Abstract
Background
Standardized pain-intensity measurement across different tools would enable practitioners to have confidence in clinical decision-making for pain management.
Objectives
The purpose was to examine the degree of agreement among unidimensional pain scales, and to determine the accuracy of the multidimensional pain scales in the diagnosis of severe pain.
Methods
A secondary analysis was performed. The sample included a convenience sample of 480 cancer patients recruited from both the internet and community settings. Cancer pain was measured using the Verbal Descriptor Scale (VDS), the Visual Analog Scale (VAS), the Faces Pain Scale (FPS), the McGill Pain Questionnaire-Short Form (MPQ-SF) and the Brief Pain Inventory-Short Form (BPI-SF). Data were analyzed using a multivariate analysis of variance (MANOVA) and a receiver operating characteristics (ROC) curve.
Results
The agreement between the VDS and VAS was 77.25%, while the agreement was 71.88% and 71.60% between the VDS and FPS, and VAS and FPS, respectively. The MPQ-SF and BPI-SF yielded high accuracy in the diagnosis of severe pain. Cutoff points for severe pain were > 8 for the MPQ-SF and > 14 for the BPI-SF, which exhibited high sensitivity and relatively low specificity.
Conclusion
The study found substantial agreement between the unidimensional pain scales, and high accuracy of the MPQ-SF and the BPI-SF in the diagnosis of severe pain.
Implications for Practice
Use of one or more pain screening tools that have been validated diagnostic accuracy and consistency will help classify pain effectively and subsequently promote optimal pain control in multi-ethnic groups of cancer patients.
Keywords: cancer, pain, self-report, measurement accuracy
Introduction
Standardized pain-intensity measurement across different tools would enable practitioners to have confidence in clinical decision-making for pain management.1 A number of instruments are being used to measure intensity or severity of self-reported pain using a unidimensional approach. These methods include a Visual Analog Scale (VAS), a Verbal Descriptor Scale (VDS), a Faces Pain Scale (FPS), and a numerical rating scale (NRS). In addition, multidimensional instruments have been developed and used to reflect the multidimensionality of the pain experience; these include the McGill Pain Questionnaire (MPQ) and the Brief Pain Inventory (BPI). Although these scales have been validated and were found reliable to measure pain, Jones et al. contend that these multiple scales have varying numbers of pain levels with different wordings for pain scores, making comparisons across multiple instruments difficult.1 They examined the equivalency of the three unidimensional pain scales (the VDS, NRS, and FPS) among nursing home residents and found 69.6 to 83.7% agreement rates between the instruments; this indicates highly correlated pain intensity measurement across all three instruments. However, the study also revealed that there was extensive variability in individual pain reporting and nursing home residents tended to underrate pain intensity on the FPS.1
Others examined the applicability of PainDetect, a self-report questionnaire used to screen neuropathic pain in patients with fibromyalgia.2 They constructed a receiver operating characteristics (ROC) curve to determine cutoff points for PainDetect and the ROC curve showed low specificity (0.53) with sensitivity of 0.79 and poor accuracy with the value of area under curve (AUC) of 0.69. This indicated the PainDetect is not useful as a screening tool for neuropathic pain.3
There have been no studies on agreement among the pain scales or on the cutoff-points of multiple pain scales in multi-ethnic groups of cancer patients.3–4 However, it has been suggested that patients’ cultures and ethnicities influence variations in pain perception and expression. Thus, comparison across multiple unidimensional pain scales as well as information on the cutoff points of multidimensional pain scales among multi-ethnic groups would help to standardize pain ratings and to provide valuable information regarding applicability of the pain instruments to screen cancer pain in multicultural settings.1–2,5
The purpose of this secondary analysis study was to examine the degree of agreement among various unidimensional pain scales (the VDS, VAS, and FPS) and to determine whether multiple pain instruments accurately represent the degree of self-reported cancer pain in a multiethnic group of cancer patients. In addition, this study aimed to determine the diagnostic accuracy for severe cancer pain including sensitivity and specificity of multidimensional pain scales (the MPQ-SF and BPI-SF) using reference criteria (gold standard), which was produced by a combination of multiple unidimensional pain scales. Here, diagnostic accuracy indicates the ability of pain scales to discriminate severe pain among cancer patients. Diagnostic accuracy can be quantified in terms of sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio.6
Methods
Design
This is a secondary analysis of the data from a cross-sectional study on gender and ethnic differences in cancer pain experience. This study was approved by the Institutional Review Board of the university with which the authors are affiliated.
Sample and Setting
The sample included 480 cancer patients recruited from both the internet (n = 204) and community (n = 276) settings using a convenience sampling method. The study recruited cancer patients from cancer clinics and cancer support groups in community settings across the United States and from cancer support groups on the internet. Internet cancer support groups were identified through major internet search engines (e.g., Google, MSN, and Yahoo). Ten community consultants, identified through internet searches, helped to recruit cancer patients in community settings. Inclusion criteria for research participants were cancer patients aged at least 18 years who could read and write English and whose self-reported racial/ethnic identity was Hispanic, non-Hispanic (NH) White, African American, or Asian. The original study identified that there were no statistically significant differences in psychometric properties between the internet format and the pen-and-pencil format of the questionnaire (p > 0.05).7 With an alpha of 0.05 and an area under the ROC curve (AUC) of 0.725, a total of 57 participants would be needed for the ROC curve analysis.8 Therefore, 480 patients in the original study were deemed sufficient for the analysis.
Instruments
The study instruments included questions on socio-demographic characteristics, self-reported health and disease status, and multiple instruments measuring self-reported cancer pain, and functional status of cancer patients. The questions on socio-demographic characteristics included age, gender, education, employment status, and race/ethnicity. The questions on disease status included those on cancer diagnosis and treatment (e.g. site/type/stage of cancer, and use of pain medicine).
Self-reported cancer pain was measured using the Verbal Descriptor Scale (VDS), the Visual Analog Scale (VAS), the Wong-Baker Faces Pain Scale (FPS), the McGill Pain Questionnaire-Short Form (MPQ-SF) and the Brief Pain Inventory-Short Form (BPI-SF). The VDS, which measures self-reported pain, has numerical values from 0 (no pain) to 5 (worst possible pain). The VAS measures pain intensity using a 10-cm horizontal line with word anchors at each end of the line (i.e., no pain and worst pain possible), and the FPS consists of six faces that are assigned numerical values from 0 (a very happy smiling face) to 5 (a sad, tearful face).9 These unidimensional instruments have been reported to be reliable and valid in multiple ethnic groups of cancer patients.5,10 In order to make comparison across multiple pain intensity scales, the VDS, VAS, and FPS were re-categorized into no pain, mild, moderate, and severe pain based on previous studies.1,4,11
The VDS rated pain as no pain, mild pain, moderate pain, severe pain, very severe pain, and worst possible pain. It was re-categorized into no pain, mild pain, moderate pain, and severe pain by combining three pain categories (severe pain, very severe pain, and worst possible pain) into a single new severe pain category.1,11 The FPS was re-categorized based on previous studies,1,12 and accordingly, no hurt (smiling face) was re-categorized as no pain, hurts a little bit and hurts a little more were re-categorized as mild pain, hurts even more was re-categorized as moderate pain, and hurts a whole lot and hurts worst (tearful face) were re-categorized as severe pain. The process of categorizing VAS was provided in the results section.
Use of standardized pain categories (no pain, mild, moderate, and severe pain) is critically important.1 Researchers argued that multiple pain scales measure pain using varying numbers and different wordings of pain intensity scores and therefore a comparison across pain scales is problematic.1 A standardized metric for pain intensity would improve clinical decision making and enhance communication about pain among clinicians, and between the clinician and patient, and the investigators could compare pain evaluation outcomes across groups that have used different scales with confidence.1
The MPQ-SF was used as a multidimensional pain assessment scale with three components: pain rating index (PRI), VAS, and present pain intensity.13 Only the PRI part was used in this study. The PRI consists of 15 descriptors including 11 sensory and 4 affective pain intensity scales and was measured with a 4-point Likert scale (0 = none, 1 = mild, 2 = moderate, or 3 = severe). Total scores were calculated by adding up all 15 items in the PRI part of the MPQ-SF for analysis in the current study (range, 0–45). The Cronbach’s Alpha of the MPQ-SF was 0.94 in this sample.7
The BPI-SF consists of 15 items to measure sensory, affective, physiologic, and behavioral pain.14 Among the 15 items in the BPI-SF, only the four items in the pain intensity part (pain now, and its worst, least and average during the past week) were used in the analysis. Because, a previous study suggested that four pain intensity items of the BPI can be combined to give an index of pain severity.15 Thus, total BPI-SF scores were produced by adding up the four items (range, 0–40). These items were measured with 0–10 numerical rating scales (0 = no pain or does not interfere and 10 = pain as bad as you can imagine or completely interferes). The Cronbach’s Alpha of the BPI-SF was 0.96 in this sample.7
The Functional Assessment of Cancer Therapy Scale (FACT-G) was used to measure functional status of patients. The FACT-G scores were used to determine optimal cutoff points of mild, moderate, and severe pain for VAS, in order to enable comparisons across multiple unidimensional pain scales. The FACT-G consists of 33 items in five domains: physical, social and family, emotional, and functional well-being, and relationship with the physician.16 Among the 33 items, 28 items were measured with a 5-point Likert scale (0= not at all, and 4 = very much), while the other five items were measured with a linear analogue scale from 0 (not at all) to 10 (very much so). The total FACT-G scores were determined by adding up the 33 item scores (range = 0–162). The Cronbach’s Alpha was 0.70 in this sample.7
Reliability and validity of the multidimensional pain scales use in multi-ethnic groups were verified in previous studies for the MPQ-SF,17–18 the BPI-SF,18–19 and the FACT-G.11–20
Procedures
In the original study, a web-based survey was conducted via a project website developed by the research team members of the original study.7 Potential participants, recruited through internet cancer support groups, visited the study website and completed the questionnaire after agreement through an informed consent on-line. Pen-and-pencil questionnaires were mailed to community consultants, who distributed the questionnaire to cancer patients in person. Community consultants collected completed questionnaires with signed informed consent and then mailed them to the research team. Data collections in both the internet and community settings relied on participants’ self-reports. Community consultants were encouraged to recruit ethnic minority cancer patients so that the study could include similar numbers across ethnic groups. However, ethnic differences in types of cancer pain and symptoms accompanying cancer pain were beyond the scope of the current study which were previously reported using the same dataset.7 On average, the questionnaire took 30–40 minutes to complete, either on the internet or using pen-and-pencil. Data was collected in 2006. The data from both internet and pen-and-pencil surveys were included for this secondary analysis.
Data Analysis
The Statistical Package for the Social Sciences 20.0 for Windows was used for data analysis (SPSS, Inc., an IBM Company, Chicago, Illinois, USA). The data on background characteristics, disease characteristics were analyzed using descriptive statistics such as means, standard deviations, frequencies, and percents. Cross-tabulations were formulated to assess the degree of agreement between two different single-item pain scales (the VDS vs. VAS, the VDS vs. FPS, and the VAS vs. FPS).
A multivariate analysis of variance (MANOVA) was conducted to determine cutoff points for the VAS. The criteria used to determine the optimal boundaries were pain categories that yielded a larger F ratio for the between-subject effect on the five dimensions of the FACT-G (physical, social/family, emotional, and functional well-being, and relationship with the physician).Those criteria yielded fairly consistent results across the four ethnic groups (small F ratio) on the three tests (Pillai’s trace, Hotelling’s trace, and Wilks’ lamda). These were used to determine the criterion ratio (the F ratio for main effects of pain category divided by the F ratio for the interaction term [pain category × ethnic group]).4 To determine cutoff points of mild, moderate, and severe pain for NRS, Serlin et al. used 7-items of interference with life in the BPI as dependent variables in the MANOVA. They assumed that mild, moderate, and severe pain would differently impact multiple functions of cancer patients.4 Accordingly, to determine cutoff points for VAS, the current study used multiple dimensions of FACT-G as dependent variables in the MANOVA.
The ROC curve was constructed to evaluate the accuracy of the multidimensional pain scales in the diagnosis of severe pain. A previous study reported that since pain is subjective in nature, patients’ self-reports are the gold standard for pain assessment.1 Thus, the gold standard for severe pain was produced by combining VDS and VAS, which showed the highest agreement rate between the unidimensional scales in the current study. Using the ROC curve, the AUC scores were examined to evaluate the diagnostic accuracy, then sensitivity and specificity scores were produced to determine cutoff values for individual instruments. The ROC curve is used in medicine to express the diagnostic accuracy of clinical tests, which are graphical plots of sensitivity (y-axis) and 1 – specificity (x-axis), and the AUC is the gross area under the ROC curve, which is used as a global indicator of diagnostic performance.21 The AUC between 0.90 and 1.0 indicates high accuracy, that between 0.70 and 0.90 indicates moderate accuracy, and that between 0.50 and 0.70 indicates low accuracy.22 Null hypotheses of no differences were rejected if p-values were less than 0.05.
Results
General and Clinical Characteristics of the Patients
The mean age of the participants was 51.92 years (SD=12.27) and 79.4% were females. The race/ethnicity composition of the participants indicated that 30.8% were non-Hispanic Whites, 24.6% were Asians, 22.7% were African Americans, and the other 21.9% were Hispanics. Forty-five percent of all patients were diagnosed with breast cancer, 9.6% had gastrointestinal cancer, and 8.1% of the patients had cancer in female reproductive organs. Twenty-five percent were at cancer stage II, and 15.0% and 12.7% were at cancer stage III and IV, respectively. Thirty-seven percent of the patients were taking pain medication (Table 1).
Table 1.
Sociodemographic and Disease Characteristics of the Participants (N=480)
| Characteristics | Distribution | N (%) | M (SD) |
|---|---|---|---|
| Age (year) | 51.92 (12.27) | ||
| Gender | Male | 97 (20.2) | |
| Female | 381 (79.4) | ||
| Race/ethnicity | Hispanic | 105 (21.9) | |
| NH White | 148 (30.8) | ||
| African American | 109 (22.7) | ||
| Asian | 118 (24.6) | ||
| Education | ≤ Elementary | 27 (5.6) | |
| ≤ High school | 156 (32.5) | ||
| College ≥ | 297 (61.9) | ||
| Employment | Yes | 190 (39.6) | |
| Cancer site | Breast | 217 (45.2) | |
| Gastrointestinal | 46 (9.6) | ||
| Female reproductive organs | 39 (8.1) | ||
| Head and neck | 32 (6.7) | ||
| Lung | 30 (6.3) | ||
| Hematologic | 14 (2.9) | ||
| Lymph nodes | 11 (2.3) | ||
| Prostate | 10 (2.1) | ||
| Combined | 42 (8.8) | ||
| Others | 35 (7.2) | ||
| Cancer stage | 0 | 23 (4.8) | |
| I | 79 (16.5) | ||
| II | 122 (25.4) | ||
| III | 72 (15.0) | ||
| IV | 61 (12.7) | ||
| Recurrent | 24 (5.0) | ||
| Not staged | 5 (1.0) | ||
| Unknown | 24 (5.0) | ||
| Pain medication | Yes | 179 (37.3) |
Unanswered responses were excluded from the analysis
Abbreviations: NH = non-Hispanic.
Agreement between Unidimensional Pain Scales: the VDS, VAS, and FPS
The VDS was re-categorized from a 6-point Likert scale (0–5) to a 4-point Likert scale (no pain, mild, moderate, and severe pain), and the FPS was also re-categorized using a 4-point Likert scale as well. The VAS was categorized using the method proposed by Serlin et al.4 With the VAS, Hirschfeld and Zernikow identified seven pairs of combinations for potential cutoff points of mild, moderate, and severe pain (40–70, 35–70, 45–70, 35–60, 40–75, 30–60, and 30–70) that were found in more than 5% of the samples.3 Using these seven pairs of potential cutoff points, seven separate MANOVAs were conducted. Ethnic group (Hispanic, NH Whites, African Americans, and Asians) and pain severity with four levels (no, mild, moderate, and severe pain) were entered as between-subject factors, while five dimensions of FACT-G were entered as dependent variables. The cutoff point of the 35–70 pair had the highest criterion ratio in all of the three tests (Pillai’s trace = 17.44, Hotelling’s trace = 27.37, and Wilks’ lamda = 22.17). Thus, the optimal cutoff points for the VAS were set at 0 (no pain), 1–35 (mild), 36–70 (moderate), and 71–100 (severe) in the current study.
Using the re-categorized VDS and FPS, the agreement between the VDS and FPS was 71.88% (340/473) with a Cohen’s Kappa = 0.624. With the cutoff points of 35–70 for the VAS, the agreement between the VDS and VAS was 77.25% (326/422) with a Cohen’s Kappa = 0.695, while the agreement between the VAS and FPS was 71.60% (300/419) with a Cohen’s Kappa = 0.617. Cohen’s Kappa values between 0.61 and 0.80 indicate substantial agreement between the two scales.23 Existence of cancer pain, at least mild pain, was reported in 67.8% (VAS), 71.4% (VDS), and 74.9% (FPS) of the participants (Table 2).
Table 2.
Agreement between the VDS, FPS, and VAS (N=480)
| FPS |
|||||
|---|---|---|---|---|---|
| VDS |  |
 |
 |
 |
Total |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| No pain | 107 (90.7) | 24 (14.3) | 0 (0.0) | 1 (1.1) | 132(27.9) |
| Mild | 7 (5.9) | 99 (58.9) | 8 (8.7) | 4 (4.2) | 118(24.9) |
| Moderate | 2 (1.7) | 43 (25.6) | 56 (60.9) | 12 (12.6) | 113(23.9) |
| Severe | 2 (1.7) | 2 (1.2) | 28 (30.4) | 78 (82.1) | 110(23.3) |
| Total | 118 (100.0) | 168(100.0) | 92(100.0) | 95(100.0) | 473 (100.0) |
| VAS |
|||||
| VDS | 0 | 1–35 | 36–70 | 71–100 | Total |
| No pain | 113 (83.7) | 12 (11.4) | 1 (1.0) | 0 (0.0) | 126(29.9) |
| Mild | 9 (6.7) | 84 (70.6) | 6 (7.0) | 1 (1.2) | 100(23.7) |
| Moderate | 7 (5.2) | 23 (19.3) | 56 (65.1) | 8 (9.8) | 94(22.2) |
| Severe | 6 (4.4) | 0 (0.0) | 23 (26.7) | 73 (89.0) | 102(24.2) |
| Total | 135(100.0) | 119(100.0) | 86(100.0) | 82(100.0) | 422(100.0) |
| FPS |
|||||
| VAS | Smile | Hurts a little | Hurts even more | Crying | Total |
| 0 | 97(89.8) | 27(18.9) | 3(3.8) | 5(5.6) | 132(31.5) |
| 1–35 | 9(8.3) | 91(63.6) | 14(17.7) | 5(5.6) | 119(28.4) |
| 36–70 | 2(1.9) | 23(16.1) | 47(59.5) | 14(15.7) | 86(20.5) |
| 71–100 | 0(0.0) | 2(1.4) | 15(19.0) | 65(73.0) | 82(19.6) |
| Total | 108(100.0) | 143(100.0) | 79(100.0) | 89(100.0) | 419(100.0) |
Unanswered responses were excluded from analysis.
Abbreviations: FPS, Faces Pain Scale; VAS, Visual Analog Scale; VDS, Verbal Descriptor Scale.
Accuracy of the MPQ-SF and BPI-SF in the Diagnosis of Severe Pain
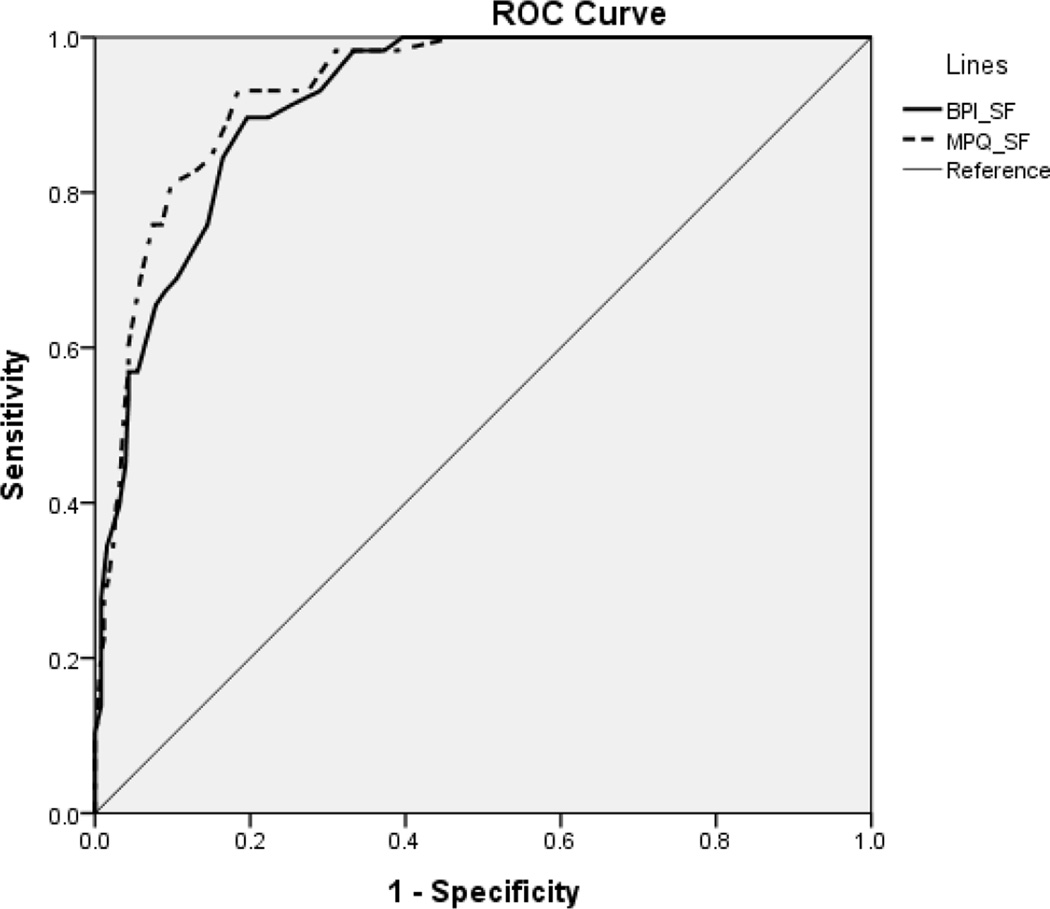
In the ROC curve analysis, combined scores of the VDS and VAS were used as the gold standard for diagnostic criteria of severe pain because the highest agreement rate was found between the VDS and VAS than between the VDS and FPS, and the VAS and FPS. Those who consistently responded in the same pain category (no pain-no pain, mild-mild, moderate-moderate, and severe-severe pain) were included in the ROC curve (n=326). The results indicated that the MPQ-SF and BPI-SF showed high accuracy in diagnosis of severe pain with AUC values greater than 0.9 (Figure) (Table 3).
Figure.
ROC Curve of the MPQ-SF and BPI-SF Scores
When Used to Define the Presence of Severe Pain.
Abbreviations: BPI-SF, Brief Pain Inventory-Short Form; MPQ-SF, McGill Pain Questionnaire-Short Form; ROC, receiver operator characteristic.
Table 3.
Area Under the Curve of the MPQ-SF and BPI-SF for Severe Pain (n=326)
| AUC | 95% CI | |
|---|---|---|
| MPQ-SF | .933 | .906 – .961 |
| BPI-SF | .918 | .886 – .950 |
Abbreviations: AUC, area under the curve; BPI-SF, Brief Pain Inventory-Short Form; CI, confidence interval; MPQ-SF, McGill Pain Questionnaire-Short Form.
Sensitivity and Specificity of the MPQ-SF and BPI-SF for Severe Pain
The optimal cutoff point for severe pain was > 8 for the MPQ-SF according to the ROC curve, which resulted in 93% sensitivity and 82% specificity, and the optimal cutoff point was > 14 for the BPI-SF with 90% and 80% of sensitivity and specificity, respectively. The positive likelihood ratio (LR+) ranged from 4.41 (the BPI-SF) to 5.03 (the MPQ-SF), indicating that the results for the scales were associated with the presence of severe pain, while the negative likelihood ratio (LR−) ranged from 0.09 (the MPQ-SF) to 0.12 (the BPI-SF), meaning that the results were associated with the absence of severe pain (Table 4).
Table 4.
Sensitivity and Specificity of the MPQ-SF and BPI-SF (n=326)
| Cutoff point | Sensitivity (95% CI) |
Specificity (95% CI) |
LR+ (95% CI) |
LR− (95% CI) |
|
|---|---|---|---|---|---|
| MPQ-SF | > 8 | 0.93 (0.84 – 0.97) |
0.82 (0.76 – 0.86) |
5.03 (3.86 – 6.57) |
0.09 (0.04 – 0.21) |
| BPI-SF | > 14 | 0.90 (0.80 – 0.95) |
0.80 (0.74 – 0.84) |
4.41 (3.42 – 5.68) |
0.12 (0.06 – 0.27) |
Abbreviations: BPI-SF, Brief Pain Inventory-Short Form; CI, confidence interval; LR+, positive likelihood ratio; LR−, negative likelihood ratio; MPQ-SF, McGill Pain Questionnaire-Short Form.
Discussion
The current analysis sought to examine agreement between unidimensional pain scales (the VDS, VAS, and FPS) and to evaluate the accuracy of multidimensional pain scales (MPQ-SF and BPI-SF) in the diagnosis of severe pain. The study results illustrated substantial agreement between the VDS and VAS (77.25%), between the VDS and FPS (71.88%), and between the VAS and FPS (71.60%). In the ROC curve, the MPQ-SF and BPI-SF yielded high accuracy in the diagnosis of severe pain. This analysis is unique because the data came from four major ethnic groups in a comparable sample size in each ethnic group. The results of this study will provide valuable information regarding multiple pain scales that could be utilized with cancer patients from multi-ethnic groups. There have been no studies on the cutoff-points of multiple pain scales in a multi-ethnic group of cancer patients.3–4
The result of the present study was similar to the previous study, which reported 68.9% agreement between the VDS and FPS measured with nursing home residents.1 They concluded that patients underrated higher intensity pain on the FPS. Similarly, cancer patients underestimated pain using the FPS when compared with the VDS in our study. For example, 25.6% of those in the mild pain category of the FPS (hurts a little) belonged to the moderate pain category in the VDS, and 30.4% of those in the moderate pain category on the FPS (hurts even more) belonged to the severe pain category of the VDS. Others also reported that there is a weak correlation between FPS and other pain scales.10 Therefore, researchers should be aware of the tendency of underestimated pain using the FPS and be cautious in the interpretation of pain measured with the FPS.
In our study, 19.4 – 23.2% of the cancer patients had severe pain measured with the VDS, VAS, and FPS, while a previous study reported that among cancer patients, 45.6% had severe pain, 24 and others reported that 26.2% had severe pain.25 Previous researchers examined cancer pain targeting metastatic cancer patients, thus they may have reported a higher proportion of severe pain than the present study.24 Others assessed cancer pain targeting all cancer patients admitted to the hospital, thus they might have more severe pain compared to cancer patients dwelling in the community in our study.25
Accurate pain assessment is a prerequisite for quality pain control, thus by using multiple tools, practitioners could gain confidence in clinical decision-making, while standardized cutoff points could enable researchers to compare across different groups.26 The current study found that there were wide variations in VAS pain scores within the same pain category of VDS: mild pain = 0–90, moderate pain = 0–95, and severe pain = 0–100, which is similar to a previous study.1 Jones et al. argued that individual interpretations of faces, numbers, and verbal descriptors differ significantly thus extensive variability existed.1
Former researchers suggested 36–60 as optimal cutoff points for mild, moderate, and severe pain for VAS using the same method as in the current study.3 To set up cutoff points, Serlin et al. included the degree of interferences in multiple aspects of life (enjoyment of life, activity, walking, mood, sleep, work, and relations with others) as dependent variables in the MANOVA.4 Other researchers used the VDS as criteria to determine cutoff points for the VAS, and contended that a VAS score in excess of 30 mm could be recorded at least as moderate; over that point 85% of the patients reported moderate pain measured with the VDS.27 If we used a different method to determine cutoff points for the VAS, different cutoff points would be produced, and in turn, would have yielded different results in agreement rates between the pain scales. Similarly, if we used different dependent variables other than the FACT-G in the MANOVA to determine cutoff points, we might have produced different cutoff points. Thus, researchers and practitioners need to be aware that cutoff points for the VAS would be varied by the method used to determine cutoffs, as well as by the health condition and population under study. Although the VAS is regarded as one of the best methods of evaluating pain intensity with high sensitivity,28 it has practical issues including wide variations in VAS scores within the same pain intensity category when examined with the VDS, difficulty in set up cutoff points, and the varying cutoff points provided by different researchers.7,27,29
The VDS and categorized VAS, however, were combined to produce a new pain category in our study that was used as the gold standard to test the diagnostic accuracy of the multidimensional scales (MPQ-SF and BPI-SF). Also, those who consistently responded to the same pain categories across the two scales were included in the ROC curve. The current study found that the MPQ-SF and the BPI-SF showed high accuracy (AUC at least 0.90) in the diagnosis of severe pain, indicating that the MPQ-SF and the BPI-SF could be used as valid pain screening tools with precision. This study also suggested optimal cutoff points for these two multidimensional pain scales. Vallerand suggested including one or more multidimensional instruments that assess pain, when two or more dimensions of the pain experience are to be measured.30 The MPQ provides a precise and comprehensive measure of pain characters, while the BPI provides a holistic picture of how pain affects patients’ lives.30 Thus, these multidimensional instruments could be used selectively according to the purpose of pain assessment.
In the present study, the cutoff point was > 8 for the MPQ-SF (sensitivity = 0.93, and specificity = 0.82) and > 14 for the BPI-SF (sensitivity = 0.90, and specificity = 0.80) that distinguish between moderate and severe pain. Based on the ROC curve coordinates of MPQ-SF, if we want to increase specificity by 0.90, sensitivity would be decreased to 0.81 with a cutoff point > 13. Thus, practitioners should consider weighing whether sensitivity or specificity holds more value in determining cancer pain categories. Researchers have contended that likelihood ratios are more useful than sensitivity and specificity for instrument accuracy.6 The positive likelihood ratio of 5.03 for the MPQ-SF indicates that those who have severe pain are 5.03 times more likely to have positive test results compared to those without severe pain, and the present study revealed that according to positive likelihood ratio, the MPQ-SF is more accurate compared with the BPI-SF (LR+ = 4.41).
Limitations
A limitation of the present study is that the study only included those who speak English. If we evaluated pain using other languages, different verbal descriptors and interpretations would yield different pain intensity scores and cutoff points. Another limitation may be that 37.3% of the patients used pain medication and we could not control the effects of pain medication on self-reported pain. Similarly, we did not consider cancer site, cancer stage, duration of cancer diagnosis, and previous medical treatments in the measure of cancer pain, all of which may influence pain intensity, thus may limit the study results.
Implications for Practice
The current study evaluated consistency and diagnostic accuracy of multiple pain screening tools, and found substantial agreement between the VDS and VAS, the VDS and FPS, and the VAS and FPS. The study also revealed high accuracy of the MPQ-SF and the BPI-SF in the diagnosis of severe cancer pain. Due to subjective nature of pain,1 issues remain in properly quantifying pain intensity using self-reported measures.31 Previous researchers suggested that because cancer pain has a multidimensional nature, cancer pain could thus be best evaluated by combining multidimensional screening tools with pain intensity rating scales such as the VAS.31 The use of one or more pain screening tools whose diagnostic accuracy and consistency have been validated will help classify pain effectively and subsequently promote optimal pain control in multi-ethnic groups of cancer patients.4 Further research is needed to examine consistency and diagnostic accuracy of unidimensional and multidimensional scales in assessing pain targeting other than that in cancer patients, such as arthritis pain and neuropathic pain.
Acknowledgments
Disclosures and Acknowledgements
The data for this secondary analysis came from a larger study funded by the National Institute of Health (1RO1NR007900-01A1).
This work was supported by INHA UNIVERSITY Research Grant.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Jones KR, Vojir CP, Hutt E, Fink R. Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. J Rehabil Res Dev. 2007;44(2):305–314. doi: 10.1682/jrrd.2006.05.0051. [DOI] [PubMed] [Google Scholar]
- 2.Gauffin J, Hankama T, Kautiainen H, Hannonen P, Haanpaa M. Neuropathic pain and use of PainDETECT in patients with fibromyalgia: a cohort study. BMC neurol. 2013;13:21. doi: 10.1186/1471-2377-13-21. PubMed PMID: 23409793. Pubmed Central PMCID: 3582578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirschfeld G, Zernikow B. Cut points for mild, moderate, and severe pain on the VAS for children and adolescents: What can be learned from 10 million ANOVAs? Pain. 2013 doi: 10.1016/j.pain.2013.05.048. PubMed PMID: 23742796. [DOI] [PubMed] [Google Scholar]
- 4.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 5.Ramer L, Richardson JL, Cohen MZ, Bedney C, Danley KL, Judge EA. Multimeasure pain assessment in an ethnically diverse group of patients with cancer. J Transcult Nurs. 1999;10(2):94–101. doi: 10.1177/104365969901000202. [DOI] [PubMed] [Google Scholar]
- 6.Alsaadi SM, McAuley JH, Hush JM, et al. Detecting insomnia in patients with low back pain: Accuracy of four self-report sleep measures. BMC Musculoskelet Disord. 2013;14:196. doi: 10.1186/1471-2474-14-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Im EO, Chee W, Guevara E, et al. Gender and ethnic differences in cancer pain experience: A multiethnic survey in the United States. Nurs Res. 2007;56(5):296–306. doi: 10.1097/01.NNR.0000289502.45284.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiol. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 9.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17. [PubMed] [Google Scholar]
- 10.Ware LJ, Epps CD, Herr K, Packard A. Evaluation of the Revised Faces Pain Scale, Verbal Descriptor Scale, Numeric Rating Scale, and Iowa Pain Thermometer in Older Minority Adults. Pain Manag Nurs. 2006;7(3):117–125. doi: 10.1016/j.pmn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Mullin V, Cella D, Chang CH, et al. Development of three African language translations of the FACT-G. Qual Life Res. 2000;9(2):139–149. doi: 10.1023/a:1008903304950. [DOI] [PubMed] [Google Scholar]
- 12.Jaywant SS, Pai AV. A comparative study on pain measurement scales in acute burn patients. Indian J Occup Ther. 2003;35(3):13–17. [Google Scholar]
- 13.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 14.Cleeland CS. Measurement and prevalence of pain in cancer. Semin Oncol Nurs. 1985;1(2):87–92. doi: 10.1016/s0749-2081(85)80041-3. [DOI] [PubMed] [Google Scholar]
- 15.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 16.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 17.Lázaro C, Bosch F, Torrubia R, Baños J-E. The development of a Spanish questionnaire for assessing pain: Preliminary data concerning reliability and validity. Eur J Psychol Assess. 1994;10(2):145–151. [Google Scholar]
- 18.Shin H, Kim K, Young Hee K, Chee W, Im EO. A comparison of two pain measures for Asian American cancer patients. West J Nurs Res. 2007;29(5):545–560. doi: 10.1177/0193945906298696. [DOI] [PubMed] [Google Scholar]
- 19.Ger LP, Ho ST, Sun WZ, Wang MS, Cleeland CS. Validation of the brief pain inventory in a Taiwanese population. J Pain Symptom Manage. 1999;18(5):316–322. doi: 10.1016/s0885-3924(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 20.Yu CLM, Fielding R, Chan CLW, et al. Measuring quality of life of Chinese cancer patients: A validation of the Chinese version of the Functional Assessment of Cancer Therapy-General (FACT-G) scale. Cancer. 2000;88(7):1715–1727. [PubMed] [Google Scholar]
- 21.Zou KH, O’Malley J, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 22.Swets JA. Measuring the accuracy of diagnostic systems. Sci. 1988;240(4857):1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biom. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 24.Paul SM, Zelman DC, Smith M, Miaskowski C. Categorizing the severity of cancer pain: Further exploration of the establishment of cutpoints. Pain. 2005;113(1–2):37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Bhuvan KC, Mohd Yusoff ZB, Alrasheedy AA, Othman S. The characteristics and the pharmacological management of cancer pain and its effect on the patients’ daily activities and their quality of life:A cross-Sectional study from Malaysia. J Clin Diagn Res. 2013;7(7):1408–1413. doi: 10.7860/JCDR/2013/5450.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palos GR, Mendoza TR, Mobley GM, Cantor SB, Cleeland CS. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J Pain. 2006;7(1):49–56. doi: 10.1016/j.jpain.2005.07.012. PubMed PMID: 16414555. [DOI] [PubMed] [Google Scholar]
- 27.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain. 1997;72(1–2):95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson AM. Assessment of chronic pain I Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 29.Emshoff R, Bertram S, Emshoff I. Clinically important difference thresholds of the visual analog scale: A conceptual model for identifying meaningful intraindividual changes for pain intensity. Pain. 2011;152(10):2277–2282. doi: 10.1016/j.pain.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Vallerand AH. Measurement issues in the comprehensive assessment of cancer pain. Semin Oncol Nurs. 1997;13(1):16–24. doi: 10.1016/s0749-2081(97)80045-9. [DOI] [PubMed] [Google Scholar]
- 31.Deschamps M, Band PR, Coldman AJ. Assessment of adult cancer pain: Shortcomings of current methods. Pain. 1988;32(2):133–139. doi: 10.1016/0304-3959(88)90061-9. [DOI] [PubMed] [Google Scholar]