Abstract
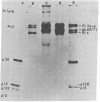
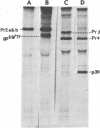
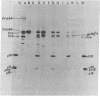
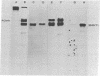
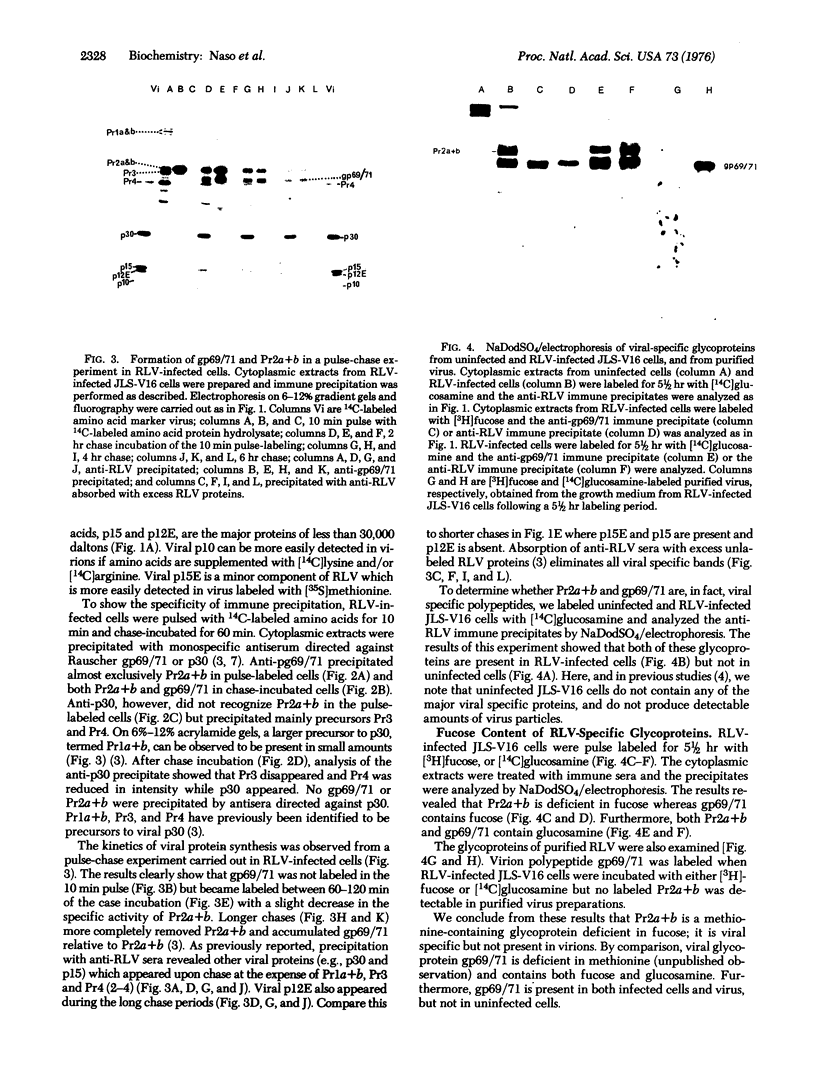
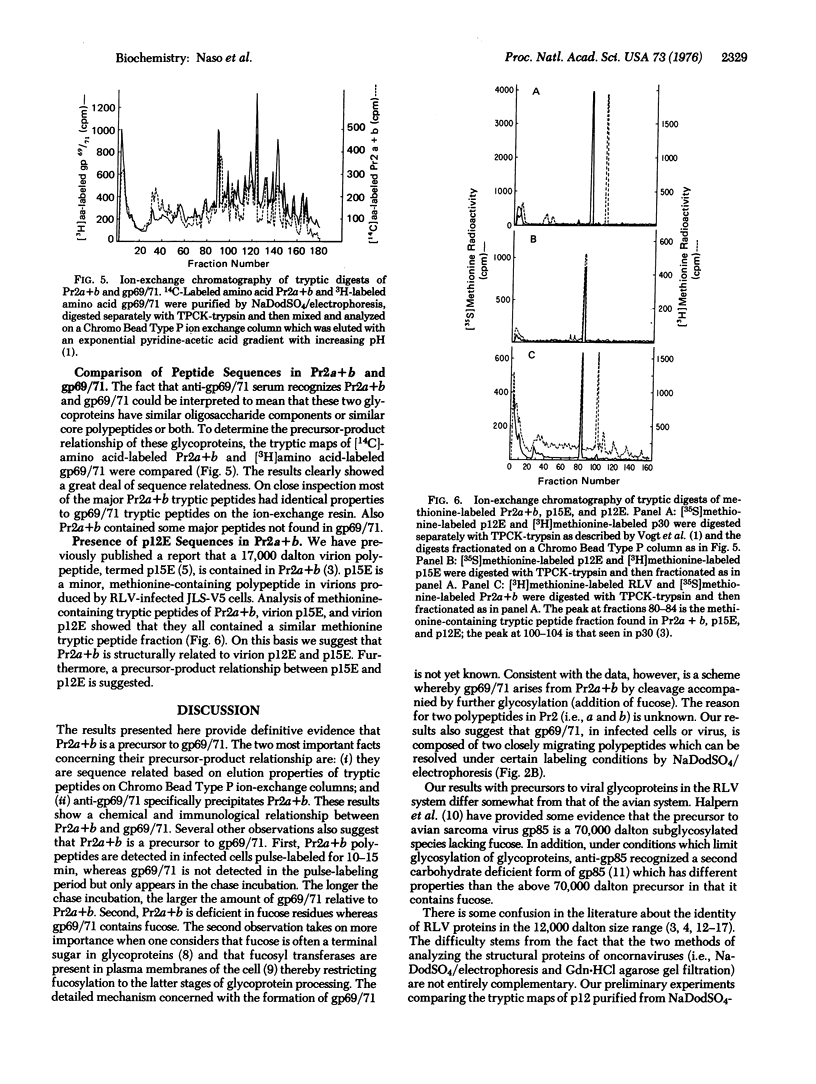
Rauscher leukemia virus glycoprotein gp69/71 is synthesized in virus-infected cells by way of a 90,000 dalton glycoprotein precursor, termed Pr2a+b. This precursor could be labeled with radioactive glucosamine and methionine but not with fucose; whereas gp69/71 could be detected by labeling with radioactive glucosamine, fucose, or a mixture of amino acids but seemed to be deficient in methionine relative to Pr2a+b. Pr2a+b and gp69/71, were specifically precipitated by an antiserum prepared against phosphocellulose purified Rauscher gp69/71. Other virus-specific precursors, in addition to Pr2a+b, could be precipitated by antiserum prepared against detergent disrupted virus. Neither Pr2a+b nor gp69/71 was precipitated from cell extracts by antisera to Rauscher p30. Tryptic maps of Pr2a+b and gp69/71 showed that these glycoproteins share many tryptic peptides. Pulse-chase experiments with 14C-labeled amino acids indicated that gp69/71 was not radio-labeled during the pulse-labeling period but slowly appeared during the chase incubations. Pr2a+b, however, was rapidly labeled and tended to disappear during long chases. Furthermore, two nonglycosylated viral proteins, termed p15E and p12E, are structurally related to Pr2a+b. Viral p15E and p12E contained the same methionine-containing tryptic peptide fraction as Pr2a+b as determined by ion-exchange chromatography. These results provide evidence that Pr2a+b is a precursor to gp69/71 and establish a structural and possible precursor-product relationship between Pr2a+b, p15E, and p12E.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Huper G., Green R. W., Graf T. Biochemical properties of oncornavirus polypeptides. Biochim Biophys Acta. 1974 Dec 31;355(3-4):220–235. doi: 10.1016/0304-419x(74)90011-0. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Glycoprotein biosynthesis: the characterization of two glycoprotein:frucosyl transferases in HeLa cells. Arch Biochem Biophys. 1968 Nov;128(2):470–481. doi: 10.1016/0003-9861(68)90053-2. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Lewandowski L. J. Isolation of the major viral glycoprotein and a putative precursor from cells transformed by avian sarcoma viruses. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2342–2346. doi: 10.1073/pnas.71.6.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Hardy W., Jr, Tress E., Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. V. Identification of a new murine viral protein, p15(E). J Virol. 1975 Jul;16(1):53–61. doi: 10.1128/jvi.16.1.53-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G., Karshin W. L., Naso R. B., Arcement L. J., Arlinghaus R. B. Proteins of Rauscher murine leukemia virus: resolution of a 70,000-dalton, Nonglycosylated polypeptide containing p30 peptide sequences. Virology. 1975 Nov;68(1):135–145. doi: 10.1016/0042-6822(75)90155-5. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Smith R. E., Bolognesi D. P., Halpern M. S. Viral glycoprotein synthesis under conditions of glucosamine block in cells transformed by avian sarcoma viruses. Virology. 1975 Aug;66(2):347–355. doi: 10.1016/0042-6822(75)90208-1. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Pal B. K., McAllister R. M., Gardner M. B., Roy-Burman P. Comparative studies on the structural phosphoproteins of mammalian type C viruses. J Virol. 1975 Jul;16(1):123–131. doi: 10.1128/jvi.16.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W., Hunsmann G., Moennig V., Noranha F., Bolognesi D. P., Green R. W., Hüper G. Polypeptides of mammalian oncornaviruses. II Characterization of murine leukemia virus polypeptide (p 15) bearing interspecies reactivity. Virology. 1975 Jan;63(1):48–59. doi: 10.1016/0042-6822(75)90369-4. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Syrewicz J. J., Naso R. B., Wang C. S., Arlinghaus R. B. Purification of large amounts of murine ribonucleic acid tumor viruses produced in roller bottle cultures. Appl Microbiol. 1972 Sep;24(3):488–494. doi: 10.1128/am.24.3.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]