Abstract
Combination antiretroviral therapy (cART) has improved the longevity and quality of life for people living with HIV, however, it does not target virus that persists in long-lived cells, such as macrophages (MΦ)s. This allows for the development of viral reservoirs in various anatomical compartments where these cells reside, including the central nervous system (CNS), where perivascular MΦs and resident microglia constitute the principle cellular reservoir of HIV. How HIV persists in MΦs/microglia is not completely understood, however, pro-survival signaling that protects infected MΦs/microglia from apoptosis is likely important to viral persistence. Macrophage colony stimulating factor (M-CSF) is an important factor in MΦ survival and has been implicated in HIV neuropathogenesis through its ability to enhance the susceptibility of MΦs to infection and promote virus production. While M-CSF has been detected in cerebrospinal fluid of HIV infected patients, the cellular source of M-CSF in the CNS is unknown. Here, we demonstrate for the first time, that MΦs comprising perivascular cuffs and nodular lesions in SIV encephalitis (SIVE) brain are the principle source of M-CSF. These cells also serve as the primary reservoir of productive SIV infection in brain. We further demonstrate that M-CSF and IL-34, which signal through the same receptor, cFMS, enhances HIV-1 production by microglia in vitro. This is attenuated by the addition of a receptor tyrosine kinase inhibitor with high specificity for cFMS, GW2580. Together, these data suggest that cFMS signaling may be an attractive target for eliminating long-lived MΦ reservoirs of HIV in brain and other tissues.
Keywords: M-CSF, IL-34, HIV reservoirs, cFMS
Introduction
As a major target of HIV and the first cell type believed to be infected, macrophages (MΦ)s facilitate the establishment of HIV infection through virus production and dissemination to T cells and other tissue MΦs. In addition, infected MΦs aid in viral persistence by serving as long-lived cellular reservoirs of HIV in tissues where these cells reside, such as the central nervous system (CNS). Here, perivascular MΦs and resident microglia are the only cells found to harbor productive HIV, with perivascular MΦs constituting the principle reservoir of productive virus in the brain (Fischer-Smith et al., 2001). Indeed, although HIV DNA can be detected in astrocytes and microglia in brain of individuals with HIV infection without CNS disease, it is most consistently observed in perivascular MΦs, even in the absence of detectable virus production in the brain (Thompson et al., 2011).
In addition to presenting a major obstacle in virus eradication, long-lived MΦ reservoirs of HIV in the brain are believed to contribute to the development and progression of HIV-associated neurocognitive disorders (HAND), which remain a significant clinical concern, even among those successfully controlling viremia with pharmacological intervention (Sacktor et al., 2002). Although the pathogenesis of HAND is not completely understood, infected and non-infected activated MΦs and microglia are significant to the disease process through the spread of virus and production of soluble factors that may impair neuronal and glial cell functions, as well as enhance and maintain neuroinflammation. As such, investigations into how the MΦ viral reservoir is established and maintained are necessary for designing therapeutic strategies aimed at this cellular reservoir and the prevention or treatment of HAND.
A significant factor in MΦ development and survival, macrophage colony-stimulating factor (M-CSF), may also play an important role in the development and maintenance of long-lived MΦ reservoirs of HIV infection in the brain. M-CSF, which is increased in cerebrospinal fluid (CSF) of adults and children with HIV infection (Gallo et al., 1990, Gallo et al., 1991), enhances the susceptibility of MΦs to HIV infection and promotes virus replication (Kalter et al., 1991, Bergamini et al., 1994, Gruber et al., 1995). In turn, HIV infection promotes M-CSF production (Gruber et al., 1995), which supports MΦ survival through its normal biological activity. Significant to HAND, M-CSF levels in CSF of some individuals with HIV infection has been shown to associate with neurocognitive decline (Lentz et al., 2010).
M-CSF, which signals through cFMS, has recently been shown to share its receptor with interleukin (IL)-34 (Lin et al., 2008), but with reported differences in receptor affinity, signaling strength, and expression profiles (Chihara et al., 2010). cFMS ligation by either M-CSF or IL-34, however, results in phosphorylation of the same tyrosine residues within the intracellular portion of the receptor, suggesting potential shared functions by the two ligands (Chihara et al., 2010, Wei et al., 2010). It is reasonable, therefore, to suspect that both IL-34 and M-CSF support the development and maintenance of MΦ HIV reservoirs through cFMS signaling. In the work presented here, we show that perivascular and nodular MΦs are the primary source of M-CSF in SIVE. This coincides with cells that we and others have previously shown are the principle reservoir of productive HIV and SIV infection in brain (Fischer-Smith et al., 2001, Williams et al., 2001). Robust IL-34 expression is seen in the brain parenchyma, however, it is not expressed by MΦs accumulating perivascularly or within nodular lesions to the same degree as M-CSF, suggesting important differences in the two populations of brain MΦs. We further demonstrate that both M-CSF and IL-34 enhance HIV-1 production in microglia in vitro, as compared to non-treated microglia, which is attenuated by the addition of a receptor tyrosine kinase inhibitor with high specificity for cFMS, GW2580. These findings suggest that cFMS signaling may be an attractive target for eliminating long-lived MΦ reservoirs of HIV infection in brain, as well as other tissues.
Materials and Methods
Immunohistochemistry
Archived brain tissue sections (4 μm) from frontal lobe and basal ganglia of eight SIVmac251 infected (four with and four without encephalitis) and two non-infected rhesus macaques were kindly provided by Dr. Jay Rappaport. Immunohistochemistry was performed as described by us previously (Fischer-Smith et al., 2001, Fischer-Smith et al., 2004, Fischer-Smith et al.). Briefly, deparaffinized and rehydrated 4 μm brain tissue sections underwent high heat non-enzymatic antigen retrieval, followed by blocking with 20% normal goat (Lampire Biological Laboratories) or horse (Fisher Scientific) serum and overnight incubation with primary mouse monoclonal M-CSF (1:12.5; Novus Biologicals) or rabbit polyclonal IL-34 (1:200; Abcam) antibodies. Spleen from seronegative rhesus macaques was used as a positive control. Negative controls consisted of isotype antibodies used in place of the primary and tissues incubated in buffer without primary antibody. Antigen-specific staining was detected with goat-α-rabbit or horse-α-mouse biotinylated antibodies (Vector), followed by Vectastain ABC Alkaline Phosphatase and Vector Red Alkaline Phosphatase Substrate Kit (Vector Laboratories), according to the manufacturer’s instructions. Tyramide signal amplification (Perkin Elmer) was used for IL-34 detection, according to the manufacturers instructions. Following a light counterstain with haematoxylin, sections were dehydrated in xylenes, coverslipped with Permount, and analyzed under light microscopy. Immunohistochemistry for each antigen of interest was performed in a single run for all animals/groupings.
Immunohistochemistry quantification
Quantification of M-CSF and IL-34 expression in brain among the three test animal groupings was completed using a bioquantification software system (Bioquant Image Analysis Program). A total of twelve 20X microscopic fields of 0.31mm2 each were assessed per brain section using a microscope (Nikon) with a motorized x, y stage and a digital camera (Q-Imaging Retiga) that were linked to a computer with the software program. An unbiased quantification approach was used, with a random start, and then systematic sampling of 12 adjacent (non-overlapping) sites within the brain region of interest using the motorized stage option. Analysis conditions were retained across animals/groupings for each antigen of interest by white-balancing the camera prior to data acquisition and maintaining the same light intensity of the microscope for each slide. The Videocount Area Array and color thresholding options of the Bioquant software were utilized for these measurements, as previously defined in detail (Al-Shatti et al., 2005). Briefly, videocount (VC) area is defined as the number of pixels in a field that meet a user-defined color threshold of staining. The user-defined color threshold of immunostaining for each antigen (i.e., positivity of each antigen) was defined by setting the red-green-blue values to detect the range of positive signal values for a particular immunostained antigen, while excluding color ranges of other antigens and background noise. The threshold values for each antigen were stored in the computer program and maintained for each immunohistochemical analysis across all animals and groupings. Percent positivity for each field and antigen was determined by dividing the number of positive pixels (those that matched the defined color threshold) by the total number of pixels within the VC area, and multiplying by 100. Data are expressed as the percentage of antigen positivity per 0.31mm2 field. The assessment was carried out in a blinded fashion.
In-situ hybridization
SIV in-situ hybridization was performed, as described by us previously (Fischer-Smith et al., 2008a). Briefly, following deparaffinization and rehydration, tissues were permeabilized with proteinase K (Dako) and washed in diethylpyrocarbonate (DEPC)-treated PBS. Afterwards, tissues were post-fixed, quenched of endogenous peroxidase, and acetylated. Slides were washed between treatments using DEPC-treated PBS. Tissues were pre-hybridized by incubating with hybridization solution (50% foramide, saline-sodium citrate buffer of 0.3M NaCl and 0.03M sodium citrate, Denhardt’s solution, 0.25M tris(hydroxymethyl)aminomethane hydrochloride, 0.25mg/mL tRNA, 18.8mM sodium pyrophosphate, 17.3mM sodium dodecyl sulfate, 50% dextran sulfate and single-stranded salmon sperm DNA) at 45°C for one hour. This was followed by hybridization with 10ng SIV sense or α-sense digoxigenin (DIG)-labeled RNA probe (Lofstrand Labs, Ltd.), with the kind permission of Dr. Vanessa Hirsch (Hirsch et al., 1997), at 45°C overnight. Following overnight incubation, tissues were placed through a series of stringent washes and exposed to 20μg/ml RNase A. Detection of the probe was accomplished using a hydrogen peroxidase labeled antibody against DIG, followed by tyramide signal amplification (Perkin Elmer), according to the manufacturers directions. Sections were counterstained with aqueous Mayer’s Hematoxylin Solution (Sigma-Aldrich) and analyzed under light microscopy.
Primary microglia acquisition and culture
Primary human microglia were obtained through the Basic Science Core II of the Comprehensive NeuroAIDS Center (P30 MH09217; Kamel Khalili, Program Director). Briefly, fetal brain tissue (gestational age 16–18 weeks) is obtained from elective abortion procedures performed in full compliance with National Institutes of Health and Temple University ethical guidelines. Tissue is washed with cold Hanks balanced salt solution (HBSS) and meninges and blood vessels are removed. To propagate glial cultures, tissue in HBSS is digested with 0.25% trypsin (Life Technologies) and 10U/mL DNASE I (Sigma Aldrich) for 30 min at 37°C. Following neutralization of trypsin with fetal bovine serum (FBS), the tissue is further dissociated to obtain single-cell suspensions. Cells are plated in mixed glial growth media (DME:F12 media supplemented with insulin, FBS, L-glutamine, and gentamicin). The mixed culture is maintained under 10% CO2 for 5 days, and the medium is fully replaced to remove any cell debris. To enrich for microglia, flasks are placed on an orbital shaker for 14–18 hours at 200 rpm in growth media, inside the tissue culture incubator. The detached cells constitute the microglial component of the culture, which are collected and plated into a new flask in the same media used during shaking. Here, they are allowed to settle 90 minutes before re-feeding with microglial media (DME:F12, supplemented with 15% FBS, L-glutamine, gentamicin, fungizone, insulin, and D-biotin. Microglia purity is assessed by immunolabeling with anti-GFAP and GLAST1 to identify astrocytes, and anti-Iba1 and CD11b for microglia.
Microglia were plated in 6-well cell culture treated plates at approximately 600,000 cells/well and infected at MOI=0.5 with an M-tropic HIV-1 isolate, 92US727, obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, from The Multi-center AIDS Cohort Study. Following a three hour infection, cells were washed three times in culture media and re-fed with fresh microglia media with and without the addition of 5ng/ml recombinant human (rh)M-CSF or IL-34. At 3 and 5 days post-infection, supernatants were collected and the media fully replaced under the same treatment conditions but this time with and without the addition of the tyrosine kinase inhibitor, GW2580 (Sigma) at 47nM, 470nM and 4.7 μM. All conditions were performed in triplicate.
HIV-1 p24 quantification
HIV-1 production was measured in cell culture supernatant by enzyme-linked immunosorbant assay (ELISA) using the HIV-1 p24 Antigen Capture Assay (Advanced Bioscience Laboratories). Assays were performed in duplicate and according the manufacturer’s instructions. The optical density of each well was measured at 450nm using a plate reader (Dynex).
Statistical analyses
The frequencies of M-CSF and IL-34 expressing cells in brain among the different groupings were evaluated using one-way ANOVA with Tukey’s multiple comparisons post-test. Outliers were identified using Grubbs’ test or the ROUT method. HIVp24 measures from cultured microglia with and without cytokine treatment were assessed using one-way ANOVA with Tukey’s multiple comparisons post-test and a post-test for linear trend. Multiple t-tests were performed for pairwise comparisons of HIV p24 production of infected microglia without cFMS inhibition (100%) versus HIV p24 production after addition of GW2580. Statistical analyses were performed using Prism 6 for Mac OS X (GraphPad Software, version 6.0d). P-values ≤ 0.05 were considered statistically significant.
Results
Increased M-CSF expression is associated with MΦs that accumulate perivascularly and within nodular lesions in SIVE, however, expression by resident microglia is decreased in SIV infection, irrespective of the presence or absence of encephalitis
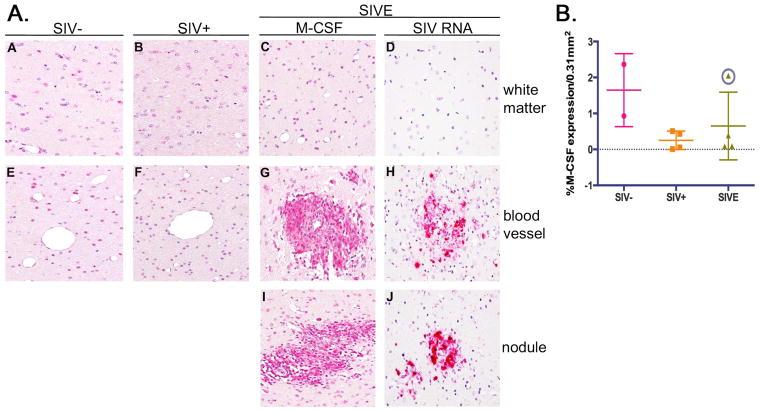
Previous studies have demonstrated that M-CSF promotes expression of CD16 by monocytes in vitro (Munn and Cheung, 1989, Ritter et al., 1999), which we’ve shown co-localizes markedly with CD163 in brain of patients with HIVE (Fischer-Smith et al., 2008a). Because of the pathological association of CD16+CD163+ MΦs and microglia with HIVE, and the potential role of M-CSF in the expansion of this subset, as well as promoting and/or advancing HIV/SIV infection and production, we examined brain tissue from seronegative and SIV infected rhesus macaques by immunohistochemistry for M-CSF expression. These studies show that M-CSF is expressed by cells in cerebral white matter of seronegative and SIV infected animals with and without encephalitis (Figure 1A, Panels A-C). Interestingly, quantitative analysis, which excluded perivascular cuffs and nodular lesions, reveals this is decreased in SIV and SIVE animals, as compared to seronegative controls (Figure 2B). This decrease does not reach statistical significance due to the small number of tissues studies and the presence of a single outlier among the SIVE animals, which was identified using Gubbs’ test for outliers (Figure 2B, circled data point). When analyzed without this outlier, a significant decrease in M-CSF is observed among the SIV infected, as compared to non-infected, animals (p=0.03).
Figure 1. Accumulating MΦs are the principle source of M-CSF in SIVE brain and are associated with productive virus.
A) M-CSF is normally expressed by glia in frontal white matter of seronegative rhesus macaques (Panels A and E). Expression is also detected in SIV infected animals (Panels B and F), including those with encephalitis (SIVE) (Panels C, G, and I). MΦs that comprise perivascular cuffs (Panel G) and nodular lesions (Panel I) appear to be the primary source of M-CSF in SIVE brain and coincide with the principle reservoir of productive SIV infection (Panels H and J). All panels shown at 400X under oil. B) Interestingly, bioquantification of M-CSF expressing cells in brain shows a decrease in SIV infection with and without encephalitis, as compared to seronegative animals. This decrease is not statistically significant due to the presence of a single outlier in the SIVE grouping (circled data point), identified by Grubbs’ test. ANOVA with this outlier removed shows a significant decrease in M-CSF expressing cells in the brain parenchyma of SIV infected animals (p=0.03). Immunohistochemical staining of twelve randomly selected 0.31mm2 fields of CNS tissue from two seronegative (●), four SIV+ (■) and four SIVE (▲) animals was quantified. Nodules and cuffs were excluded in quantification of M-CSF expression in SIVE to determine the degree of expression by resident cells. Data points represent the mean percent expression for each animal.
Figure 2. The frequency of IL-34+ cells is unchanged in brain of seronegative and SIV infected rhesus macaques.
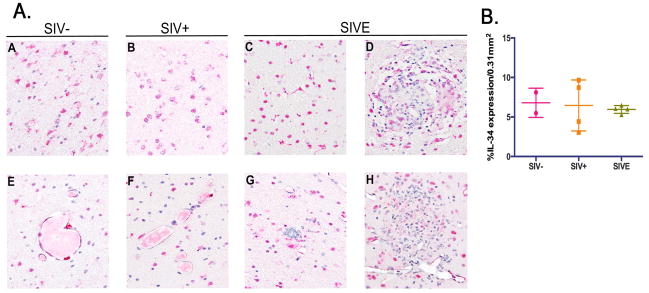
A) IL-34+ cells are observed in the white matter parenchyma of seronegative (Panel A), SIV+ (Panel B), and SIVE (Panel C) rhesus macaques. IL-34+ cells found around blood vessels in healthy animals (Panel E) and those with SIV infection (Panel F) may be perivascular MΦs and/or endothelial cells based on their location and morphology. IL-34 expression is observed within perivascular cuffs and nodular lesions in SIVE (Panels D and H), but to a considerably lesser degree than that seen with M-CSF (compare Figure 1, Panels G and I with Figure 2, Panels D and H). Multinucleated giant cells were rarely observed and do not appear to express IL-34, (Panel G). All panels are shown at 400X magnification under oil. B) In contrast to M-CSF, quantification reveals no significant changes in parenchymal IL-34 expression among the animal groupings. Immunohistochemical staining of twelve randomly selected 0.31mm2 fields of CNS tissue from two seronegative (●), four SIV+ (■) and four SIVE (▲) animals was quantified. Nodules and cuffs were excluded in quantification of IL-34 expression in SIVE to determine the degree of expression by resident cells. Data points represent the mean percent expression for each animal.
In contrast to parenchymal microglia, MΦs accumulating perivascularly and comprising nodular lesions in SIVE brain, show considerable M-CSF expression (Figure 1A, Panels G and I). Of particular interest is the finding that these cells also appear to be the major reservoir of productive SIV infection in brain (Figure 1A, Panels H and J).
IL-34 expression in cerebral white matter is unchanged in SIV infection and is not associated with MΦs accumulating perivascularly and within nodular lesions in SIVE
Recently IL-34 has been shown to utilize the same receptor as M-CSF, cFMS, and exert similar biological effects on monocytes and MΦs (Lin et al., 2008, Chihara et al., 2010), suggesting a potential role for IL-34 in HIV and SIV-associated neuropathogenesis. Immunohistochemical analysis of IL-34 expression in the brains of seronegative and SIV infected rhesus macaques shows that, like M-CSF, IL-34 is normally expressed by perivascular MΦs and resident microglia (Figure 2A, Panels A and E). This is largely unchanged in SIV infected animals with and without encephalitis (Figure 2A, Panels B, C and F; Figure 2B) and is not associated with SIVE pathology (Figure 2A, Panels D, G, and H). In stark contrast to M-CSF, the frequency of IL-34+ cells comprising perivascular cuffs and nodular lesions is considerably less than those expressing M-CSF (compare Figure 1A, Panels G and I with Figure 2A, Panels D and H).
M-CSF and IL-34 enhance HIV production in vitro, which is attenuated by inhibition of their shared receptor, cFMS
To begin to understand the effect of M-CSF and IL-34 on HIV-1 replication in microglia, we infected primary human microglia with an M-tropic HIV-1 isolate, 92US727, with and without 5ng/ml of rhM-CSF or rhIL-34. Both cytokines appear to enhance to virus production, as determined by HIV-1 p24 quantification (Figure 3, Panel A). This is greater than that observed in microglia cultured without M-CSF or IL-34 treatment, however, statistical significance was not seen in this study. Using the ROUT method for identification of outliers among the different culture conditions revealed a single outlier in the “media only” grouping (Figure 3A, circled data point). The exclusion of this single point reveals a linear trend between the column means (media<M-CSF<IL-34) that is statistically significant (p=0.046).
Figure 3. M-CSF and IL-34 enhance virus production in microglia in vitro, which is attenuated by cFMS inhibition.
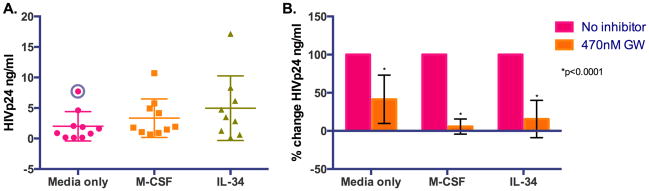
Primary human microglia were infected at MOI=0.5 with an M-tropic HIV-1 isolate, 92US727, with and without 5ng/ml of rhM-CSF or rhIL-34. Both cytokines appear to enhance to virus replication, compared to ‘media alone’, as determined by HIV-1 p24 quantification (Panel A). Marginally higher virus production is seen with IL-34, over M-CSF, which may reflect the higher affinity IL-34 has for their shared receptor, cFMS. A significant linear trend (media<M-CSF<IL-34) is seen when a single outlier, identified by the ROUT method, in the “media only” grouping (circled data point) is removed (p=0.046). Virus production is attenuated by the addition of GW2580, a tyrosine kinase inhibitor with high specificity for the receptor cFMS (Panel B), supporting a role for downstream cFMS signaling events in virus replication.
Although it is unclear why greater virus production is seen with IL-34 treatment in this small study, it may be a reflection of the higher affinity of IL-34 for cFMS (Chihara et al., 2010). This notion is supported by the finding that enhanced HIV-1 production by M-CSF or IL-34 is attenuated by the addition of GW2580, a tyrosine kinase inhibitor with high specificity for the receptor cFMS that is shared by both cytokines (Figure 3, Panel B). Using the reported IC50 of GW2580 for growth inhibition of M-CSF treated primary monocytes of 470nM (Conway et al., 2005), a significant decrease in HIV p24 is seen in all culture conditions (Figure 3B) without appreciable cell loss. These data suggest a role for cFMS signaling through either of its known ligands in virus production by brain MΦs and microglia.
Discussion
Efforts toward HIV eradication have been thwarted by the ability of the virus to persist in cellular reservoirs in various anatomical compartments. Even in patients under successful pharmacological therapy, low level virus production from these viral reservoirs can contribute to disease pathogenesis through the continued spread of virus, chronic immune activation, and disruption or impairment of cellular processes. Furthermore, continued virus replication while on cART risks the development of viral quasi-species that are resistant to cART, allowing for expansion of cART-resistant strains and the development of AIDS and AIDS-related pathologies.
Tissue MΦs, including brain perivascular MΦs and microglia, are well-recognized as an important reservoir of viral persistence in HIV infection. In the brain, infected MΦs and microglia pose additional concerns due to the presence of virus and viral proteins, as well as chronic inflammation in the CNS, all of which have been implicated in the development and progression of HIV-associated neurocognitive impairment. M-CSF, an important hematopoietic cytokine involved in monocyte/MΦ proliferation, differentiation, and survival, is believed to promote and/or contribute to HIV neuropathogenesis through its ability to increase the susceptibility of MΦs and microglia to HIV infection and promote virus production in these cells, as well as induce chemotaxis of activated monocytes/MΦs into the CNS. M-CSF is also an important modulator of MΦ/microglial activity and has been implicated in the pathogenesis of other neurodegenerative diseases, including Alzheimer’s disease (Laske et al., 2010).
Although microglia, the primary modulators of immune activity in the CNS, express M-CSF, it has also been reported to be expressed by neurons and astrocytes (Frei et al., 1992, Raivich et al., 1998). As such, any or all of these cells may contribute to the pool of CSF M-CSF reported in HIV infection. The cellular source of M-CSF in brains of HIV infected humans or SIV infected rhesus macaques, however, has not yet been identified. Here, we show for the first time, significant M-CSF positivity by MΦs accumulating perivascularly and within nodular lesions in SIVE. Previously, we reported that these MΦs are phenotypically similar to cells that have recently entered the brain in HIVE and SIVE (Fischer-Smith et al., 2001, Fischer-Smith et al., 2008b), suggesting that infiltrating monocytes and MΦs from the peripheral blood are a rich source of M-CSF in SIVE brain. In addition, we found the number of M-CSF-expressing resident (parenchymal) cells is significantly decreased in SIV infection, as compared to non-infected animals, regardless of the presence of encephalitis. These findings suggest infiltrating monocytes and MΦs that accumulate perivascularly and within nodular lesions, rather than resident cells, are the primary source of M-CSF in SIVE brain.
The finding that M-CSF expression is decreased in the brain parenchyma is surprising and what is behind this decrease is unclear. Recently, we reported prominent neuroinflammation in brains of HIV infected subjects without encephalitis or productive infection that is similar but less severe than that seen in HIVE (Tavazzi et al., 2014). It is possible that the decrease in M-CSF we observe in SIV brain is due to increased secretion of the protein by astrocytes and/or microglia in response to chronic inflammation, even in the absence of gross pathological alterations or productive virus. In support of this notion, in vitro studies using mouse astrocytes report increased astrocytic M-CSF secretion in response to stimulation with tumor necrosis factor (TNF)-α or IL-1 (Frei et al., 1992), cytokines found to be elevated in CSF and post-mortem brain tissue of HIV infected individuals with severe neurocognitive impairment (Tyor et al., 1992). This same study demonstrated that, while specific stimuli promoted M-CSF release by astrocytes in vitro, it did not upregulate M-CSF mRNA (Frei et al., 1992). This may suggest that astrocytes contribute to an initial burst of M-CSF expression that would coincide with a rapid upregulation of its receptor, cFMS, by microglia, which has been reported in a mouse model of acute CNS injury (Raivich et al., 1998), but does not sustain high levels of production in chronic neuroinflammation, such as that seen in HIV and SIV infection.
In addition to serving as the primary source of M-CSF in SIVE brain, perivascular MΦs and nodular lesions appear to be the primary reservoir of productive SIV infection in the brain, in agreement with earlier studies by us and others (Fischer-Smith et al., 2001, Williams et al., 2001). In HIV infection, the relationship between the HIV and M-CSF constitutes a positive-feedback loop, where HIV infection of MΦs increases M-CSF production, which, in turn, enhances the susceptibility of MΦs to HIV infection and promotes virus replication (Kalter et al., 1991, Bergamini et al., 1994, Gruber et al., 1995). This increase in susceptibility occurs through alterations in monocyte differentiation and/or activation by M-CSF, rather than a direct effect of M-CSF on the virus (Kalter et al., 1991, Bergamini et al., 1994, Gruber et al., 1995). Additionally, M-CSF serves as a key factor in MΦ survival and as such, it is plausible that M-CSF expression by infiltrating infected MΦs plays a major role the development and maintenance of long-lived viral MΦ/microglial reservoirs within the CNS compartment.
IL-34, which utilizes the same M-CSF receptor, cFMS, (Lin et al., 2008) is also seen in brain, but does not appear to have significant overlap with M-CSF. Recent findings in mice indicate IL-34 is the dominant cFMS ligand within tissues, while M-CSF has been more extensively studied in association with hematopoiesis and bone development (Wei et al., 2010, Wang et al., 2012). In the studies reported here, we find that while significant expression of M-CSF by perivascular and nodular MΦs is seen in SIVE, these MΦs do not produce appreciable levels of IL-34. This supports the notion of a spatial relationship between the two cytokines (Wei et al., 2010), where IL-34 expression and function is limited to specific tissues (Wang et al., 2012) and M-CSF plays a larger role in the skeletal and hematopoietic systems. Accordingly, M-CSF, which is normally detected in plasma, may contribute to the increased frequency of circulating CD16+CD163+ monocytes in viremic HIV infected subjects reported by ourselves and others (Allen et al., 1991, Locher et al., 1994, Thieblemont et al., 1995, Fischer-Smith et al., 2008b), as M-CSF increases the frequency of this subset in vitro, while IL-34 contributes more to microglial activation and CD16/CD163 expression in HIV brain (Fischer-Smith et al., 2001, Tavazzi et al., 2014). M-CSF may also support the significant accumulation of CD16+/CD163+ MΦs from the peripheral blood in HIVE/SIVE brain that serves as the principle reservoir of productive virus in the CNS (Fischer-Smith et al., 2001, Fischer-Smith et al., 2008a). Since M-CSF enhances both virus production and the susceptibility of MΦs to infection (Bergamini et al., 1994, Gruber et al., 1995), M-CSF expression by accumulating MΦs harboring HIV/SIV infection would, presumably, promote virus replication in these cells and enhance the spread of virus to non-infected perivascular MΦs in HIVE/SIVE, as well as resident microglia.
In the work described here, we report both cFMS ligands, M-CSF and IL-34 are expressed in brain of rhesus macaques, but only M-CSF expression appears to be altered in animals with SIV and is associated with cells that serve as the principle reservoir of virus production. M-CSF and IL-34 support monocyte production and differentiation to MΦs, monocyte/MΦ chemotaxis via C-C chemokine receptor type 2 (CCR2) and (C-C motif) ligand 2 (CCL2), and MΦ survival (Lin et al., 2008, Chihara et al., 2010, Barve et al., 2013, Foucher et al., 2013), supporting a role for both cytokines in the pathogenesis of neuroAIDS. Furthermore, M-CSF and IL-34 enhance virus production by microglia in vitro, as compared to those without cytokine treatment. This affect is attenuated by inhibiting cFMS signaling, suggesting that downstream signaling events promote virus replication. Although additional studies are required to understand the mechanisms behind cFMS signaling and inhibition in HIV replication, as well as its potential role in maintaining activation of MΦs and microglia, these early studies identify cFMS as an attractive target for therapeutic design aimed at eliminating MΦ reservoirs of HIV infection.
Acknowledgments
Funding sources: RO1 NS063605; T32 MH079785; P30 MH09217
The authors would like to thank Dr. Mary Barbe and the Basic Science Core II of the Comprehensive NeuroAIDS Center (CNAC; P30 MH0921777) for assisting with the immunohistochemical quantification. CNAC Basic Science Core I provided the primary microglial cell cultures.
Footnotes
Conflict of Interest Statement
Dr. Tracy Fischer-Smith and Lindsey Gerngross have no ethical or financial conflicts of interest to declare.
References
- Al-Shatti T, Barr AE, Safadi FF, Amin M, Barbe MF. Increase in inflammatory cytokines in median nerves in a rat model of repetitive motion injury. J Neuroimmunol. 2005;167:13–22. doi: 10.1016/j.jneuroim.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JB, Wong HL, Guyre PM, Simon GL, Wahl SM. Association of circulating receptor Fc gamma RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-beta. J Clin Invest. 1991;87:1773–1779. doi: 10.1172/JCI115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barve RA, Zack MD, Weiss D, Song RH, Beidler D, Head RD. Transcriptional profiling and pathway analysis of CSF-1 and IL-34 effects on human monocyte differentiation. Cytokine. 2013;63:10–17. doi: 10.1016/j.cyto.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Bergamini A, Perno CF, Dini L, Capozzi M, Pesce CD, Ventura L, Cappannoli L, Falasca L, Milanese G, Calio R, et al. Macrophage colony-stimulating factor enhances the susceptibility of macrophages to infection by human immunodeficiency virus and reduces the activity of compounds that inhibit virus binding. Blood. 1994;84:3405–3412. [PubMed] [Google Scholar]
- Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, Kimura F, Okada S. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010;17:1917–1927. doi: 10.1038/cdd.2010.60. [DOI] [PubMed] [Google Scholar]
- Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, Jansen M, Lin P, Payne A, Crosby RM, Johnson JH, Frick L, Lin MH, Depee S, Tadepalli S, Votta B, James I, Fuller K, Chambers TJ, Kull FC, Chamberlain SD, Hutchins JT. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005;102:16078–16083. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008a;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, Rappaport J. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol. 2004;164:2089–2099. doi: 10.1016/S0002-9440(10)63767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008b;24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects of GM-CSF and IFNgamma. PLoS ONE. 2013;8:e56045. doi: 10.1371/journal.pone.0056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei K, Nohava K, Malipiero UV, Schwerdel C, Fontana A. Production of macrophage colony-stimulating factor by astrocytes and brain macrophages. J Neuroimmunol. 1992;40:189–195. doi: 10.1016/0165-5728(92)90133-6. [DOI] [PubMed] [Google Scholar]
- Gallo P, Laverda AM, De Rossi A, Pagni S, Del Mistro A, Cogo P, Piccinno MG, Plebani A, Tavolato B, Chieco-Bianchi L. Immunological markers in the cerebrospinal fluid of HIV-1-infected children. Acta Paediatr Scand. 1991;80:659–666. doi: 10.1111/j.1651-2227.1991.tb11926.x. [DOI] [PubMed] [Google Scholar]
- Gallo P, Pagni S, Giometto B, Piccinno MG, Bozza F, Argentiero V, Tavolato B. Macrophage-colony stimulating factor (M-CSF) in the cerebrospinal fluid. J Neuroimmunol. 1990;29:105–112. doi: 10.1016/0165-5728(90)90152-d. [DOI] [PubMed] [Google Scholar]
- Gruber MF, Weih KA, Boone EJ, Smith PD, Clouse KA. Endogenous macrophage CSF production is associated with viral replication in HIV-1-infected human monocyte-derived macrophages. J Immunol. 1995;154:5528–5535. [PubMed] [Google Scholar]
- Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins WR, Montefiori DC. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter DC, Nakamura M, Turpin JA, Baca LM, Hoover DL, Dieffenbach C, Ralph P, Gendelman HE, Meltzer MS. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J Immunol. 1991;146:298–306. [PubMed] [Google Scholar]
- Laske C, Stransky E, Hoffmann N, Maetzler W, Straten G, Eschweiler GW, Leyhe T. Macrophage colony-stimulating factor (M-CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2010;7:409–414. doi: 10.2174/156720510791383813. [DOI] [PubMed] [Google Scholar]
- Lentz MR, Degaonkar M, Mohamed MA, Kim H, Conant K, Halpern EF, Sacktor N, Barker PB, Pomper MG. Exploring the relationship of macrophage colony-stimulating factor levels on neuroaxonal metabolism and cognition during chronic human immunodeficiency virus infection. J Neurovirol. 2010;16:368–376. doi: 10.3109/13550284.2010.513029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- Locher C, Vanham G, Kestens L, Kruger M, Ceuppens JL, Vingerhoets J, Gigase P. Expression patterns of Fc gamma receptors, HLA-DR and selected adhesion molecules on monocytes from normal and HIV-infected individuals. Clin Exp Immunol. 1994;98:115–122. doi: 10.1111/j.1365-2249.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Cheung NK. Antibody-dependent antitumor cytotoxicity by human monocytes cultured with recombinant macrophage colony-stimulating factor. Induction of efficient antibody-mediated antitumor cytotoxicity not detected by isotope release assays. J Exp Med. 1989;170:511–526. doi: 10.1084/jem.170.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Haas S, Werner A, Klein MA, Kloss C, Kreutzberg GW. Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: a quantitative immunofluorescence study using confocal laser microscopy. J Comp Neurol. 1998;395:342–358. doi: 10.1002/(sici)1096-9861(19980808)395:3<342::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ritter M, Buechler C, Langmann T, Orso E, Klucken J, Schmitz G. The scavenger receptor CD163: regulation, promoter structure and genomic organization. Pathobiology. 1999;67:257–261. doi: 10.1159/000028105. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain Inflammation is a Common Feature of HIV-Infected Patients Without HIV Encephalitis or Productive Brain Infection. Curr HIV Res. 2014 doi: 10.2174/1570162x12666140526114956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179:1623–1629. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, Lin H, Stanley ER. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]