT1 mRNA and protein in the frontal cortex [53,54]. These latter findings contradict our results showing that PNS had no significant effect on DNTM1 protein in adult male C57BL/6 offspring. These discrepancies may result from established differences in strain sensitivity among mouse strains [55] or to differences in the effects of restraint and chronic unpredictable stressors. Nevertheless, the increase in DNMT1 levels in PNS females in the present study, together with the large reduction in histone H3 acetylation observed in PNS females, suggests that PNS produces a more closed chromatin state in the genome of female offspring than in males. How this putative transcriptional repression relates to memory, however, is unclear, given the fact that the impairment in platform crossings was evident in both sexes. Thus, the increased epigenetic susceptibility of females to PNS did not appear to translate into a greater deficit in females in the spatial water maze task. It is possible that the task was not sensitive enough to detect a difference between the sexes, or that sex differences in epigenetic processes and/or corticosterone levels induced by PNS regulate another type of memory mediated by the hippocampus or another brain region. Alternatively, PNS may produce changes in DNA methylation and histone acetylation at specific gene promoters involved in spatial memory that our more global measures could not detect. As such, future studies should be conducted to examine the effects of PNS on epigenetic regulation of specific genes involved in synaptic plasticity in the hippocampus and other cognitive brain regions.
The observed inverse correlation between AcH3 and DNMT1 levels is not surprising, as DNMT1 is associated with HDAC1 in a large macromolecular complex which uses the deacetylase as a substrate for DNA methylation [42]. Hence, lower histone acetylation promotes higher DNA methylation and vice versa, with consequent decreases and increases in gene expression and promoter occupancy. In the present study, AcH3Lys14 levels were reduced by PNS in both males and females, but PNS significantly increased DNMT1 only in females. Therefore, the data suggest that prenatally stressed female mice may be more susceptible than males to the potentially repressive effects of PNS on gene expression. At this point, however, the extent to which PNS-induced changes in H3 acetylation and DNMT1 levels lead to altered gene expression is unclear. One study showed that PNS reduced sex differences in various growth factor mRNA levels (i.e. EGF, IGF, FGF, VEGF, PDGF) in the frontal cortex and hippocampus of rats [56]. Another study reported that PNS produced an 89% reduction in the number of genes differentially expressed in the hippocampus of male and female rats [57]. These findings suggest that PNS-induced alterations in DNMT1 and AcH3Lys14 may not necessarily produce genome-wide alterations in gene expression, but rather regulate the expression of targeted genes in a sex-specific manner. Furthermore, other data suggest that PNS-induced changes in DNA methylation may depend on the origin and severity of the PNS. For example, Myschasiuk et al. [58] found that mild prenatal elevated platform stress significantly increased DNA methylation in the hippocampus and frontal cortex of adult rat offspring, whereas severe prenatal elevated platform stress decreased DNA methylation in these brain regions. These same investigators found that paternal stress decreased global DNA methylation in the frontal cortex of female rat offspring, but increased methylation in the hippocampus of both male and female offspring [59]. Thus, it would appear that the source of the prenatal stress (maternal or paternal) and the severity of the stressor play a role in regulating DNA methylation patterns in male and female offspring. Other methodological issues surely contribute as well, including type of stressor, duration of stress, and time during gestation the stressor is initiated. Clearly, much more will need to be done to determine the implications for gene expression of the epigenetic changes observed here, and the extent to which such changes influence learning and memory.
Compared with epigenetic processes, the effects of PNS on standard measures of HPA axis functioning are better understood, with evidence suggesting that maternal glucocorticoids cross the placental border and affect HPA axis reactivity of the offspring [4,60,61]. Elevated corticosterone levels are indicative of dysregulated HPA activity, and our study found the highest levels of corticosterone levels in PNS females. Interestingly, PNS females also exhibited the most significant epigenetic alterations. Given the important role of the hippocampus in inhibiting HPA activity, these alterations may have contributed to the elevated corticosterone levels observed in PNS females. Alternatively, PNS-induced HPA axis dysregulation may have contributed to epigenetic alterations in the hippocampus that reduce the expression of genes related to synaptic plasticity, thereby, impairing long-term potentiation and hippocampal-dependent memory. That the detrimental effects of PNS appear to occur preferentially in females agrees with previous work showing little or no effect of PNS on HPA axis sensitivity in males [62]. Future research should examine PNS-induced sex-specific changes in gene expression in plasticity- and learning-related genes to determine exactly which epigenetic changes lead to impaired hippocampal memory in prenatally stressed offspring and how these changes may be associated with HPA axis and glucocorticoid activity.
Despite the fact that the effects of PNS on memory can vary based on methodological factors including type and length of stressor, sex, type of memory, and testing parameters, PNS does appear to have a generally deleterious effect on hippocampal learning and memory in adult offspring. Importantly, the present study provides the first evidence that prenatal CUS administered to pregnant dams can impair spatial memory and affect both histone acetylation and an index of DNA methylation in the hippocampus of adult offspring. Prenatally stressed females were the most significantly affected, exhibiting the greatest changes in AcH3Lys14 and DNMT1 levels, as well as heightened serum corticosterone levels. Overall, these findings suggest that the female brain may be more susceptible to the detrimental effects of PNS on both the HPA axis and epigenetic processes. Indeed, previous epidemiological work has suggested that prenatal stress induced during the Israeli–Arab 6-day war of 1967 may have increased rates of schizophrenia in female offspring of mothers in the 2nd month of pregnancy [63], thereby linking prenatal stress during a defined period to an outcome of mental illness in women. Because this susceptibility could contribute to the increased risks of many psychiatric disorders (e.g., depression, anxiety) in women, additional future studies of PNS in females could provide important insight into the etiology of these disorders.
HIGHLIGHTS.
Effects of chronic unpredictable prenatal stress were tested in adult offspring.
Prenatal stress impaired spatial memory in adult male and female offspring.
Prenatally stressed females had less H3 acetylation and higher DNMT1 levels.
Prenatally stressed females had higher plasma corticosterone levels than males.
The female brain may be more susceptible to the effects of prenatal stress.
Acknowledgments
This project was supported by Yale University, the University of Wisconsin-Milwaukee, R01-AG022525 to K.M.F, the Kavli Institute for Neuroscience at Yale University, and R01-DA023999 and R01-NS038296 to P.R. We would like to thank Eileen Manning, Francesca Yi, Dr. Lu Fan, Dr. Patrick Orr, and Brianne Kent for assistance with stress administration and behavioral testing.
References
- 1.Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Behav Rev. 2002;26:457–70. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 2.Braastad B. Effects of prenatal stress on behaviour of offspring of laboratory and farmed animals. Appl Anim Behav Sci. 1998;61:159–80. [Google Scholar]
- 3.Cabrera R, Rodriguez-Echandia E, Jatuff A, Foscolo M. Effects of prenatal exposure to a mild chronic variable stress on body weight, preweaning mortality and rat behaviour. Braz J Med Biol Res. 1999;32:1229–37. doi: 10.1590/s0100-879x1999001000009. [DOI] [PubMed] [Google Scholar]
- 4.Vallee M, Maccari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur J Neurosci. 1999;11:2906–16. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 5.Mesquita A, Wegerich Y, Patchev A, Oliveira M, Leao P, Sousa N, et al. Glucocorticoids and neuro- and behavioural development. Semin Fetal Neonatal Med. 2009;14:130–5. doi: 10.1016/j.siny.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Bowman R, MacLusky N, Sarmiento Y, Frankfurt M, Gordon M, Luine V. Sexually dimorphic effects of stress on cognition, hormonal responses and central neurotransmitters. Endocrinology. 2004;45:3778–87. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 7.Kay G, Tarcic N, Poltyrev T, Weinstock M. Prenatal stress depresses immune function in rats. Physiol Behav. 1998;63:397–402. doi: 10.1016/s0031-9384(97)00456-3. [DOI] [PubMed] [Google Scholar]
- 8.Fride E, Weinstock M. The effects of prenatal exposure to predictable or unpredictable stress on early development in the rat. Dev Psychobiol. 1984;17:651–60. doi: 10.1002/dev.420170607. [DOI] [PubMed] [Google Scholar]
- 9.Entringer S, Epel E, Kumsta R, Lin J, Helhammer D, Blackburn E, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci USA. 2011;108:513–8. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low handling in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–36. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experience and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res. 2000;107:133–44. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 12.Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci. 2010;25:173–6. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomon S, Bejar C, Schorer-Apelbaum D, Weinstock M. Corticosterone mediates some but not other behavioural changes induced by prenatal stress in rats. J Neuroendocrinol. 2011;23:118–28. doi: 10.1111/j.1365-2826.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- 14.Szuran T, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–62. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- 15.Gue M, Bravard A, Meunier J, Veyrier R, Gaillet S, Recasens M, et al. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behav Brain Res. 2004;150:149–57. doi: 10.1016/S0166-4328(03)00250-X. [DOI] [PubMed] [Google Scholar]
- 16.Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–85. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Fride E, Dan Y, Gavish D, Weinstock M. Prenatal stress impairs maternal behaviour in a conflict situation and reduces hippocampal benzodiazepine receptors. Life Sci. 1985;22:2103–9. doi: 10.1016/0024-3205(85)90306-6. [DOI] [PubMed] [Google Scholar]
- 18.De Quervain D, Roozendaal B, McGaugh J. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–90. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 19.Son G, Geum D, Chung S, Kim E, Jo J, Kim C, et al. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. J Neurosci. 2006;26:3309–18. doi: 10.1523/JNEUROSCI.3850-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Q, Zhu Z, Huang S, Li H, Fan X, Jia N, et al. Sex and region difference of the expression of ERK in prenatal stress hippocampus. Int J Dev Neurosci. 2007;25:207–13. doi: 10.1016/j.ijdevneu.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Lemaire V, Koehl M, Le Moal M, Abrous D. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. PNAS. 2000;97:11032–7. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 23.Levenson J, Sweatt JD. Epigenetic mechanisms in memory formation. Nature. 2005;1:108–18. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 24.Levenson J, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci. 2006;63:1009–16. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller C, Campbell S, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood M, Hawk J, Abel T. Combinatorial chromatin modifications and memory storage: a code for memory. Learn Mem. 2006;13:241–4. doi: 10.1101/lm.278206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–7. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colvis C, Pollock J, Goodman R, Imprey S, Dunn J, Mandel G, et al. Epigenetic networks and gene regulation in the nervous system. J Neurosci. 2005;24:10379–89. doi: 10.1523/JNEUROSCI.4119-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.05.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18:71–9. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawk JD, Florian C, Abel T. Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory. Learn Mem. 2011;18:367–70. doi: 10.1101/lm.2097411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106:9447–52. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci. 2010;107:5605–10. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crosio C, Heitz E, Allis C, Borelli E, Sassone-Corsi P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci. 2003;116:4905–16. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- 36.Nan X, Ng H, Johnson C, Lahgerty C, Turner B, Eisenman R, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–90. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 37.Li E, Bestor T, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–26. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 38.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–7. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 39.Chen R, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–95. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- 40.Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;13:1319–23. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dunn G, Morgan C, Bale T. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59:290–5. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, et al. Evidence that DNA (Cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–73. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 42.Robertson K, Uzvolgi E, Liang G, Talmadge C, Sumegi J, Gonzales F, et al. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27:2291–8. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuks F, Burgers W, Brehm A, Hughes-Davies L, Kouzarides T. DNA methyl-transferase Dnmt1 associates with histone deacetylase activity. Nature. 2000;24:88–93. doi: 10.1038/71750. [DOI] [PubMed] [Google Scholar]
- 44.Morris R, Garrud P, Rawlins J, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 45.Schenk F, Morris R. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp Brain Res. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- 46.Markowska A, Long J, Johnson C, Olton D. Variable-interval probe test as a tool for repeated measurements of spatial memory in the water maze. Behav Neurosci. 1993;107:627–32. doi: 10.1037//0735-7044.107.4.627. [DOI] [PubMed] [Google Scholar]
- 47.Moser M, Moser E. Functional differentiation in the hippocampus. Hippocampus. 1998:608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Han H, Cao J, Lingjiang L, Xu L. Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus. 2006;16:431–6. doi: 10.1002/hipo.20181. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Song TB, Li YJ, He KS, Ge L, Wang LR. Prenatal restraint stress impairs learning and memory and hippocampal PKCbeta1 expression and translocation in offspring rats. Brain Res. 2007;1141:205–13. doi: 10.1016/j.brainres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Sierksma A, Prickaerts J, Chouliaras L, Rostamian S, Delbroek L, Rutten B, et al. Behavioural and neurobiological effects of prenatal stress exposure in male and female APPswe/PS1dE9 mice. Neurobiol Aging. 2013;34:319–37. doi: 10.1016/j.neurobiolaging.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Winston JH, Li Q, Sarna SK. Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterol Motil. 2014;5:715–30. doi: 10.1111/nmo.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen Penña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;6:39791–9. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matrisciano F, Tueting P, Maccari S, Nicoletti F, Guidotti A. Pharmacological activation of group-II metabotropic glutamate receptors corrects a schizophrenia-like phenotype induced by prenatal stress in mice. Neuropsychopharmacology. 2012;4:929–38. doi: 10.1038/npp.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, et al. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2012;68:184–94. doi: 10.1016/j.neuropharm.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;1:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- 56.Mychasiuk R, Gibb R, Kolb B. Prenatal stress produces sexually dimorphic and regionally specific changes in gene expression in hippocampus and frontal cortex of developing rat offspring. Dev Neurosci. 2011;33:531–8. doi: 10.1159/000335524. [DOI] [PubMed] [Google Scholar]
- 57.Biala Y, Bogoch Y, Bejar C, Linial M, Weinstock M. Prenatal stress diminishes gender differences in behavior and in expression of hippocampal synaptic genes and proteins in rats. Hippocampus. 2011;21:1114–25. doi: 10.1002/hipo.20825. [DOI] [PubMed] [Google Scholar]
- 58.Mychasiuk R, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R. Intensity matters: brain, behaviour and the epigenome of prenatally stressed rats. Neuroscience. 2011;180:105–10. doi: 10.1016/j.neuroscience.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 59.Mychasiuk R, Harker A, Ilyntskyy S, Gibb R. Paternal stress prior to conception alters DNA methylation and behaviour of developing rat offspring. Neuroscience. 2013;241:100–5. doi: 10.1016/j.neuroscience.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 60.Matthews S. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–80. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- 61.Wellberg L, Seckl J, Holmes M. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–9. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 62.Morgan C, Bale T. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–55. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, et al. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:1–9. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]