Abstract
In spite of the clinical utility of buprenorphine, parenteral abuse of this medication has been reported in several laboratory investigations and in the real world. Studies have demonstrated lower abuse liability of the buprenorphine/naloxone combination relative to buprenorphine alone. However, clinical research has not yet examined the utility of the combined formulation to deter intranasal use in a buprenorphine-maintained population. Heroin-using volunteers (n = 12) lived in the hospital for 8–9 weeks and were maintained on each of three sublingual buprenorphine doses (2, 8, 24 mg). Under each maintenance dose, participants completed laboratory sessions during which the reinforcing and subjective effects of intranasal doses of buprenorphine (8, 16 mg), buprenorphine/naloxone (8/2, 8/8, 8/16, 16/4 mg) and controls (placebo, heroin 100 mg, naloxone 4 mg) were assessed. Intranasal buprenorphine alone typically produced increases in positive subjective effects and the 8 mg dose was self-administered above the level of placebo. The addition of naloxone dose-dependently reduced positive subjective effects and increased aversive effects. No buprenorphine/naloxone combination dose was self-administered significantly more than placebo. These data suggest that within a buprenorphine-dependent population, intranasal buprenorphine/naloxone has reduced abuse potential in comparison to buprenorphine alone. These data strongly argue in favor of buprenorphine/naloxone rather than buprenorphine alone as the more reasonable option for managing the risk of buprenorphine misuse.
Keywords: Abuse Liability, Buprenorphine, Intranasal, Opioids, Self-administration
Introduction
Buprenorphine (Bup) is a partial mu opioid agonist and kappa antagonist used as a maintenance medication to reduce the morbidity and mortality associated with opioid abuse and dependence (Mattick et al. 2008). Sublingual (SL) buprenorphine maintenance is one of the most commonly utilized treatments for opioid abuse around the world (Auriacombe et al. 2004; Carrieri et al. 2006; Malinoff et al, 2005; Maxwell et al. 2010). Like other mu agonists, Bup has the potential to be abused. However, its abuse potential can vary substantially depending on route of administration and state of opioid dependence (Jasinski et al., 1978; Comer et al. 2008; Mello & Mendelson, 1985, Umbricht et al. 2004; Walsh et al. 1994, 1995). Several studies have shown that with parenteral administration, Bup produces effects similar to full mu agonists and is self-administered (Bedi et al. 1998; Comer et al. 2002, 2005, 2010; Duke et al. 2010; Middleton et al. 2011; Strain et al. 1997; Zacny et al. 1997).
Consistent with laboratory findings suggesting that Bup does have abuse potential, several regions of the world have noted Bup abuse including: Australia (Jenkinson et al. 2005; Nielsen et al. 2007), Europe (Alho et al. 2007; Auriacombe et al. 2004; Carrieri et al. 2006; Hakansson et al. 2007; Obadia et al. 2001; Vidal-Trecan et al. 2003), South East Asia (Chua & Lee, 2006; Horyniak et al. 2011; Lee 2006; Vicknasingam et al. 2010) and the U.S. (Nordmann et al. 2012; Young et al. 2010). To address this concern, the opioid antagonist naloxone (Nal), which has low sublingual bioavailability, was combined with Bup in a 4:1 ratio (Suboxone) to reduce its abuse liability. The addition of naloxone is intended to discourage misuse of Bup by parental routes by either precipitating withdrawal or through direct antagonist effects (Preston et al. 1990).
Several clinical studies have sought to determine if, in fact, naloxone does decrease the abuse liability of Bup. Duke and colleagues (2010) administered various Bup/Nal dose combinations via the intramuscular (IM) and SL (4/1, 8/2, and 16/4 mg) routes to non-dependent opioid users. Their study found no evidence that the inclusion of naloxone altered the positive subjective effects of IM Bup. Middleton and colleagues (2011) tested five intranasal (IN) doses [Bup (2, 8 mg), Bup/Nal (0/0, 2/0.5, 8/2 mg)] and one intravenous (IV) dose (0.8/0.2 mg) among non-dependent opioid users. Both formulations produced increases in subjective and physiological effects, suggesting that the addition of naloxone did little to alter the abuse liability of Bup in individuals who are not dependent on opioids.
Mendelson and colleagues (1999) examined the subjective experience of intravenous Bup/Nal (2/1, 2/0.5, 2/0.25 mg) in morphine-maintained volunteers (IM, 60 mg/day). Among these opioid-dependent participants, they found that Bup/Nal (2/1 and 4/1 mg) produced significant increases in opiate withdrawal, bad drug effect, and sickness, while Bup/Nal (8/1 mg) produced mild withdrawal (see also Fudala et al. 1998 and Mendelson et al. 1996). Stoller and colleagues (2001) studied the effects of IM and SL Bup/Nal (1/0.25, 2/0.5, 4/1, 8/2, 16/4 mg) in hydromorphone-maintained participants (oral, 40 mg/day). Sublingual Bup/Nal produced no opioid antagonist effects, but IM Bup/Nal did produce dose-related antagonist effects. In methadone-maintained participants, IV Bup/Nal but not Bup alone precipitated withdrawal (Mendelson et al. 1997). Combined, the literature appears to indicate that in individuals who are dependent on morphine, hydromorphone, or methadone, parenteral Bup/Nal can precipitate withdrawal and it has less abuse liability than Bup alone.
In contrast to their ability to precipitate withdrawal in individuals dependent on methadone or shorter-acting opioids, parenteral Bup and Bup/Nal do not precipitate withdrawal in Bup-maintained individuals (IM Bup, Strain et al. 1997; IV Bup, Comer et al. 2010). However, IV administration of the Bup/Nal combination did produce less robust positive subjective effects, increased aversive effects, and was self-administered less than Bup alone in Bup-maintained individuals. Despite reports that Bup is abused intransally, investigators have yet to address how naloxone alters the abuse liability of Bup when the combination is administered in this manner to a Bup-dependent population (Hakansson et al. 2007; Nordmann et al. 2012; Roux et al. 2008; Young et al. 2010). Therefore, the purpose of the present study was to test the abuse liability of intranasal (IN) Bup/Nal compared to IN Bup in heroin users under varying doses of SL Bup maintenance.
Methods
Participant Selection and Qualification
Participants were recruited from the New York City metropolitan area through various print media advertisements. Respondents who met study inclusion/exclusion criteria, based upon the initial telephone interview, were scheduled to come to the New York State Psychiatric Institute for additional screening procedures. Screening consisted of both self-report and clinical interviews administered by a team of research assistants, psychologists, nurses, and physicians. Assessments were made of drug use, general health, and medical history, and multiple laboratory tests (hematology, blood chemistry panel, liver and thyroid functioning, urinalysis, syphilis serology) were performed. Rapid urine drug screens assessed recent use of opioids, cocaine, benzodiazepines, cannabinoids, and amphetamines.
Participants were required to be physically and mentally healthy intravenous or intranasal heroin users between the ages of 21 and 55 years. All participants were required to meet DSM-IV criteria for opioid abuse. Potential participants were excluded from the study if they were seeking treatment for their drug use, physiologically dependent on alcohol or illicit drugs (other than opioids), or had a severe Axis I psychiatric diagnosis (other than opioid, nicotine or caffeine dependence).
Participants resided on a locked inpatient unit during the study. During the first week after admission, they were stabilized on 2 mg sublingual Bup, which was administered once daily, at approximately 2000 hrs. During the first week after admission into the hospital, participants were treated for emergent withdrawal symptoms with various supplemental medications until withdrawal symptoms dissipated based on self-report and observer ratings. During the second week after admission into the hospital, while still being maintained on 2 mg SL Bup, participants completed a qualification phase of the study. During this phase, they were given each of 4 active IN Bup doses in ascending order (2, 4, 8, and 16 mg). One dose was tested on each day, and a placebo (Pbo, 0 mg) dose was randomly inserted into this order. Participants then completed sample and choice self-administration laboratory sessions, described in detail below. Participants who self-administered more active Bup than placebo were allowed to continue with the study. The qualification phase ensured that we sampled a population of heroin users in which we could demonstrate the reinforcing effects of Bup. Following completion of subsequent study procedures, participants were discharged from the hospital, and provided with referrals for drug treatment if they were interested. Opioid detoxification on the inpatient unit was also available to all participants at the end of the study.
As compensation, participants were paid $25/day with a $25/day bonus for completing the study. In addition to the per diem payment, participants had the opportunity to earn money during the experimental sessions ($20 per sample session plus up to $20 per self-administration session). All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute.
Principal Study Design
This within-subjects investigation was placebo-controlled and conducted under double-blind conditions. The dosing order of the 9 IN challenge drugs/doses, as well as the order of the 3 maintenance doses, was determined using a nested Latin-square randomization procedure. The IN challenge drugs/doses were: placebo, naloxone (4 mg), heroin (100 mg), Bup (8, 16 mg), and buprenorphine/naloxone (8/2, 8/8, 8/16, 16/4 mg). By using these Bup and Bup/Nal challenge doses, we hoped to determine whether different Bup-to-Nal dose ratios were more effective than the currently approved 4:1 ratio in deterring IN Bup abuse. By manipulating the dose ratio of Bup-to-Nal, we also hoped to learn more concerning how naloxone deters IN abuse i.e., through precipitating withdrawal, direct opioid antagonist effects, or both. Challenge doses were administered at approximately 1100 hrs during the morning sample session and again at approximately 1500 hrs during an afternoon choice session. Only a single IN challenge drug/dose was tested each day. In order to gather data on the interaction between the challenge drug and the maintenance drug, each of the IN doses was tested under each of three SL Bup maintenance conditions (2, 8, 24 mg). We allowed for 5–7 days of stabilization on each SL dose prior to testing the IN doses (Figure 1). Administration of the SL Bup maintenance dose occurred at 2000 hrs each evening. These SL doses were not adjusted (increased or decreased) as a function of the challenge drug administered earlier that day.
Figure 1.
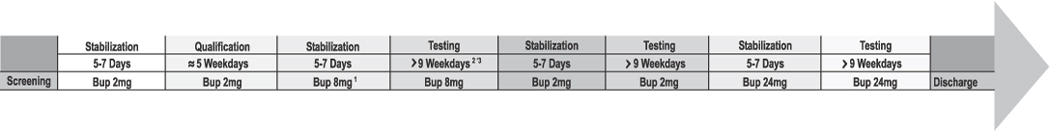
Illustration of the study design from screening to discharge. 1 The order in which participants received each of the 3 SL maintenance doses was randomized. 2 The order of testing the 9 IN doses also was randomized. 3 A minimum of 9 days was required to complete testing for each of the 9 IN challenge doses. Though this number of days could vary significantly between maintenance conditions and between participants, depending on holidays, weekends, etc.
Sample and Choice Self-Administration Procedure
Testing consisted of two types of laboratory sessions: sample and choice. At approximately 1000 hrs participants were brought to the laboratory to complete a sample session. Forty minutes (min) prior to drug administration, physiological monitoring began. A pulse oximeter continuously measured arterial oxygen saturation (%SpO2); heart rate, systolic blood pressure, and diastolic blood pressure were measured every five minutes throughout the session. Participants received full doses of the IN challenge drug and money (U.S. $20) at 0 min, which occurred at approximately 1100 hrs. Participants were instructed to insufflate the entire dose through a plastic straw within a 30-second period in one or both nostrils. Shown in Table 1 are the time points at which right eye pupil diameter was measured, blood was collected and participants completed subjective effects batteries and performance tasks. At the end of the sample session, participants returned to the inpatient unit where they received lunch.
Table 1.
Sample Session Events
Time points at which physiological, plasma, performance and subjective assessments were made throughout the sample session.
| −40 | Physiological monitoring (oxygen saturation, blood pressure), DSST, DAT, VAS, SOWS |
| 0 | Sample IN drug and $20, Pupils, Bloods |
| 5 | Pupils, Bloods, VAS, DEQ |
| 15 | Pupils, Bloods, DSST, DAT, VAS, DEQ |
| 30 | Pupils, Bloods |
| 45 | Pupils, Bloods, VAS, DEQ |
| 60 | Pupils, Bloods, DSST, DAT, VAS, DEQ |
| 90 | Pupils, Bloods |
At approximately 1400 hrs participants were brought to the laboratory to complete a choice session. The baseline assessments during each choice session were identical to those used in the sample session; participants then completed a self-administration task to receive portions of the dose of drug or money they had sampled earlier in the day (0 to 100%, in increments of 10%). Participants were told that they could work for all or part of the sampled dose or the sampled money amount by choosing the drug or money option each time a choice was available. The alternative money value (U.S., $20) was chosen based on previous studies conducted in our laboratory (Comer et al. 1997). Drug and money were available at each choice trial. Thus, if the dose for that day was 16 mg, at each opportunity participants could respond for 1.6 mg (10% of 16 mg) or $2 (10% of $20). Completion of the ratio requirement for each choice trial was accompanied by a visual stimulus on the computer screen. After a choice was made for one option, by clicking on its visual representation on the computer screen, responding for the other option was not possible until the ratio was completed and another trial was initiated. Responses to complete the ratio requirement consisted of finger presses on a computer mouse. The operant response requirement for each of the two options increased independently. The initial ratio requirement for each option was 50 responses, which then increased progressively each time the option was selected (50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, and 2800). At the end of the self-administration task (approximately 1600 hrs), the participant received whatever (s)he had chosen. Money was added to their study payment, and the IN drug was given to the participant for nasal insufflation while under observation by a physician.
Tasks and Measures
Subjective Effects
Three questionnaires were used to assess subjective drug effects and opioid withdrawal symptoms. A 26-item visual analog scale (VAS) was used to assess subjective and physiological drug effects such as “I feel a good effect” and “I feel high”. Participants rated each item on the scale from ‘Not at all’ (0 mm) to ‘Extremely’ (100 mm). In addition, a 6-item drug effects questionnaire (DEQ) was used to measure drug effects (strength of drug effects, good effects, bad effects, willingness to take the drug again, drug liking, and similarity to other drugs). Participants selected among a series of possible answers ranging from 0 (‘No Effect’) to 4 (‘Very Strong Effect’), except for the drug liking questionnaire, which ranged from −4 (‘Dislike Very Much’) to 4 (‘Like Very Much’). The Subjective Opioid Withdrawal Scale (SOWS) was used to identify the presence and severity of opioid withdrawal symptoms (Handelsman et al. 1987).
Participants also completed a questionnaire designed to assess specific aspects of how their nose and throat felt after each intranasal drug administration (Middleton et al. 2011). Participants rated the presence of 9 sensations such as “burning,” “stinging,” and “pain” on a scale from 0 (Not at all) to 4 (Extremely). Because this questionnaire was added later in the study, for exploratory purposes, only 6 of the 12 participants completed it.
Performance effects
The performance battery consisted of two tasks: a 10-min divided attention task (DAT) and a 3-min digit-symbol substitution task (DSST). Custom-made software was used for these performance tasks (see Comer et al. 1999 for details). The divided attention task consisted of concurrent pursuit-tracking and vigilance components. Participants tracked a moving stimulus on the video screen using the mouse and also signaled when a small black square appeared at any of the four corners of the video screen. The distance between the cursor and moving stimulus was measured, as was the speed of the moving stimulus (with greater accuracy, the stimulus moved at a faster rate). The digit-symbol substitution task consisted of nine 3-row by 3-column squares (with one black square per row) displayed across the top of the computer screen. A randomly generated number indicated which of the nine patterns should be emulated on a keypad by the participant on a particular trial. Participants were required to emulate as many patterns as possible by entering the pattern associated with randomly generated numbers appearing on the bottom of the screen. Prior to testing for the main study, participants would have encountered the performance tasks twice, during a mock lab session performed while screening and during the qualification session.
Physiological Measures
Miosis was assessed as a physiological indicator of mu agonist effects using a NeurOptics™ Pupillometer under ambient lighting conditions. For safety, a pulse oximeter continuously monitored oxygen saturation (%SpO2) during sessions, while respiration (breaths per minute), heart rate, and blood pressure (systolic and diastolic) were measured every 5 minutes. Supplemental oxygen also was provided throughout the session.
Pharmacokinetic Measures and Blood Sample Collection
Pharmacokinetic parameters for Bup, its metabolite norbuprenorphine (NorBup), and naloxone were also quantified as study outcome measures (Cmax: mean peak concentration achieved, Tmax: mean time to reach peak concentration, and AUC0–90mins: mean area-under-the curve from dose administration (time 0) to 90 min after dose administration). Throughout the sample session, blood (~8 ml/sample) was collected from an intravenous catheter (catheters could remain in place for up to 96 hours). The window of observation and time points of the pharmacokinetic assessments closely followed those of the subjective measures in order to observe how changes in plasma Bup and Nal concentrations were related to changes in subjective effects. Whole blood was collected in tubes containing 15% EDTA and centrifuged. Plasma was separated and frozen at −70°C. Frozen plasma samples were batched and transferred to Worldwide Clinical Trials for analysis. The lower limits of quantitation for Bup, NorBup, and naloxone were 0.025 ng/ml, 0.2 ng/ml and 1.0 pg/ml (respectively).
Drugs
Bup tablets (2 mg) for SL administration, and buprenorphine hydrochloride (HCl) powder and naloxone HCl powder for IN administration were provided by Reckitt Benckiser Pharmaceuticals, Inc. (Richmond, VA) through the Research Triangle Institute. The doses used were chosen based on previous studies of Bup conducted in our laboratory as well as in other laboratories (Comer & Collins 2002; Comer et al. 2005, 2010; Middleton et al. 2011; Umbricht et al. 2004). Heroin HCl powder was obtained from Macfarlan Smith Limited (Edinburgh, Scotland, UK). Intranasal placebo consisted of 100 mg lactose powder. A constant weight of 100 mg of drug and/or lactose was used for all IN dose administrations in order to maintain the blind. All intranasal doses were insufflated through a plastic straw within 5–10 seconds. Naloxone HCl solution for IM administration to confirm opioid dependence during screening was obtained from International Medication System Limited Amphastar (South Elmonte, CA). IM naloxone was administered in doses between 0.2–0.8 mg prior to admission into the study. All test drugs were prepared by the New York State Psychiatric Institute Pharmacy and administered by a physician.
Statistical Analyses
An omnibus repeated-measures analyses of variance (ANOVA) was first used to compare peak or trough drug effects as a function of the nine IN Dose conditions (IN Dose) and the three SL Bup maintenance conditions (SL Dose). Separate univariate ANOVAs compared each of the nine IN drug effects within each SL dosing condition. Univariate ANOVAs were also utilized to compare drug effects among the nine IN challenge doses, summed across the SL doing conditions. Post-hoc tests were performed between the individual IN doses to determine where significant differences occurred.
In order to examine the time course of drug effects, ANOVAs were used to assess differences among the 9 IN challenge drugs over the various time points throughout a session. An α of p<0.05 was considered statistically significant, while p<0.10, was considered as trending towards significance. All data analyses were performed using SPSS version 18 (SPSS 2009) and SuperANOVA (Gagnon et al. 1990).
Results
Participants
Twenty-seven participants were enrolled into the study. Eleven participants either voluntarily withdrew from the study or were dropped by the investigators due to a number of factors including: elevated liver function tests (n = 2), participants’ legal/personal issues (n = 5), and disgruntlement with the unit or duration of the inpatient period (n = 4). Four participants were dropped from the study after they failed to meet study entry criteria during the qualification phase.
Complete data sets were obtained from 12 participants for inclusion in this analysis (11M, 1F; 5 White, 3 Black, 3 Latino, 1 Multiracial). The mean age of this sample was 41.4 years. All participants enrolled in the study were daily heroin users. The majority (7) of study completers were intranasal heroin users, 3 reported current intravenous heroin use, and 2 used heroin through both routes. The mean duration of heroin use was 18.8 years (range: 1–36 years), the mean daily amount spent on heroin was $56.40 (range: $20– $150), and participants used an average of 7.9 bags per day (range: 1.5–30). According to the latest information from the U.S. Drug Enforcement Administration (DEA), heroin in NYC cost an average of $0.99 per mg pure (DEA, 2013). As our participants report an average heroin price of ~$10 per bag, we roughly estimate that a bag consists of ≈10mg of pure heroin.
In addition to daily heroin use, 11 completers were daily tobacco smokers (they were allowed to smoke immediately before the sample session and after the choice session), 9 were regular cocaine/crack users (“a few times a week”), 5 were occasional alcohol drinkers (“a few times a month” or more without meeting criteria for dependence), and 3 were occasional marijuana users (“a few times a month”). (Ab)use of prescription opioids, benzodiazepines, hallucinogens and amphetamines was also reported, but use of these drugs was very sporadic.
Positive Subjective Effects
Peak VAS and DEQ measurements of positive subjective effects were significantly increased following IN heroin administration (under all SL Bup dosing conditions). Significant increases were seen following IN Bup administration, though this effect was attenuated with larger SL Bup doses. None of the IN Bup/Nal combinations, or IN naloxone alone, produced any observable positive subjective effects. Details of the analyses for each of the individual measures are below.
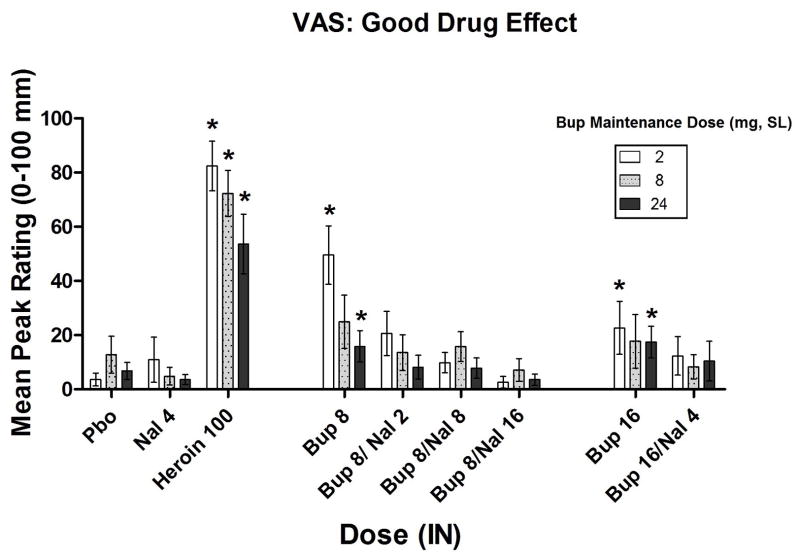
Omnibus ANOVA found no overall significant SL dose x IN dose interaction on peak VAS ratings of “Good” effect (p=0.35), though some differences were found among the SL dosing conditions. When participants were maintained on 2 mg SL Bup, IN doses of heroin (100 mg) and Bup alone (8, 16 mg) significantly increased peak VAS ratings of “Good” effect when compared to placebo, (p<0.001, p<0.01, p<0.01, respectively; Figure 2). None of the Bup/Nal dose combinations differed significantly from placebo on this measure. This pattern of results differed slightly when participants were maintained on 8 mg SL Bup: while IN heroin significantly increased “Good” drug effect (p<0.001), the effects of the 8 mg dose of Bup alone only approached significance (p<0.10) and no significant effect of Bup 16 mg was found. None of the Bup/Nal dose combinations differed significantly from placebo on this measure. When participants were maintained on 24 mg SL Bup, heroin continued to significantly increase ratings on this measure (p<0.01) along with Bup alone (8 mg, 16 mg, p’s<0.05). None of the Bup/Nal dose combinations differed significantly from placebo on this measure.
Figure 2.
Mean peak (± SEM) Visual Analog Scale (VAS) ratings of “Good” drug effect. * Indicates a significant difference from placebo (p<.05).
Omnibus ANOVA found no significant SL dose x IN dose interaction on the VAS ratings of “High” (p=0.39). When participants were maintained on 2 mg SL Bup, IN doses of heroin (100 mg) and Bup alone (8, 16 mg) significantly increased peak ratings of “High” when compared to placebo (p<0.001, p<0.01, p<0.05, respectively). None of the Bup/Nal dose combinations differed significantly from placebo on this measure, though the effects of the 8/2 mg dose did approach significance (p<0.10). When participants were maintained on 8 mg SL Bup, IN heroin significantly increased reports of “High” (p<0.001), though none of the Bup alone or Bup/Nal combinations differed significantly from placebo on this measure. This same pattern was seen when participants were tested under the 24 mg SL Bup maintenance dose condition.
Omnibus ANOVA found no significant SL dose x IN dose interaction on the VAS ratings of “I Would Pay” (p=0.26). When participants were maintained on 2 mg SL Bup, IN doses of heroin (100 mg) and Bup alone (8 mg, 16 mg) significantly increased peak ratings when compared to placebo (p<0.001, p<0.001, p<0.05, respectively). None of the Bup/Nal dose combinations differed significantly from placebo on this measure. When participants were maintained on 8 mg SL Bup, IN heroin significantly increased ratings on this measure (p<0.001), while none of the Bup doses or Bup/Nal dose combinations significantly altered ratings of how much participants would be willing to pay for the dose. Similar results were obtained when participants were maintained on 24 mg SL Bup: only heroin significantly (p<0.001) increased reports of “I Would Pay.”
Omnibus ANOVA found no significant SL dose x IN dose interaction on the VAS ratings of drug “Quality” (p=0.22). When participants were maintained on 2 mg SL Bup, IN doses of heroin (100 mg) and Bup alone (8 mg) significantly increased peak ratings when compared to placebo (p<0.001, p<0.001, respectively). None of the Bup/Nal dose combinations differed significantly from placebo on this measure. When participants were maintained on 8 mg SL Bup, IN heroin and IN Bup (8 mg) significantly increased ratings on this measure (p<0.001, p<0.05, respectively), while none of the Bup/Nal dose combinations significantly altered rating on this measure. This pattern of results was seen again when participants were maintained on 24 mg SL Bup: only IN heroin (100 mg) and IN Bup (8 mg) significantly increased peak ratings of drug “Quality” (p<0.001, p<0.05, respectively).
Omnibus ANOVA found no significant SL dose x IN dose interaction on the VAS ratings of “Liked the Choice” (p=0.18). When participants were maintained on 2 mg SL Bup, IN doses of heroin (100 mg) and Bup alone (8, 16 mg) and Bup/Nal 8/2 mg significantly increased peak VAS ratings of “Liked the Choice” when compared to placebo (p<0.001, p<0.001, p<0.05, p<0.05 respectively). None of the other Bup/Nal dose combinations differed significantly from placebo on this measure. This pattern of results differed when participants were maintained on 8 mg SL Bup: IN heroin significantly increased ratings (p<0.001), but none of the Bup doses or Bup/Nal dose combinations significantly altered rating on this measure. This pattern of results was also found when participants were maintained on 24 mg SL Bup: only IN heroin produced a significant effect (p<0.001).
Omnibus ANOVA did find a significant SL dose x IN dose interaction on the DEQ ratings of “Like” (p<0.05). When participants were maintained on 2 mg SL Bup, IN doses of heroin (100 mg), Bup alone (8 mg), and Bup/Nal 8/2 mg significantly increased peak ratings of “Like” when compared to placebo (p<0.001, p<0.001, p<0.05, respectively). Neither the 16 mg IN dose of Bup nor any of the other Bup/Nal dose combinations differed significantly from placebo on this measure. When participants were maintained on 8 mg SL Bup, IN heroin significantly increased ratings on this measure (p<0.001), but none of the Bup doses or Bup/Nal dose combinations significantly altered ratings on this measure. This pattern of results was also found when participants were maintained on 24 mg SL Bup: only IN heroin produced a significant effect (p<0.001).
Omnibus ANOVA found a trend for an interaction between the SL Bup dose and the IN doses on DEQ ratings of “Would Take Again” (p <0.10). When participants were maintained on 2 mg SL Bup, IN doses of heroin (100 mg) and Bup alone (8 mg) significantly increased peak ratings when compared to placebo (p<0.001, p<0.01, respectively). Neither the 16 mg IN dose of Bup nor any of the other Bup/Nal dose combinations differed significantly from placebo on this measure. Under maintenance on 8 mg SL Bup, IN heroin significantly increased ratings of “Would Take Again” (p<0.001), but none of the Bup doses or Bup/Nal dose combinations significantly altered ratings on this measure. This pattern of results was also found when participants were maintained on 24 mg SL Bup: only heroin significantly increased reports of “Would Take Again” (p<0.001).
Aversive Subjective Effects
VAS, DEQ and SOWS assessment of aversive subjective effects were significantly increased following IN naloxone administration (under all SL Bup dosing conditions). Significant increases in peak aversive effects were only observed after IN administration of Bup in combination with Nal. However, under some SL maintenance conditions, aversive ratings of the Bup 16 mg dose did approach and exceed significance. Details of the analyses for each of the individual measures are below.
Omnibus ANOVA found no significant SL dose x IN dose interaction on peak VAS ratings of “Bad” drug effect (p=0.28). Under maintenance on 2 mg SL Bup, peak VAS ratings of “Bad” drug effect increased only after administration of Bup in combination with naloxone compared to placebo (8/2 mg, p<0.01; 8/8 mg, p<0.01; 8/16 mg, p<0.01; 16/4 mg, p<0.10). This pattern of results was also obtained in the 8 mg SL maintenance dose condition: peak VAS ratings of “Bad” drug effect increased only after the administration of Bup/Nal (8/2 mg, p<0.10; 8/8 mg, p<0.001; 8/16 mg, p<0.01; 16/4 mg, p<0.10). In contrast, under the 24 mg SL Bup maintenance dose condition, not only did all of the Bup/Nal dose combinations increase ratings on this measure, (8/2 mg, p<0.01; 8/8 mg, p<0.001; 8/16 mg, p<0.001; 16/4 mg, p<0.10), but the 16 mg dose of IN Bup alone did as well (p<0.01).
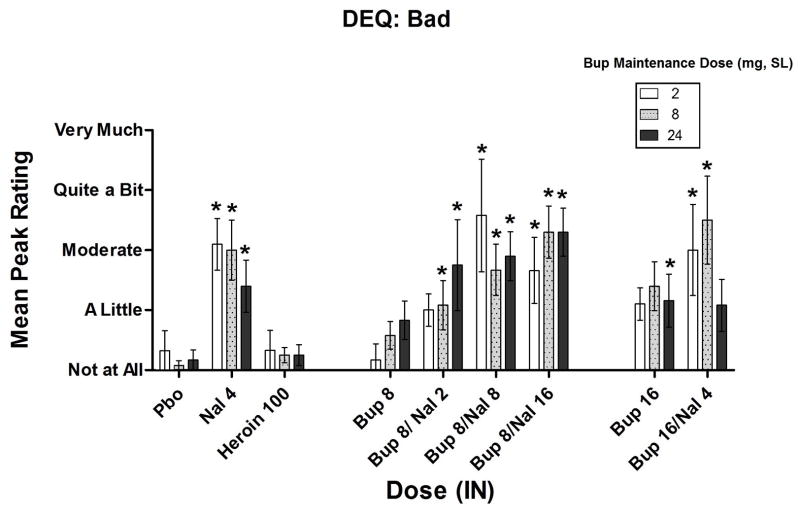
Omnibus ANOVA found no significant SL dose x IN dose interaction on DEQ ratings of “Bad” drug effect (p=0.45). As shown in Figure 3, when participants were maintained on 2 mg SL Bup, increases in “Bad” drug effect were seen with all of the Bup/Nal combinations compared to placebo (8/2 mg, p<0.10; 8/8 mg, p<0.01; 8/16 mg, p<0.01; 16/4 mg, p<0.05) and a trend in that direction was seen with the larger dose of Bup alone (16 mg, p<0.10). This pattern was observed again with the 8 mg SL Bup maintenance condition: not only did all of the Bup/Nal combinations increase ratings on this measure (8/2 mg, p<0.05; 8/8 mg, p<0.001; 8/16 mg, p<0.001; 16/4 mg, p <0.001), and the IN 16 mg dose (p<0.10). This pattern of results was again found when participants were maintained on 24 mg SL Bup (8/2 mg, p<0.001; 8/8 mg, p<0.001; 8/16 mg, p<0.001; 16 mg, p < 0.05; 16/4 mg, p<0.10). IN naloxone alone significantly increased VAS and DEQ ratings of “Bad” without significant variation among the SL dosing conditions (p<.01).
Figure 3.
Mean peak (± SEM) Drug Effects Questionnaire (DEQ) ratings of “Bad” drug effect * Indicates a significant difference from placebo (p<.05).
Average SOWS scores prior to the sample sessions, which ranged between approximately 3 and 4 (out of a total possible score of 64), were low and did not differ across the SL dosing conditions. SOWS scores prior to the afternoon choice session revealed that participants who received IN naloxone (4 mg) and Bup/Nal (8/16 mg) during the morning sample session may have still been experiencing mild withdrawal symptoms compared to sessions where they received placebo during the sample session. Overall however, these SOWS scores were minimal, ranging between 5–10 across all SL and IN conditions. IN naloxone alone significantly increased “SOWS” scores (p<.01). However, this effect was significantly greater while participants were maintained under 2mg SL Bup when compared to the 24 mg of SL Bup (p<.05).
Mean peak ratings on all measures can be found in Table 2. These data are collapsed across the SL maintenance Bup conditions in order to give the reader a sense of the between-dose differences among the IN challenge drugs.
Table 2.
Mean Peak Drug Effects
Mean peak (± SEM) physiological, performance and subjective measures for each of the IN challenge drugs, averaged across the 3 SL maintenance conditions. Bolded numbers indicate a significant difference from placebo.
| Pbo 0 mg | Nal 4 mg | Heroin 100 mg | Bup 8 mg | Bup/Nal 8/2 mg | Bup/Nal 8/8 mg | Bup/Nal 8/16 mg | Bup 16 mg | Bup/Nal 16/4 mg | |
|---|---|---|---|---|---|---|---|---|---|
| Dependent Measure | |||||||||
| SOWS: Sum (0–64) | |||||||||
| Sample Session Baseline | 2.69 (.81) | 2.56 (.70) | 2.67 (.73) | 2.67 (.67) | 3.74 (1.2) | 2.59 (.67) | 2.92 (.97) | 3.29 (.91) | 3.15 (.78) |
| Choice Session Baseline | 2.25 (.53) | 7.16 (1.4) | 2.08 (.36) | 2.75 (.55) | 3.41 (.86) | 3.41 (.79) | 5.13 (1.1) | 3.00 (.68) | 2.78 (.53) |
| Subjective: VAS (0–100) | |||||||||
| Alert | 39.3 (4.9) | 43.8 (5.3) | 57.2 (5.3) | 46.5 (5.6) | 44.6 (5.6) | 44.7 (5.4) | 37.8 (5.1) | 46.4 (5.1) | 41.7 (5.6) |
| Anxious | 15.9 (4.5) | 30.7 (6.6) | 29.4 (6.2) | 17.1 (4.9) | 27.9 (6.4) | 24.1 (5.7) | 26.1 (6.07) | 23.2 (5.5) | 25.1 (6.0) |
| Bad | 7.5 (3.2) | 47.4 (6.2) | 7.22 (3.2) | 17.8 (4.3) | 26.22 (5.6) | 42.3 (6.6) | 55.8 (6.3) | 27.4 (5.7) | 32.7 (5.9) |
| Depressed | 3.3 (1.4) | 7.5 (3.6) | 5.9 (3.0) | 3.5 (1.3) | 3.3 (1.7) | 4.9 (2.9) | 8.7 (2.9) | 4.3 (1.8) | 5.1 (1.8) |
| Energetic | 40.9 (5.3) | 38.3 (5.2) | 56.1 (5.6) | 46.2 (6.1) | 41.2 (5.7) | 37.6 (5.2) | 34.1 (5.0) | 42.3 (5.2) | 43.3 (5.6) |
| Good | 7.72 (2.5) | 3.72 (2.5) | 69.3 (5.4) | 28.0 (5.4) | 17.1 (4.6) | 8.25 (2.50 | 5.27 (1.8) | 22.1 (5.3) | 9.97 (3.2) |
| Gooseflesh | 4.2 (1.6) | 25.1 (4.6) | 6.8 (3.1) | 2.9 (1.2) | 7.1 (2.1) | 18.8 (4.5) | 28.0 (5.2) | 2.6 (1.2) | 7.7 (2.3) |
| High | 6.1 (2.1) | 3.4 (1.6) | 61.7 (5.8) | 19.8 (5.1) | 18.5 (5.8) | 6.0 (2.0) | 5.1 (2.1) | 19.7 (5.4) | 10.7 (3.6) |
| Irritable | 8.6 (3.6) | 39.2 (6.8) | 20.2 (5.5) | 8.1 (3.6) | 22.0 (5.8) | 31.0 (6.3) | 38.0 (6.5) | 20.9 (6.2) | 22.3 (5.7) |
| Like | 7.69 (2.6) | 6.44 (3.0) | 69.4 (5.7) | 30.1 (5.6) | 14.1 (3.8) | 11.1 (2.5) | 4.42(1.7) | 19.3 (4.9) | 10.4 (3.6) |
| Mellow | 21.3 (4.8) | 14.4 (4.2) | 55.9 (5.3) | 28.2 (4.8) | 19.8 (4.5) | 18.4 (4.5) | 13.1 (4.1) | 27.2 (5.1) | 20.9 (5.1) |
| Nauseous | 2.6 (1.1) | 13.2 (3.8) | 10.1 (4.4) | 5.6 (2.9) | 8.1 (4.0) | 7.3 ( 1.9) | 12.0 (3.9) | 8.0 (4.0) | 6.9 (3.1) |
| Of High Quality | 6.1 (1.8) | 6.9 (2.9) | 64.1 (5.5) | 28.7 (4.9) | 16.1 (3.6) | 12.0 (3.3) | 6.9 (2.1) | 16.8 (4.2) | 13.2 (3.7) |
| Potent | 8.1 (2.9) | 12.5 (4.2) | 64.6 (5.6) | 25.3 (4.8) | 20.4 (4.5) | 15.8 (4.4) | 12.5 (4.2) | 19.2 (4.6) | 16.3 (4.6) |
| Restless | 14.4 (4.6) | 33.6 (5.8) | 18.0 (4.9) | 11.7 (3.3) | 15.5 (4.7) | 23.6 (5.5) | 32.1 (6.5) | 14.9 (4.6) | 20.1 (4.9) |
| Sedated | 7.0 (2.3) | 5.4 (2.2) | 34.5 (5.5) | 13.4 (3.6) | 17.0 (4.3) | 5.6 (1.8) | 5.1 (1.4) | 13.3 (3.5) | 10.5 (3.7) |
| Sleepy | 25.5 (5.2) | 36.3 (5.6) | 29.8 (5.20 | 30.4 (5.1) | 39.0 (5.3) | 31.8 (5.3) | 30.9 (5.0) | 31.4 (5.7) | 28.6 (4.7) |
| Stimulated | 10.3 (2.9) | 14.9 (3.9) | 38.8 (5.4) | 19.1 (4.5) | 12.7 (4.0) | 14.5 (4.1) | 15.1 (4.3) | 17.9 (4.1) | 16.3 (4.4) |
| Talkative | 30.5 (5.3) | 26.7 (4.5) | 40.6 (5.6) | 36.7 (5.6) | 34.5 (5.70 | 31.2 (4.7) | 27.0 (5.2) | 34.7 (4.9) | 32.5 (5.1) |
| Would Pay | 1.2 (.55) | .42 (.19) | 13.4 (1.2) | 3.9 (1.1) | 2.3 (.75) | 1.1 (.46) | .50 (.22) | 3.7 (.95) | 1.9 (.80) |
| Subjective: DEQ (0–5) | |||||||||
| Bad | .47 (.26) | 1.8 (.25) | .28 (.13) | .53 (.14) | 1.2 (.30) | 2.0 (.36) | 2.1 (.27) | 1.3 (.21) | 1.8 (.38) |
| Good | .67 (.27) | .19 (.07) | 2.6 (.19) | 1.0 (.21) | .91 (.28) | 1.0 (.34) | .31 (.08) | .92 (.21) | .69 (.27) |
| Like | .69 (.29) | .25 (.13) | 2.8 (.21) | 1.2 (.23) | .80 (.29) | .41 (.14) | .22 (.09) | .78 (.21) | .61 (.27) |
| Strong | .64 (.17) | 1.3 (.22) | 2.67 (.19) | 1.22 (.20) | 1.55 (.29) | 1.66 (.36) | 1.5 (.24) | 1.4 (.21) | 1.4 (.29) |
| Take Again | .83 (.29) | .44 (.15) | 2.7 (.21) | 1.2 (.22) | .75 (.17) | 1.2 (.35) | .42 (.12) | .86 (.20) | .69 (.27) |
| Pupil Diameter (mm, Trough) | 3.2 (.12) | 3.2 (.15) | 2.6 (.02) | 2.8 (.11) | 2.7 (.10) | 3.1 (.14) | 3.1 (.12) | 2.9 (.11) | 2.8 (.10) |
| Pupil Diameter (mm, Peak) | 4.1 (.42) | 4.4 (.68) | 3.6 (.71) | 3.8 (1.0) | 4.1 (.59) | 4.3 (.91) | 4.2 (.57) | 3.9 (.65) | 4.0 (.67) |
| Performance: DAT | |||||||||
| # Hits | 19.3 (.17) | 19.1 (.16) | 19.1 (.16) | 19.2 (.16) | 19.3 (.18) | 19.3 (.16) | 19.1 (.19) | 19.2 (18) | 19.3 (.19) |
| # Misses | .39 (.11) | 1.31 (.49) | 2.3 (.52) | .94 (.29) | .94 (.25) | 1.44 (.55) | 1.36 (.35) | .94 (.23) | 1.22 (.28) |
| Tracking Distance (mm) | 9,395 (962) | 12,572 (1300) | 15,374 (1476) | 12,593 (1215) | 12,337 (1048) | 12,603 (1119) | 13,653 (1254) | 12,362 (1264) | 12,569 (1194) |
| Performance: DSST | |||||||||
| Total # Attempted | 83.1 (1.9) | 91.6 (9.4) | 81.5 (2.7) | 82.6 (2.7) | 97.0 (14.3) | 82.8 (2.2) | 84.9 (3.5) | 82.5 (2.3) | 81.0 (2.1) |
| Total # Correct | 81.0 (1.8) | 89.2 (9.5) | 79.9 (2.7) | 80.6 (2.7) | 80.4 (2.1) | 95.1 (14.4) | 82.4 (3.4) | 80.3 (2.6) | 78.9 (2.0) |
When asked to rate how their nose and throat felt after each dose was insufflated, none of the participants reported that any of the drugs were “Difficult to Snort.” The most commonly reported effects of snorting the drugs were “Burning,” “Tingling,” “Stinging,” “Dry Mouth,” and “Thirsty.” No significant differences in the prevalence of these effects were observed among the doses, and all of the participants reporting experiencing only “A Little Bit” of each. This absence of an effect was consistent across the 3 SL Bup maintenance conditions (p=0.47).
Reinforcing Effects
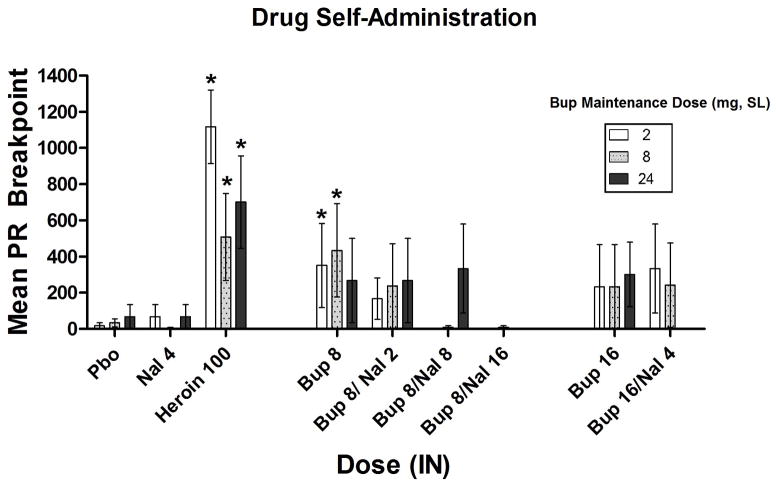
Omnibus ANOVA found no significant SL dose x IN dose interaction on progressive ratio breakpoint (BP) values (p=0.83). Across all of the SL maintenance conditions, heroin elicited a significant increase in BP compared to placebo (p<0.001). The 8 mg BUP dose also produced a BP significantly above that of placebo (p<0.05) although this only occurred under the 2 mg and 8 mg SL Bup maintenance conditions (Figure 4). Across all three SL Bup maintenance conditions, the BP value for the 16 mg dose of BUP did not significantly differ from placebo nor did any combination of Bup/Nal. Reinforcing effects examined as a function of percentage of drug choices (% of the 10 trials on which drug was chosen vs. money) showed the same pattern of results. IN naloxone alone was not reinforcing under any SL dosing condition.
Figure 4.
Mean (± SEM) progressive ratio breakpoint (BP). * Indicates a significant difference from placebo.
Physiological Measures
There was no SL Dose x IN Dose interaction when comparing trough pupil constriction (p=0.24). In general, Bup alone decreased pupil diameter when compared to placebo, but increasing the dose of naloxone typically antagonized this effect. When participants were maintained on SL Bup 2 mg: IN Bup 8 mg (p<0.001), IN Bup/Nal 8/2 mg (p<0.01), Bup 16 mg (p<0.001), and Bup/Nal 16/4 mg (p<0.001), significantly decreased pupil size. Under SL Bup 8 mg significant miosis was observed for: IN Bup 8 mg (p< 0.05), IN Bup/Nal 8/2 mg (p< 0.05), and Bup/Nal 16/4 mg (p<0.01). Meanwhile, when maintained on SL Bup 24 mg, only the IN 8/8 mg dose (p<0.10) altered pupil diameter, leading to an increase that trended towards significance. When compared to placebo, IN naloxone had no significant effect on pupil diameter.
Pharmacokinetic Outcomes
Due to concerns regarding participant health (i.e., low hemoglobin levels), we did not draw plasma samples on 4 of the 12 completers. Therefore, the following data were obtained using a subset of 8 completers. Mean plasma Bup concentrations peaked 30–45 minutes post-drug administration, with Bup 16 mg producing the highest plasma levels (p<0.05). The highest plasma norbuprenorphine (NorBup) concentrations were found at the end of our measurement period, approximately 90 minutes following drug administration, but did not significantly differ among the drug/dose conditions tested. Naloxone plasma concentrations peaked 15 minutes after dosing and increased dose dependently (p<0.01). Shown in Table 3 are the: Tmax, Cmax and AUC0–90mins for these drugs/doses under each SL maintenance condition.
Table 3.
Pharmacokinetic Profiles for Intranasal Buprenorphine, Norbuprenorphine, and Naloxone Under Each Sublingual Buprenorphine Maintenance Dose Condition
Tmax, Cmax and AUC0–90mins for Bup, NorBup, and Nal shown as a function of each IN drug/dose and SL maintenance condition (N = 8).
| 2 mg | 8 mg | 24 mg | |||
|---|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | Mean (SEM) | |||
| Tmax (min) | Bup | 8 mg | 35.00 (3.16) | 37.50 (4.33) | 40.71 (4.29) |
| 8/2 mg | 34.00 (9.14) | 37.50 (4.33) | 40.71 (2.77) | ||
| 8/8 mg | 51.00 (11.22) | 30.00 (0.00) | 47.50 (14.19) | ||
| 8/16 mg | 45.00 (12.55) | 37.50 (7.50) | 40.00 (10.72) | ||
| 16 mg | 39.00 (6.00) | 33.75 (7.18) | 38.57 (4.46) | ||
| 16/4 mg | 33.00 (8.75) | 41.25 (9.44) | 36.43 (6.43) | ||
| NorBup | 8 mg | 67.50 (7.50) | 56.25 (12.81) | 26.43 (13.17) | |
| 8/2 mg | 49.00 (13.73) | 41.25 (14.20) | 30.71 (12.84) | ||
| 8/8 mg | 51.00 (17.49) | 40.00 (26.46) | 33.33 (12.09) | ||
| 8/16 mg | 66.00 (10.17) | 63.75 (17.72) | 45.00 (12.85) | ||
| 16 mg | 42.00 (5.61) | 56.25 (12.81) | 72.86 (11.54) | ||
| 16/4 mg | 81.00 (9.00) | 60.00 (18.37) | 60.00 (13.09) | ||
| Nal | 8 mg | 9.00 (2.45) | 17.32 (10.00) | 29.00 (15.92) | |
| 8/2 mg | 11.00 (2.45) | 26.26 (13.13) | 12.86 (3.43) | ||
| 8/8 mg | 15.00 (0.00) | 5.77 (3.33) | 12.50 (4.03) | ||
| 8/16 mg | 13.00 (2.00) | 8.66 (4.33) | 11.67 (2.11) | ||
| 16 mg | 10.00 (5.00) | 18.93 (9.46) | 15.00 (4.23) | ||
| 16/4 mg | 19.00 (6.78) | 5.00 (2.50) | 22.14 (6.89) | ||
| Cmax (ng/ml) | Bup | 8 mg | 6.14 (.416) | 7.19 (1.99) | 7.29 (.689) |
| 8/2 mg | 4.63 (.670) | 8.15 (1.97) | 5.90 (.764) | ||
| 8/8 mg | 5.33 (.819) | 8.05 (1.69) | 7.64 (1.92) | ||
| 8/16 mg | 5.72 (2.27) | 7.07 (.950) | 9.55 (2.12) | ||
| 16 mg | 6.57 (.848) | 8.30 (1.57) | 11.53 (1.68) | ||
| 16/4 mg | 5.79 (.827) | 9.53 (2.22) | 9.00 (1.16) | ||
| NorBup | 8 mg | 1.14 (.506) | 2.19 (.717) | 4.44 (.928) | |
| 8/2 mg | 0.96 (.144) | 4.38 (2.01) | 5.20 (1.21) | ||
| 8/8 mg | 1.21 (.267) | 2.86 (1.32) | 5.38 (1.62) | ||
| 8/16 mg | 1.03 (.229) | 2.51 (.830) | 5.10 (1.54) | ||
| 16 mg | 1.32 (.552) | 2.34 (.973) | 5.68 (1.02) | ||
| 16/4 mg | 1.10 (.338) | 2.53 (.848) | 4.67 (.972) | ||
| Nal | 8 mg | .036 (.030) | .076 (.057) | .004 (.001) | |
| 8/2 mg | 3.55 (.959) | 5.26 (1.78) | 3.35 (1.22) | ||
| 8/8 mg | 7.57 (.962) | 11.43 (1.85) | 10.47 (3.05) | ||
| 8/16 mg | 17.37 (5.57) | 17.38 (4.99) | 24.73 (7.79) | ||
| 16 mg | .142 (.135) | .007 (.002) | .084 (.063) | ||
| 16/4 mg | 2.38 (1.25) | 5.24 (2.53) | 2.11 (.761) | ||
| AUC (0–90 min) | Bup | 8 mg | 391.26 (23.26) | 467.56 (129.79) | 521.89 (47.70) |
| 8/2 mg | 352.25 (23.38) | 508.52 (128.02) | 438.24 (53.47) | ||
| 8/8 mg | 315.79 (44.03) | 542.05 (109.20) | 533.91 (128.05) | ||
| 8/16 mg | 363.39 (134.61) | 443.04 (71.45) | 621.49 (117.31) | ||
| 16 mg | 428.76 (51.03) | 430.94 (82.00) | 791.16 (100.36) | ||
| 16/4 mg | 382.19 (50.81) | 572.70 (89.67) | 593.98 (57.38) | ||
| NorBup | 8 mg | 87.19 (38.20) | 180.52 (58.69) | 366.50 (75.71) | |
| 8/2 mg | 74.8 (14.19) | 251.79 (75.59) | 420.99 (90.71) | ||
| 8/8 mg | 89.8 (21.35) | 218.96 (103.16) | 433.55 (131.11) | ||
| 8/16 mg | 78.86 (16.22) | 206.04 (69.54) | 396.97 (110.97) | ||
| 16 mg | 78.09 (18.95) | 184.66 (71.89) | 464.34 (86.16) | ||
| 16/4 mg | 80.87 (23.64) | 185.29 (63.73) | 370.18 (83.13) | ||
| Nal | 8 mg | 1.342 (1.13) | 1.78 (.780) | .210 (.087) | |
| 8/2 mg | 125.76 (37.32) | 179.20 (54.58) | 129.32 (32.11) | ||
| 8/8 mg | 340.76 (42.70) | 444.86 (57.30) | 443.31 (109.12) | ||
| 8/16 mg | 676.52 (173.29) | 802.03 (161.23) | 887.48 (175.86) | ||
| 16 mg | 7.49 (7.21) | .229 (.486) | 1.38 (.903) | ||
| 16/4 mg | 94.75 (47.40) | 200.92 (99.97) | 100.31 (37.8) | ||
Discussion
This study examined the abuse liability of various combinations of intranasal Bup and naloxone. Specifically, we sought to assess conditions under which the abuse potential of intranasally administered Bup could be minimized e.g., using a higher SL Bup maintenance dose, increasing the dose of co-administered Nal, or decreasing the ratio of Bup to Nal. The current investigation found that intranasal buprenorphine (8 mg, 16 mg) produced significant increases in positive subjective effects including: drug “Liking,” “High,” “Good” effect and amount of money participants reported they “Would Pay” for the dose. Oftentimes, this IN drug effect was only found when participants were maintained on the lowest SL Bup dose (2 mg). In several cases, when participants were maintained on the larger SL Bup doses (8 mg, 24 mg), positive subjective effects then failed to differ significantly from placebo (e.g., “Good,” “Liking,” “High,” “Would Pay” and “Would Take Again”).
Of all the IN Bup and Bup/Nal combinations tested, the 8 mg Bup dose appeared to have the most consistent positive subjective effects (across the most measures, and under the most SL maintenance conditions). The 8 mg Bup dose also failed to produce aversive effects that differed significantly from placebo (under any SL condition). In contrast however, significant “Bad” effects were reported with the 16 mg IN Bup dose. This effect was increasingly pronounced under larger SL Bup maintenance dose conditions. Reports of significant aversive effects were consistently found when participants received an IN Bup/Nal combination. In fact, all of the Bup/Nal dose combinations significantly increased ratings of “Bad” drug effect as measured on both the VAS and DEQ scales.
Only the 8 mg IN dose of Bup was self-administered significantly more than placebo, and this only occurred under the 2 mg and 8 mg SL Bup maintenance dose conditions. Across all three SL Bup maintenance dose conditions, the BP value for the 16 mg IN dose of BUP did not significantly differ from placebo, nor did any dose combination of Bup/Nal. Combined with the subjective effects data, our findings demonstrate the relatively low abuse liability of 8 and 16 mg intranasal Bup among a Bup-dependent population. The addition of naloxone to Bup dose dependently attenuated the positive subjective effects of Bup and increased aversive effects. Larger SL maintenance doses of Bup also appeared to attenuate the positive subjective effects of IN Bup (8 mg, 16 mg) while increasing the aversive effects (16 mg). No intranasal Bup/Nal combination was found to produce positive subjective or reinforcing effects above placebo levels. As such, IN Bup/Nal appears to have less abuse potential than Bup alone.
Consistent with a previous clinical investigation (Middleton et al., 2011), the present study demonstrated the abuse potential of intranasal Bup. In their non-dependent opioid-abusing participants, 8 mg IN Bup produced peak VAS ratings of “Liking” and “Good” of 46.4 and 39.8 mm, respectively. Among our Bup-maintained participants, peak ratings of “Liking (Liked the Choice)” produced by 8 mg IN Bup under the different SL Bup maintenance doses were: 46.3 mm (2 mg SL Bup), 24.9 mm (8 mg SL Bup), 15.8 mm (24 mg SL Bup), and for “Good” were: 39.5 mm (2 mg SL Bup), 29.4 mm (8 mg SL Bup), and 17.7 mm (24 mg SL Bup). Thus, our results for peak ratings of “Liking” and “Good” effects under the 2 mg SL Bup maintenance condition were remarkably similar to the peak ratings for these two measures reported by Middleton and colleagues (2011) in non-dependent opioid abusers. The higher maintenance doses of SL Bup generally produced dose-related reductions in positive subjective ratings produced by opioid agonists, which has been noted in several previous studies (Bickel et al. 1988; Jones et al. 2011; Mello & Mendelson 1980, 1982; Walsh et al. 1995).
Comparing the effects of IN Bup/Nal 8/2 mg between the current study and the Middleton et al. study (2011) also has interesting implications. In their non-dependent sample, peak VAS ratings of drug “Liking” (43.6 mm), and “Good” drug effect (35.5 mm) were significantly greater than placebo. Their ratings of “Bad” drug effect (8.4 mm), however, were not significantly different from placebo (0.6 mm). In our dependent sample, 8/2 mg IN Bup/Nal produced peak VAS ratings of drug “Liking” [20.6 mm (2 mg SL Bup), 13.5 mm (8 mg SL Bup), and 8.2 mm (24 mg SL Bup)] and “Good” drug effect [17.7 mm (2 mg SL Bup), 20.5 mm (8 mg SL Bup), and 22.2 mm (24 mg SL Bup)] that were only significantly greater than placebo when participants were maintained on 2 mg SL Bup. These ratings of “Liking” and “Good” drug effect after administration of 8/2 mg IN Bup under the 2 mg SL Bup maintenance condition were lower than those reported by Middleton et al. (2011). Meanwhile, ratings of “Bad” drug effect after administration of 8/2 mg IN Bup in our study were greater than those reported by the non-dependent opioid abusers in the study conducted by Middleton et al. (2011) [18.5 mm (2 mg SL Bup), 23.9 mm (8 mg SL Bup), 32.7 mm (24 mg SL Bup)]. These data suggest that the Bup + Nal combination may be less effective in deterring parenteral abuse in non-dependent individuals or those maintained on low Bup doses. The IN administration of Bup/Nal combinations (under larger SL maintenance conditions) produced significant increases in VAS ratings of: “Anxious,” “Irritable,” “Gooseflesh (piloerection),” and “Nauseous,” (Table 2), which are common symptoms of opioid withdrawal (Doyon 2004).
The magnitudes of positive subjective effects and drug break point for IN Bup were significantly less than IN heroin. This in itself is not surprising, though the degree of difference between IN heroin and IN Bup is far greater than what we observed in a previous study with IV heroin (25 mg) and IV Bup (Comer et al. 2010). In the earlier study, peak ratings of positive subjective effects produced by 8 or 16 mg of IV Bup (“Like,” “Would Take Again,” “High”) as well as drug breakpoint, did not significantly differ from IV heroin. In contrast, in the current study, assessments of these effects for IN Bup were approximately half of what they were for heroin (Table 2). However, a direct comparison is complicated because, between the two studies, the dose of heroin was adjusted to account for the differences in bioavailability between the two routes of administration (IV heroin dose = 25 mg, IN heroin dose = 100 mg), while the Bup and Bup/Nal doses were not.
Differences in the pharmacokinetics of these drugs via different routes of administration are a possible factor contributing to the differences in our current findings and our previous results with IV buprenorphine (Comer et al., 2010). The time to maximum concentration (3–5 mins) and time course of heroin in plasma is very rapid following IV administration (Comer et al., 1999; Rentsch et al., 2001; Rook et al. 2006). Similarly, the time to maximum concentration and time course of buprenorphine in plasma achieved after IV administration suggests a high degree of bioavailability and relatively short Tmax (10–15 mins: Bullingham et al. 1980; Kuhlman et al. 1996; Lloyd-Jones et al. 1980; Huestis et al. 2013). In contrast to IN heroin (Tmax: ≈ 5 mins: Cone et al. 1993; Skopp et al. 1997), a slower onset of the pharmacodynamic effects and poorer bioavailability following IN administration of Bup have been observed in other studies (Tmax: 30–40 mins: Eriksen et al. 1989; Middleton et al. 2011) as well as in the current study (Tmax: 33–45 mins). As such, the differences in the pharmacokinetics of IN heroin versus IN Bup may be a substantive factor contributing to the greater difference in abuse liability seen between IN heroin versus IN Bup (current study) relative to IV heroin versus IV Bup (Comer et al. 2010).
The same-day sample and choice design employed in this study also make it difficult to directly compare the self-administration of a short-acting opioid like heroin to a longer-acting opioid like Bup. With the choice session occurring only 3 hours after the sample session, it is possible that there were more carryover effects with Bup that may not have been present with heroin. Data from our previous studies, however, suggest that carryover effects from morning to afternoon sessions do not significantly alter the reinforcing effects of buprenorphine in non-dependent participants (Comer et al., 2002, 2005; Comer and Collins, 2002). Concerning subjective responses, assessments of peak effects were made using data collected only during the sample session, so acute carryover effects would have been less prominent. The fact that the subjective effects profile so closely matches the reinforcing effects observed in the present study further supports the argument that carryover effects from the sample to choice session may not have substantially confounded our self-administration outcomes.
Accumulation of buprenorphine plasma levels over the course of the study also may have had a general confounding effect by increasing variability of drug responses during the randomized sample sessions, thereby lowering our ability to detect differences among the IN doses. However, as noted previously in the discussion, when participants were maintained on the lowest dose of SL Bup (2 mg), the magnitude of the positive subjective effects elicited by 8 mg of IN Bup are almost identical to those seen when administered to a non-dependent sample (Middleton et al. 2011). The 2 mg SL Bup maintenance condition was randomized, so participants could have been tested under this dose, after having been maintained on at least 14 days of 8 mg Bup or 14 days of 24 mg Bup. If carryover effects from other maintenance doses were prominent, one might expect that the overall effects of the challenge doses under the 2 mg SL Bup condition would be much lower than those reported by Middleton and colleagues (2011) in non-opioid-dependent participants.
The levels of total Bup concentration can be seen in Table 3. The results of our pharmacokinetic analysis of these data reveal interesting findings concerning the relationship between plasma buprenorphine levels and its subjective and reinforcing effects. Specifically, increasing plasma Bup concentrations did not directly yield increases in positive subjective effects and more drug self-administration. The 16 mg Bup dose produced higher plasma levels in comparison to the 8 mg dose, yet was consistently rated below the 8 mg dose on positive subjective assessments and was less reinforcing. These data suggest that the positive subjective effects of IN Bup plateau within this dose range. This ceiling effect has been noted in several other clinical studies (Jasinski et al. 1978; Umbricht et al. 2004; Walsh et al. 1994). The 16 mg dose was also rated as being significantly more aversive than the 8 mg dose. Combined, these data suggest that there is an ideal plasma Bup concentration that maximizes positive subjective effects and minimizes aversive effects. This conclusion is further supported when one observes the interaction between the IN Bup dose and the SL Bup dose. Positive subjective ratings for Bup 8 and 16 mg trend downwards with increased SL Bup maintenance doses, while ratings of aversive effects trend upwards.
Our data also revealed the complex nature of the interaction between Bup and Nal. There were several conditions under which larger maintenance doses reduced the positive subjective and reinforcing effects of IN-administered drug (heroin, Bup 8 mg, Figures 2 & 4). These data confirm the ability of SL Bup to reduce the abuse potential of other opioids. It also demonstrates a protective effect of larger SL Bup maintenance doses against the intranasal abuse of Bup. In contrast, however, increasing the SL Bup maintenance dose often blunted the aversive effects produced by IN naloxone (Bup/Nal 8/8 mg, Figure 3). Though this difference did not meet statistical significance, it may explain the increase in the self-administration of the 8/8 mg dose, under the 24 mg SL maintenance condition (Figure 4). These data are not surprising as we know that naloxone has more difficulty displacing Bup from mu receptors than it does other mu opioid agonists (Hardman et al. 1996; O’Brien et al. 1978; SAMHSA, 2004). An additional concern, drawn from these data, may be that higher Bup maintenance doses may reduce the utility of the combined formulation to deter parenteral use. Yet, the fact that no Bup/Nal combination was self-administered, no matter how large the sublingual maintenance Bup dose, strongly argues in favor of the use of Bup/Nal in cases where diversion to parenteral use may be suspected or likely. However, minimizing the risk of parenteral Bup/Nal use is just one of many clinical considerations in determining the appropriate maintenance dose of sublingual Bup.
In summary, this study provides empirical support for the lower abuse potential of Bup/Nal by insufflation compared to Bup alone. Furthermore, the results of this study suggest that the abuse potential of IN Bup or other opioids could be further minimized with larger SL Bup or Bup/Nal maintenance doses. However, the generalizability of these findings may have been affected by the stringent criteria for study enrollment, qualification and retention. Although these criteria are vital to conducting ethical research, our selectivity may have led to us sample a subpopulation of heroin users that may not be broadly generalizable. Despite this reservation, these findings demonstrate that Bup/Nal has lower abuse liability than Bup alone among Bup-dependent individuals, and thus should be used preferentially as an opioid dependence pharmacotherapy (Comer et al. 2005; Mello et al. 1982, 1983; Mello & Mendelson, 1980).
Acknowledgments
This study was supported by an unrestricted, unsolicited investigator-initiated grant from Reckitt-Benckiser Pharmaceuticals to SDC.
Financial support for the preparation of this manuscript was provided by the National Institute on Drug Abuse grant K01DA030446 to JDJ.
Over the past three years all of the authors (with the exception of Dr. Metz) have received compensation (in the form of partial salary support) from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals, Schering-Plough Corporation, Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and MediciNova. In addition, SKV has served as a consultant for and is currently employed by Grunenthal USA. SDC has also served as a consultant to the following companies: AstraZeneca, Grunenthal USA, Guidepoint Global, Mallinckrodt, Neuromed, Orexo, Pfizer, and Salix.
The medical assistance of Janet Murray, Claudia Tindall, and Audrey Perez, along with the technical assistance of Phillip Saccone, Joseph Lazar, Greta Bielaczyc-Raglan, Gabriel Madera, Jessica Fogel, Paula Askalsky, Brian Wade and Andrew Segoshi is gratefully acknowledged.
Footnotes
Disclosures
The study sponsor had no role in study design, collection, analysis and interpretation of the data, in the writing of the manuscript, or in the decision to submit this manuscript for publication, but did review the report for scientific accuracy.
Authors’ Contribution
Dr. Comer designed the study and wrote the protocol. Drs. Sullivan, Jones, Manubay, Comer, Vosburg, Mogali and Metz were responsible for the conduct of study procedures. Dr. Jones conducted the data analysis and wrote the first draft of the manuscript. All authors contributed to and approved the final draft of the manuscript.
References
- Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Auriacombe M, Fatsea M, Dubernet J, Daulouede J, Tignol J. French field experience with buprenorphine. Am J Addict. 2004;13(Suppl 1):S17–S28. doi: 10.1080/10550490490440780. [DOI] [PubMed] [Google Scholar]
- Bedi NS, Ray R, Jain R, Dhar NK. Abuse liability of buprenorphine-a study among experienced drug users. Indian J Physiol Pharmacol. 1998;42(1):95–100. [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988;247:47–53. [PubMed] [Google Scholar]
- Bullingham RE, McQuay HJ, Moore A, Bennett MR. Buprenorphine kinetics. Clin Pharmacol Ther. 1980;28(5):667–672. doi: 10.1038/clpt.1980.219. [DOI] [PubMed] [Google Scholar]
- Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infec Dis. 2006;43:S197–S215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- Chua SM, Lee TS. Abuse of prescription buprenorphine, regulatory controls and the role of the primary physician. An Acad Med Singapore. 2006;35:492–495. [PubMed] [Google Scholar]
- Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacol. 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Manubay J, Amass L, Cooper ZD, Saccone P, Kleber HD. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010;105:709–718. doi: 10.1111/j.1360-0443.2009.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315:1320–1330. doi: 10.1124/jpet.105.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacol. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Holicky BA, Grant TM, Darwin WD, Goldberger BA. Pharmacokinetics and pharmacodynamics of intranasal “snorted” heroin. J Anal Toxicol. 1993;17:327–337. doi: 10.1093/jat/17.6.327. [DOI] [PubMed] [Google Scholar]
- Doyon S. Opioids. In: Tintinalli JE, Kelen GD, Stapczynski JS, Ma OJ, Cline DM, editors. Emergency Medicine: A Comprehensive Study Guide. 6. Chap 167. McGraw-Hill; New York: 2004. pp. 1071–1075. [Google Scholar]
- Drug Enforcement Administration. [Accessed 11 November 2013.];Heroin Domestic Monitor Program. 2013 Available at: http://info.publicintelligence.net/DEA-HeroinDMP-2011.pdf.
- Duke AN, Correia CJ, Walsh SL, Bigelow GE, Strain EC. Acute effects of intramuscular and sublingual buprenorphine and buprenorphine/naloxone in non-dependent opioid abusers. Psychopharmacol (Berl) 2010;211:303–312. doi: 10.1007/s00213-010-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Jensen NH, Kamp-Jensen M, Bjarnø H, Friis P, Brewster D. The systemic availability of buprenorphine administered by nasal spray. J Pharm Pharmacol. 1989;41:803–805. doi: 10.1111/j.2042-7158.1989.tb06374.x. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN. Effects of buprenorphine and naloxone in morphine-stabilized opioid addicts. Drug Alcohol Depend. 1998;50:1–8. doi: 10.1016/s0376-8716(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, Simpson J. Superanova accessible general linear modeling. Yale J Biolo Med. 1990;63:191–192. [Google Scholar]
- Hakansson A, Medvedeo A, Andersson M, Berglund M. Buprenorphine misuse among heroin and amphetamine users in Malmo, Sweden: purpose of misuse and route of administration. Euro Addic Res. 2007;13:207–215. doi: 10.1159/000104883. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw–Hill, Health Professions Division; 1996. p. 521.p. 555. [Google Scholar]
- Horyniak D, Dietze P, Larance B, Winstock A, Degenhardt L. The prevalence and correlates of buprenorphine inhalation amongst opioid substitution treatment (OST) clients in Australia. Int J Drug Pol. 2011;22:167–171. doi: 10.1016/j.drugpo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ, Pirnay SO, Umbricht A, Preston KL. Intravenous buprenorphine and norbuprenorphine pharmacokinetics in humans. Drug Alcohol Depend. 2013;131(3):258–262. doi: 10.1016/j.drugalcdep.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch General Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay J, Vosburg SK, Comer SD. The subjective, reinforcing, and analgesic effects of oxycodone in patients with chronic, non-malignant pain who are maintained on sublingual buprenorphine/naloxone. Neuropsychopharmacol. 2011;36(2):411–22. doi: 10.1038/npp.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman JJ, Jr, Lalani S, Magluilo J, Jr, Levine B, Darwin WD. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369–378. doi: 10.1093/jat/20.6.369. [DOI] [PubMed] [Google Scholar]
- Lee CE. Tackling Subutex abuse in Singapore. Singapore Med J. 2006;47:919–921. [PubMed] [Google Scholar]
- Lloyd-Jones JG, Robinson P, Henson R, Biggs SR, Taylor T. Plasma concentration and disposition of buprenorphine after intravenous and intramuscular doses to baboons. Eur J Drug Metab Pharmacokinet. 1980;5(4):233–239. doi: 10.1007/BF03189469. [DOI] [PubMed] [Google Scholar]
- Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12:379–384. doi: 10.1097/01.mjt.0000160935.62883.ff. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2008;16:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, McCance-Katz EF. Indicators of buprenorphine and methadone use and abuse: what do we know? Am J Addict. 2010;19:73–88. doi: 10.1111/j.1521-0391.2009.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Fernandez I, Welm S, Melby AK, Baggott MJ. Buprenorphine and naloxone interactions in opiate-dependent volunteers. Clin Pharmacol Ther. 1996;60:105–114. doi: 10.1016/S0009-9236(96)90173-3. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Welm S, Baggott M, Fernandez I, Melby AK, Nath RP. Buprenorphine and naloxone combinations: the effects of three dose ratios in morphine-stabilized, opiate-dependent volunteers. Psychopharmacol. 1999;141:37–46. doi: 10.1007/s002130050804. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Welm S, Brown J, Batki SL. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095–1101. doi: 10.1016/S0006-3223(96)00266-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J Pharmacol Exp Ther. 1983;225:378–386. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Behavioral pharmacology of buprenorphine. Drug Alcohol Depend. 1985;14:283–303. doi: 10.1016/0376-8716(85)90062-6. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on human heroin self-administration: an operant analysis. J Pharmacol Exp Ther. 1982;223:30–39. [PubMed] [Google Scholar]
- Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction. 2011;106(8):1460–1473. doi: 10.1111/j.1360-0443.2011.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Dietze P, Lee N, Dunlop A, Taylor D. Concurrent buprenorphine and benzodiazepine use and self-reported opioid toxicity substitution treatment. Addiction. 2007;102:616–622. doi: 10.1111/j.1360-0443.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Nordmann S, Frauger E, Pauly V, Orléans V, Pradel V, Mallaret M, Thirion X, Micallef J. Misuse of buprenorphine maintenance treatment since introduction of its generic forms: OPPIDUM survey. Pharmacoepidemiol Drug Saf. 2012;21:184–90. doi: 10.1002/pds.2263. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Greenstein R, Ternes J, Woody GE. Clinical pharmacology of narcotic antagonists. Ann N Y Acad Sci. 1978;311:232–239. doi: 10.1111/j.1749-6632.1978.tb16779.x. [DOI] [PubMed] [Google Scholar]
- Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti JP. Injecting misuse of buprenorphine among French drug users. Addiction. 2001;96:267–272. doi: 10.1046/j.1360-0443.2001.96226710.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Effects of sublingually given naloxone in opioid-dependent human volunteers. Drug Alcohol Depend. 1990;25:27–34. doi: 10.1016/0376-8716(90)90136-3. [DOI] [PubMed] [Google Scholar]
- Rentsch KM, Kullak-Ublick GA, Reichel C, Meier PJ, Fattinger K. Arterial and venous pharmacokinetics of intravenous heroin in subjects who are addicted to narcotics. Clin Pharmacol. 2001;70:237–246. doi: 10.1067/mcp.2001.117981. [DOI] [PubMed] [Google Scholar]
- Rook EJ, van Ree JM, van den Brink W, Hillebrand MJ, Huitema AD, Hendriks VM, Beijnen JH. Pharmacokinetics and pharmacodynamics of high doses of pharmaceutically prepared heroin, by intravenous or by inhalation route in opioid-dependent patients. Basic Clin Pharmacol Toxicol. 2006;98(1):86–96. doi: 10.1111/j.1742-7843.2006.pto_233.x. [DOI] [PubMed] [Google Scholar]
- Roux P, Villes V, Bry D, Spire B, Feroni I, Marcellin F, Carrieri MP. Buprenorphine sniffing as a response to inadequate care in substituted patients: results from the Subazur survey in south-eastern France. Addic Behav. 2008;33(12):1625–1629. doi: 10.1016/j.addbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. 2004 http://162.99.3.213/products/tools/cl-guides/pdfs/QGP_40.pdf. [PubMed]
- Skopp G, Ganssmann B, Cone EJ, Aderjan R. Plasma concentrations of heroin and morphine-related metabolites after intranasal and intramuscular administration. J Anal Toxicol. 1997;21:105–111. doi: 10.1093/jat/21.2.105. [DOI] [PubMed] [Google Scholar]
- Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacol (Berl) 2001;154(3):230–42. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacol (Berl) 1997;129:329–338. doi: 10.1007/s002130050199. [DOI] [PubMed] [Google Scholar]
- SPSS I. SPSS 18.0.0 for windows. Chicago, Illinois: 2009. [Google Scholar]
- Umbricht A, Huestis MA, Cone EJ, Preston KL. Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J Clin Psychopharmacol. 2004;24:479–487. doi: 10.1097/01.jcp.0000138766.15858.c6. [DOI] [PubMed] [Google Scholar]
- Vicknasingam B, Mazlan M, Schottenfeld RS, Chawarski MC. Injection of buprenorphine and buprenorphine/naloxone tablets in Malaysia. Drug Alc Depend. 2010;111:44–49. doi: 10.1016/j.drugalcdep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Vidal-Trecan G, Varescon I, Nabet N, Boissonnas A. Intravenous use of prescribed sublingual buprenorphine tablets by drug users receiving maintenance therapy in France. Drug Alc Depend. 2003;69:175–181. doi: 10.1016/s0376-8716(02)00312-5. [DOI] [PubMed] [Google Scholar]
- Walsh S, Preston K, Bigelow G, Stitzer M. Acute administration of buprenorphine in humans: partial agonist and blockage effect. J Pharmacolo Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Young AM, Havens JR, Leukefeld CG. Route of administration for illicit prescription opioids: a comparison of rural and urban drug users. Harm Reduct J. 2010;7:24–30. doi: 10.1186/1477-7517-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282(3):1187–1197. [PubMed] [Google Scholar]