Abstract
Too controversial to discuss only a short time ago, achieving a cure for HIV infection has become a priority in HIV research. However, substantial challenges must be overcome. Among key hurdles to be surmounted is the definition of a reliable, validated model in which to test latency reversal agents (LRAs), as current primary cell models differ in their response to such agents. Animal models such as the HIV-infected humanized BLT mouse and SIV-infected macaque will be essential to study LRAs, and to quantify their effects in anatomic reservoirs. Of several potential anatomic reservoirs, the central nervous system presents a significant obstacle, as it is known to harbor persistent HIV infection, and is difficult to access for study and therapeutic intervention.
Many obstacles stand in the way of efforts to target latent HIV infection with the goal of eradication, chief among them the dilemma of identifying all relevant cellular and anatomic reservoirs of latent, persistent infection, and determining whether virus in those reservoirs is able to be reactivated. Thus far, the cellular reservoir that exists within the resting central memory CD4+T cells is the best characterized, however many other potential sanctuaries have been described. Most recently, the newly-described stem-like T memory cells (Buzon et al, 2014), and γδ T cells (Soriano-Sarabia et al, 2013) have been reported to harbor latent HIV infection. Virus has long been thought to persist in macrophages, and specific strategies may be required to address persistence within these cells. In addition and of substantial importance, HIV may hide in anatomic compartments such as the central nervous system (CNS).
Timothy Ray Brown, known as the “Berlin Patient,” is the first reported case of HIV cure (Hutter et al, 2009). HIV-infected and diagnosed with acute myeloid leukemia, Mr. Brown underwent two stem cell transplantations from an unrelated donor who was homozygous CCR5 32 mutation, and so donor cells were absolutely resistant to CCR5-using HIV strains. More than six years after the transplant, plasma HIV viral load and PBMCs DNA remain undetectable in the absence of antiretroviral therapy (Yukl et al, 2013). This inspiring case encouraged similar studies, and thus two HIV-infected individuals also suffering from lymphoma interrupted therapy after bone marrow transplant. These “Boston Patients,” however, remained without viremia for only 12 and 31 weeks. Several reasons could have accounted for this disappointing outcome, including a less intense preconditioning protocol, and the lack of a transplant with the CCR5 32 phenotype. However, before interrupting therapy, patients were tested and found to be negative for HIV-DNA in PBMCs, as well as without plasma viremia. Perhaps viral rebound was due to release of virus from anatomic reservoirs, such as the CNS. CNS reservoirs add a level of complexity to attempts to cure HIV infection, due to the uncertain access of therapeutic interventions, and the difficulty of sampling and studying the events in this privileged site.
Genetic compartmentalization in the CNS in the absence of antiretroviral treatment failure has been observed, suggesting autonomous viral evolution within the CNS (Harrington et al, 2009). However treatment intensification with raltegravir did not alter CSF viral load measured by single-copy assay (Dahl et al, 2011), suggesting that ongoing viral evolution within the CNS may not occur during ART.
The CNS is distinguished by the selective permeability of the blood brain barrier (BBB). Comprised of a basement membrane, brain endothelial cells, pericytes and astrocytes, in addition to efflux pumps and enzymes, the BBB reduces the entry of many small molecule drugs, creating a potential pharmacologic sanctuary. The capacity to penetrate the brain differs among the different antiretroviral drugs, and an index has been developed to reflect this: CNS penetration effectiveness (Letendre et al, 2010). CPE classifies drugs in four groups according to their penetration into the CNS, ranging from 1 (poor penetration) to 4 (high penetration). This score has been correlated with cerebrospinal fluid (CSF) viremia (Cusini et al, 2013) and neurocognitive dysfunction (Ciccarelli et al, 2013). However, obvious sampling limitations exist to accurately measure the magnitude of infection in the CNS, and for instance virus levels in the brain parenchyma can only be quantitated upon autopsy, and might differ from CSF measures. It is believed that improved drug delivery to the CNS will reduce the underlying persisting infection in the CNS and thus reduce the establishments of reservoirs. A proposed strategy to achieve this enhanced delivery of ART includes the use of nanotechnology. Nanoparticles (NPs) are solid colloidal particles in the nanometer range which can be used as delivery systems by absorption, entrapment or covalent attachment of therapeutic drugs. Several different types of nanoparticles have been developed differing in their nature, such as liposomes, dendrimers, micelles and inorganic crystals (Sanvicens and Marco, 2008). In addition, some antiretroviral drugs can be nanoformulated and carried into monocyte-derived macrophages (Dou et al, 2009). These systems have proven to increase CNS drug penetration, in addition to offering potential for further benefits including the simultaneous deliver of different molecules or the increase of the drug bioavailability. Gold nanoparticles (AuNPs) comprise an interesting nanoparticle approach to be used for HIV therapy. Its small size, ~2nm diameter, inert nature and multivalency make them attractive as carrier for drugs. AuNPs can be conjugated with an entry inhibitor and exert antiviral activity (Bowman et al, 2008). In addition, entry of AuNPs has been demonstrated in lymphocytes, macrophages and microendothelial cells, without observable short-term toxicity. Further, such particles can be fabricated to display conjugates of raltegravir, an HIV integrase inhibitor, and inhibit viral replication. Finally AuNPs cross the BBB and can be found in the brain parenchyma in mice (Garrido et al, 2013)
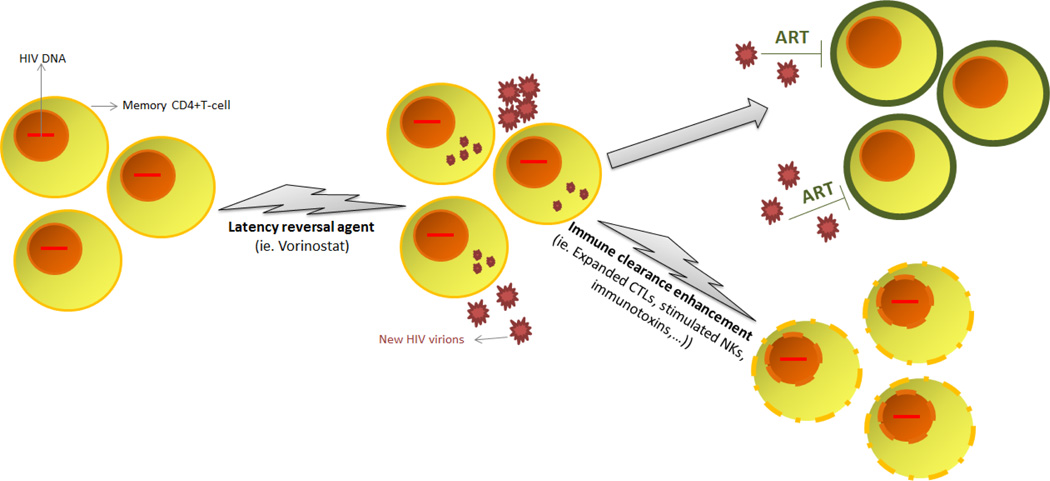
Currently, several efforts to eliminate the HIV reservoir begin with the concept of reversing latency within resting CD4+ T cells with small molecules that target host mechanisms crucial for the maintenance of latency. Not unexpectedly, recent studies suggest that disruption of latency will not necessarily result in the uniform and prompt death of infected cells from viral cytopathic effect (figure 1). Thus a consensus is emerging that enhancement of the antiviral immune response will be required as a component of eradication protocols (Shan et al, 2012).
Figure 1.
Proposed strategy to purge HIV cellular reservoirs, known as “shock and kill”. A first step consists in disrupting latency by agents such as vorinostat, which will cause HIV expression and new virions release. The presence of HAART will prevent new rounds of infection, and the reactivated cells will be cleared in a second step where immune clearance will be promoted through the activation of effector cells or the administration of targeted molecules.
Vorinostat, a histone deacetylase inhibitor (HDACi) used for the treatment of cutaneous T cell lymphoma, can disrupt HIV latency in vivo, as shown in a clinical trial in HIV-infected patients on antiretroviral therapy (Archin et al, 2012). However, in vitro studies recently suggest that a single pulse of HDACi would reactivate a tiny minority of infected cells (Cillo et al, 2014) , and with this in mind many groups are seeking combination approaches to more effectively purge latent reservoirs (Bullen et al, 2014) . A clinical trial evaluated recently the outcome of the administration of multiples doses of vorinostat , but the dosing schedule used was unable to demonstrate repeated induction of HIV RNA expression of the magnitude seen on the initial dose (Archin et al, 2014a).
Measuring the latent reservoir and documenting the effect of latency reversal agents has been unexpectedly difficult. DNA quantification by quantitative PCR (qPCR) was first used to measure the amount of infected cells, but this methodology will not give information about the proportion of replicative competent virus contained in those genomes, ultimately responsible for new cycles of infection (Eriksson et al, 2013). Therefore, the quantitative viral outgrowth assay (QVOA) is considered the gold standard to assess the magnitude of the reservoir, since it is the way of detecting replication competent virus (Archin et al, 2008; Siliciano and Siliciano, 2005). Unfortunately, viral outgrowth measured by QVOA appears to be a minimal estimate in at least 50% of patients (and a large underestimate in some), as a significant proportion of proviral DNA encodes proviruses that remain non-induced after a single maximal ex vivo stimulus, but are potentially productive of replication competent virus (Ho et al, 2013). However, besides the unquestioned relevance of replication competent proviruses, we should also consider the possibility that defective proviruses unable to produce viral particles may still express viral proteins, whose production may contribute to immune activation or trigger CD4+T cell apoptosis (Finzi et al, 2006). Thus, the clearance of infected cells may have to extend to those that produce antigen as well as functional virions.
It is essential to define experimental systems that allow the study of HIV eradication approaches. Ex vivo study of patient cells provide an important, if imperfect, model in which latency disruption can be tested, but as less than 1 in a million CD4+T cells are latently infected, it is demanding to measure the effect of drugs or compare different interventions in this setting. Nevertheless, when carefully performed, this system is robust, reproducible, and sensitive (Archin et al, 2014b).
Alternatively, many studies of viral persistence can be conducted in cell or animal models (table 1). There are several primary cell models to study latency reversal developed so far, differing in the viral strain, cell subset and mechanism used to establish latency. A recent study compared the response of several primary T cell models, J-Lat cell models and the patient cell viral outgrowth assay to a panel of various latency reversal agents. None of the in vitro cell models alone was in complete agreement with the ex vivo response of latently infected T cells from patients (Spina et al, 2013), and hence observations using even primary cell models is imperfect, each model perhaps giving a representation of a subset of the in vivo latently infected reservoir. The ultimate approach to investigate eradication strategies is perhaps the use of animal models. In the last several years, the development of humanized mouse models has added a powerful tool to study therapeutic interventions, in addition to those previously performed in non-human primates. An impressive study by Denton at al. (Denton et al, 2014) recently used a combination of ART and an immunotoxin to measure depletion of persistent infection. However, thus far true anatomic reservoirs such as the CNS have been studied most extensively in primate models (Clements et al, 2011).
Table 1.
Summary of models to study HIV latency.
| Models to study HIV latency | ||||
|---|---|---|---|---|
| Cellular models | Animal models | |||
| Cell lines | Primary cell models |
Patient resting CD4 cells |
Mouse models | Non-human primates |
| - T-cell lines: | ||||
| • Jurkat cells | - Greene [4] | - HuBLT [11] | ||
| (J89, 2D10, | - Lewin [5] | - Macaque: | ||
| J-Lat, JΔT) [1] | - Spina [6] | - Isolated from | - T-cell Only | |
| • ACH-2 [2] | - Karn [7] | HIV+ individuals | Mouse(TOM)[12] | • SIV [14] |
| - Planelles [8] | [10] | |||
| - Promonocytic | - Siliciano [9] | - HuSCID [13] | • RT-SHIV [15] | |
| • U1 [3] | ||||
Some clinical trials are taking place to evaluate therapeutic strategies towards HIV cure. Generally, outcomes of these trials are evaluated using peripheral blood samples, since blood is the most accessible compartment and represent the systemic effects. However, it is important to additionally evaluate treatment outcomes in other HIV reservoirs such as the CNS. Three main different types of assessment can be performed to evaluate HIV infection in the CNS: surrogate biomarkers in CSF, neurocognitive testing and imaging techniques. Biomarkers are usually analyzed in the CSF, and include HIV-RNA viral load, neopterin levels, neurofilament light chain protein (NFL), tau protein and precursors and products of amyloid protein (Spudich and Gonzalez-Scarano, 2012). However, CSF sampling is an invasive technique which cannot be performed frequently. Therefore, non-invasive tests are desirable for CNS evaluation. A wide range of standardized neuropsychological tests can be used to identify subclinical deficits. These exams may include psychomotor speed tests, hand-eye coordination, ability to register and recall memories, attention and concentration, problem-solving capability and language abilities. Sacktor and colleagues (Sacktor et al, 2005) developed the International HIV Dementia Scale (IHDS), consisting on three different tests - motor speed, psychomotor speed and memory-recall - which provides a score according to performance. There are also a number of neuroimaging techniques used to assess HIV-associated neurocognitive disorders, such as magnetic resonance spectroscopy (MRS), volumetric MRI, diffusion tensor imaging (DTI) and functional MRI (fMRI)(Masters and Ances, 2014). Magnetic resonance spectroscopy of the brain, also known as proton magnetic spectroscopy, has demonstrated to show virus-induced neurological deterioration (Jarvik et al, 1993; McConnell et al, 1994). Regardless of the method used to evaluate the neurocognitive state, it is essential to perform longitudinal tests over time, or in the case of a cross sectional study, include appropriate matched controls. As pilot studies to perturb latency and deplete persistent HIV infection move forward into the clinic, careful assessment of the CNS will be also important to insure that neurocognitive function is not impaired as an unwanted side effect of viral induction strategies, even as study patients maintain ART.
There are still many challenges to overcome in order to achieve an HIV cure. Therapies to target latent infection in resting CD4+ T cells are now undergoing extensive study, but at this early point in this endeavor, success is uncertain. Close behind this is the challenge of clearing infected cells, following their emergence from latency. The final frontier may be strategies to access privileged spaces such as the CNS. Initial efforts in this regard are likely to depend critically on animal model studies. Like therapy for childhood leukemia, success in eradication of established HIV infection may not come until we have developed specific therapies to reach the deepest hiding places in which the virus lurks.
Acknowledgements
This study was supported by the National Institutes of Health Grant DA030156 to D.M.M.
Footnotes
Dr. Margolis and Dr. Garrido both declare they have no conflict of interest with the published work.
References
- 1.Archin NM, Bateson R, Tripathy M, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. HIV-1 Expression within Resting CD4 T-Cells Following Multiple Doses of Vorinostat. J Infect Dis. 2014a doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archin NM, Crooks A, Bateson R, Cope A, Dahl N, Eron J, Gay C, Kuruc J, Margolis D, Bosch R. 20th Annual Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2014b. Measuring HIV Latency Over Time: Reservoir Stability and Assessing Interventions. [Google Scholar]
- 3.Archin NM, Eron JJ, Palmer S, Hartmann-Duff A, Martinson JA, Wiegand A, Bandarenko N, Schmitz JL, Bosch RJ, Landay AL, Coffin JM, Margolis DM. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. Aids. 2008;22:1131–1135. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosque A, Planelles V. Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods. 2011;53:54–61. doi: 10.1016/j.ymeth.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman M-C, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV Fusion with Multivalent Gold Nanoparticles. Journal of the American Chemical Society. 2008;130:6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20:425–429. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccarelli N, Fabbiani M, Colafigli M, Trecarichi EM, Silveri MC, Cauda R, Murri R, De Luca A, Di Giambenedetto S. Revised central nervous system neuropenetration-effectiveness score 10 is associated with cognitive disorders in HIV-infected patients with controlled plasma viraemia. Antivir Ther. 2013;18:153–160. doi: 10.3851/IMP2560. [DOI] [PubMed] [Google Scholar]
- 10.Cillo AR, Sobolewski MD, Bosch RJ, Fyne E, Piatak M, Jr, Coffin JM, Mellors JW. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements JE, Gama L, Graham DR, Mankowski JL, Zink MC. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Curr Opin HIV AIDS. 2011;6:37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clouse KA, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci AS, Folks TM. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 13.Cusini A, Vernazza PL, Yerly S, Decosterd LA, Ledergerber B, Fux CA, Rohrbach J, Widmer N, Hirschel B, Gaudenz R, Cavassini M, Klimkait T, Zenger F, Gutmann C, Opravil M, Gunthard HF. Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr. 2013;62:28–35. doi: 10.1097/QAI.0b013e318274e2b0. [DOI] [PubMed] [Google Scholar]
- 14.Dahl V, Lee E, Peterson J, Spudich SS, Leppla I, Sinclair E, Fuchs D, Palmer S, Price RW. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204:1936–1945. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, Perkey K, Archin NM, Choudhary SK, Yang K, Hudgens MG, Pastan I, Haase AT, Kashuba AD, Berger EA, Margolis DM, Garcia JV. Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART. PLoS Pathog. 2014;10:e1003872. doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, Chateau M, Nochi T, Krisko JF, Spagnuolo RA, Margolis DM, Garcia JV. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinoso JB, Rabi SA, Blankson JN, Gama L, Mankowski JL, Siliciano RF, Zink MC, Clements JE. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol. 2009;83:9247–9257. doi: 10.1128/JVI.00840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman HE. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183:661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O'Doherty U, Palmer S, Deeks SG, Siliciano JD. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finzi D, Plaeger SF, Dieffenbach CW. Defective virus drives human immunodeficiency virus infection, persistence, and pathogenesis. Clin Vaccine Immunol. 2006;13:715–721. doi: 10.1128/CVI.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 22.Garrido C, Dahl N, Simpson C, Bresee J, Feldheim D, Melander C, Margolis D. 19th Annual Conference on Retroviruses and Opportunistic Infections. Atlanta GA: 2013. Gold Nanoparticles to Improve Drug Delivery to the Central Nervous System. [Google Scholar]
- 23.Harrington PR, Schnell G, Letendre SL, Ritola K, Robertson K, Hall C, Burch CL, Jabara CB, Moore DT, Ellis RJ, Price RW, Swanstrom R. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. Aids. 2009;23:907–915. doi: 10.1097/QAD.0b013e3283299129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honeycutt JB, Wahl A, Archin N, Choudhary S, Margolis D, Garcia JV. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology. 2013;10:121. doi: 10.1186/1742-4690-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 27.Jarvik JG, Lenkinski RE, Grossman RI, Gomori JM, Schnall MD, Frank I. Proton MR spectroscopy of HIV-infected patients: characterization of abnormalities with imaging and clinical correlation. Radiology. 1993;186:739–744. doi: 10.1148/radiology.186.3.8430182. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Tian B, Saifuddin M, Agy MB, Emau P, Cairns JS, Tsai CC. RT-SHIV, an infectious CCR5-tropic chimeric virus suitable for evaluating HIV reverse transcriptase inhibitors in macaque models. AIDS Res Ther. 2009;6:23. doi: 10.1186/1742-6405-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. Embo j. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lassen KG, Hebbeler AM, Bhattacharyya D, Lobritz MA, Greene WC. A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs. PLoS One. 2012;7:e30176. doi: 10.1371/journal.pone.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 32.Masters MC, Ances BM. Role of Neuroimaging in HIV-Associated Neurocognitive Disorders. Semin Neurol. 2014;34:89–102. doi: 10.1055/s-0034-1372346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell JR, Swindells S, Ong CS, Gmeiner WH, Chu WK, Brown DK, Gendelman HE. Prospective utility of cerebral proton magnetic resonance spectroscopy in monitoring HIV infection and its associated neurological impairment. AIDS Res Hum Retroviruses. 1994;10:977–982. doi: 10.1089/aid.1994.10.977. [DOI] [PubMed] [Google Scholar]
- 34.Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 35.Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. Aids. 2005;19:1367–1374. [PubMed] [Google Scholar]
- 36.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:4161–4164. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 37.Sanvicens N, Marco MP. Multifunctional nanoparticles – properties and prospects for their use in human medicine. Trends in Biotechnology. 2008;26:425–433. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Shan L, Deng K, Shroff Neeta S, Durand Christine M, Rabi SA, Yang H-C, Zhang H, Margolick Joseph B, Blankson Joel N, Siliciano Robert F. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 40.Soriano-Sarabia N, Archin N, Margolis D. 19th Annual Conference on Retroviruses and Opportunistic Infections. Atlanta GA: 2013. Study of Transitional Memory CD4+ T Cells and Gamma-Delta T cells as Latent Reservoirs for Replication Competent HIV-1. [Google Scholar]
- 41.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, Greene WC, Kashuba A, Lewin SR, Margolis DM, Mau M, Ruelas D, Saleh S, Shirakawa K, Siliciano RF, Singhania A, Soto PC, Terry VH, Verdin E, Woelk C, Wooden S, Xing S, Planelles V. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013;9:e1003834. doi: 10.1371/journal.ppat.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang HC, Xing S, Shan L, O'Connell K, Dinoso J, Shen A, Zhou Y, Shrum CK, Han Y, Liu JO, Zhang H, Margolick JB, Siliciano RF. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, Eisele E, Haase A, Ho YC, Hutter G, Justement JS, Keating S, Lee TH, Li P, Murray D, Palmer S, Pilcher C, Pillai S, Price RW, Rothenberger M, Schacker T, Siliciano J, Siliciano R, Sinclair E, Strain M, Wong J, Richman D, Deeks SG. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]