Abstract
Antimicrobial photodynamic therapy (APDT) combined with endodontic treatment has been recognized as an alternative approach to complement conventional root canal disinfection methods on bacterial biofilms. We developed an in vitro model of bioluminescent Candida albicans biofilm inside curved dental root canals and investigated the microbial reduction produced when different light delivery methods are employed. Each light delivery method was evaluated in respect to the light distribution provided inside curved root canals. After conventional endodontic preparation, teeth were sterilized before canals were contaminated by a bioluminescent strain of C. albicans (CEC789). Methylene blue (90 µM) was introduced into the canals and then irradiated (λ=660 nm, P=100 mW, beam diameter=2 mm) with laser tip either in contact with pulp chamber or within the canal using an optical diffuser fiber. Light distribution was evaluated by CCD camera, and microbial reduction was monitored through bioluminescence imaging. Our findings demonstrated that the bioluminescent C. albicans biofilm model had good reproducibility and uniformity. Light distribution in dental tissue was markedly dependent on the light delivery system, and this strategy was directly related to microbial destruction. Both light delivery systems performed significant fungal inactivation. However, when irradiation was performed with optical diffuser fiber, microbial burden reduction was nearly 100 times more effective. Bioluminescence is an interesting real-time analysis to endodontic C. albicans biofilm inactivation. APDT showed to be an effective way to inactivate C. albicans biofilms. Diffuser fibers provided optimized light distribution inside curved root canals and significantly increased APDT efficiency.
Keywords: Photoinactivation, Photoactivated, Diffuser optical fiber, Molar, Fungi, Yeast, Bioluminescence
Introduction
Antimicrobial photodynamic therapy (APDT) has been proposed as an adjuvant to endodontic treatment since reports have showed further microbial decontamination when APDT is used after chemo-mechanical preparation [1–5]. This strategy involves the combination of a nontoxic photosensitizer (PS) and low-intensity light to kill a broad spectrum of microorganisms by local formation of reactive oxygen species (ROS), as singlet oxygen and hydroxyl radical among others [6–9]. APDT commonly has a varying killing effect against different microorganisms, being least effective against fungi, followed by gram-negative bacteria and most effective against gram-positive bacteria [8, 9]. Also, when organized in biofilms, microbes tend to present increased resistance to both chemotherapy and APDT mainly due to the high cellular density and the presence of extracellular matrix blocking drug diffusion and uptake [10].
The incidence of Candida albicans in human oral cavity has been reported to be over 30 % in healthy adults and up to 95 % in immunocompromised patients [11, 12]. Fungi can be detected in 7–18 % of infected root canals, with C. albicans being the most common species found in dental root canal [12]. The ability of Candida to strongly adhere to dentin, forming germ tubes, hyphae, and thick extracellular matrix, contributes to increased resistance against the immune system response, and therefore it may contribute to the pathogenesis of periradicular diseases and also to chemical agents such as azoles, amphotericin B, calcium hydroxide, sodium hypochlorite, hydrogen peroxide, and even ROS [10, 13, 14]. Particularly, C. albicans can be associated with refractory endodontic infection, and given to its resistance pattern and virulence, APDT would be an interesting strategy to eradicate this microorganism in endodontics [15–17].
Endodontic failures may occur when treatment procedures have not reached a pattern for control and elimination of infection [15]. Even when good technique and careful procedures are executed, failures may still occur due to difficult access to root canal regions that are not well cleaned through the existing techniques, and thus, infection can persist [15, 16]. Anatomic particularities such as curved canals, dentin tubules permeability, and apical ramifications can lead to limited diffusion of chemical agents inside the root canal [17, 18].
When light therapy is employed, the light distribution may be directly affected by the anatomical and morphological features of each tooth, and improved light delivery systems may be required [19, 20]. Recently, our group has demonstrated how diffuser optical fibers can potentiate the photodynamic inactivation of Enterococcus faecalis biofilms in single and straight root canals [21]. Once such system provided better intracanal light diffusion leading to greater ROS formation and microbial inactivation, we hypothesized whether APDT could be a sufficiently powerful technique for a higher challenging situation, i.e., to inactivate C. albicans biofilms in curved root canals. For this purpose, in the present investigation, we developed an endodontic bioluminescent C. albicans biofilm model infecting curved root canals and evaluated in real-time the effectiveness of endodontic APDT mediated by intracanal methylene blue (MB, 90 µM, 2-min pre-irradiation time) and red light comparing two light delivery systems to elucidate the role of light distribution on APDT efficacy.
Material and methods
Teeth samples preparation
Ten freshly extracted human third molar teeth with clinically intact crowns, identified as having normal anatomy, three root canals, free of calcification, and curvatures between 15 and 25 % were selected. Each molar was radiographed to evaluate canal morphology. Angles of canals curvature were determined using an image analysis program (ImageJ NIH, Bethesda, MD USA) based on established criteria [18, 19].
Canals were enlarged to an apical size of #40 using the rotary system Mtwo (VDW GmbH—Munich, Germany) and irrigated with 10 mL of 2.5 % sodium hypochlorite solution between each endodontic file. The apical foramen was subsequently closed with composite (Filtek Z250, 3 M, Brazil) and the external surfaces coated with nail polish to avoid contamination. All root canals were irrigated once with 17 % EDTA for 2 min followed by irrigation with phosphate-buffered saline (PBS) solution to remove the smear layer and thereafter sterilized by autoclaving prior to inoculation [1, 2, 18, 21–23].
Biofilm culture
Bioluminescent C. albicans (CEC 789) were grown overnight in yeast peptone dextrose (YPD) broth at 37 °C (80 rpm) to form an exponential growth phase suspension of 107 CFU/mL (confirmed by transmission spectroscopy at λ=540 nm, T= 15 % in a glass cuvette with 1 cm of optical path). Ten microliters of the yeast suspension was added into each root canal. To ensure proper microbial distribution and diffusion of the broth into each canal, a 28-gauge needle was inserted into the canal, and spiral movements from apical to cervical portion of the root were manually performed [1, 2, 21, 22]. In order of avoiding any cross-contamination, the samples were isolated in sterile falcon tubes that were subsequently sealed. The tubes were kept upright in an orbital shaker incubator set at 115 rpm for 72 h at 37 °C to encourage biofilm adhesion. The YPD broth was changed every 24 h [10, 12, 22, 23]. The same teeth samples were reused for three separated APDT assays (i.e., once for each experimental group: control, laser tip, and diffuser fiber). We decided to use the same samples in order to guarantee a better comparison between groups as the root canals’ anatomical features were identical, and consequently, the differences obtained on light propagation and distribution must be mostly linked to the employed light delivery system. Before each test, all teeth were internally cleaned with a rod-shaped brush, abundantly irrigated with NaClO (10 mL, 2.5 %) and PBS (10 mL), recoated with nail polish (consecutive autoclaving could deteriorate nail polish coating), and sterilized by autoclaving. After that, the teeth were recontaminated using the same method described above [1, 23].
Bioluminescence evaluation
The bioluminescence light emission is based on biochemical reactions that occur in metabolically active cells. The enzymes involved in this process—named luciferases—are oxygenases that utilize molecular oxygen to oxidize a substrate (luciferin), with the formation of an electronically excited molecule that emits the light when it decays to a less energetic state [23–26]. Thus, environmental influences from MB dark toxicity or luciferin, O2, and ATP consumption could directly disturb the levels of bioluminescence signal.
To obtain the bioluminescence signal stability over the total experimental time (20 min), each root canal of the five molar teeth had the remaining culture media carefully removed and filled with a mixed solution of luciferin (coelenterazine, 0.5 mg/mL, Gold Biotechnology, Inc., St. Louis, MO) and MB (90 µM, Sigma-Aldrich, WI, USA) dissolved in distilled sterile water. The total volume of 60 µL per tooth was sufficient to fill the entire internal cavity, so all cultured cells could be in contact with the MB-luciferin solution. The capture of each bioluminescence image from the samples was performed by a proper camera set (Argus, Hamamatsu Photonics KK, Bridgewater, NJ) enclosed in a light-tight chamber [23, 25, 26]. After inoculation, samples were kept inside the chamber with no light exposure or any further intervention. After the first 2 min of incubation in the dark, four images were taken every 6 min (i.e., image capture at t=2, 8, 14, and 20 min postinoculation) to cover the same experimental period used for the APDT protocol. We excluded the possibility of bioluminescence signal being influenced by the action of light alone since red light does not significantly affect C. albicans viability at low fluence rates [27].
The photon count quantification was performed using Argus software (Hamamatsu Photonics KK, Bridgewater, NJ). Each sample had the signal intensity normalized in relation to its initial measured value. Mean signal intensity was standardized by the mean of normalized photon count values obtained from each experimental group [1, 23, 26].
To confirm that the cell viability was proportional to light emission, 100 µL of C. albicans suspensions were serially diluted (from 106 to 103 cells/well) and distributed in 96-well microplates containing MB-luciferin solution, and we analyzed these samples by the same abovementioned luminescense test [24, 26].
APDT assays
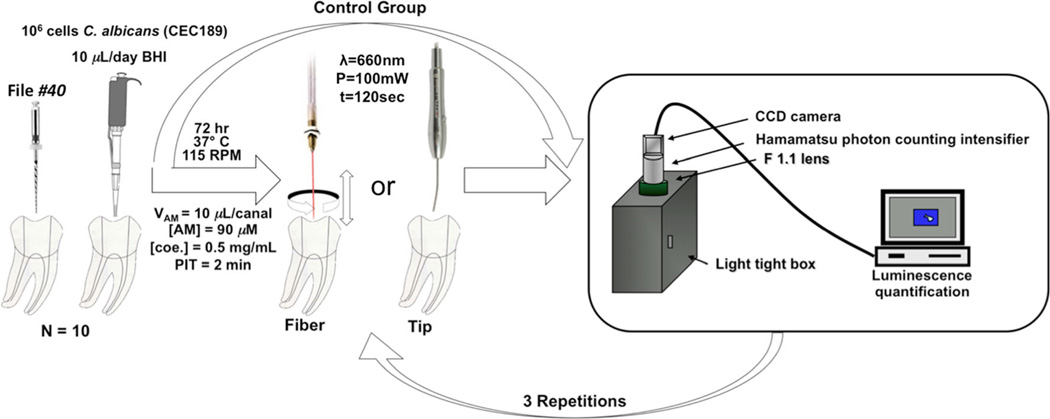
Illumination was provided by a commercial portable diode laser device (continuous wave, λ=660 nm, P=100 mW, beam diameter=2 mm, area=0.04 cm2—Laser Duo MM Optics, São Carlos, Brazil) under two distinct light delivery methods: positioning the laser handpiece tip over the pulp chamber access, aligned with each canal entrance, or inserting into the root canal a diffuser optical fiber (cone-shaped, base diameter=1 mm and apex diameter=0.3 mm, MM Optics, São Carlos, Brazil) made of flexible polymethyl methacrylate and coated with fluorinated polymer, capable of providing homogeneous radial light dispersion for 1.5 cm of its distal extremity. The optical fiber coupling presents an average loss of 7 % based on experiments performed using an integrating sphere (data not shown). The diffuser fiber was placed vertically in the apical portion of the root canal at the working length (1 mm from the apex), and spiral movements, from apical to cervical portion of the root were manually performed for approximately ten times per minute [1, 2, 22].
The irradiation procedure was divided into three steps alternated by bioluminescence imaging to allow the realtime microbial reduction follow-up. The initial image (t=0) was acquired after 2 min of MB-luciferin incubation in dark (pre-irradiation time) to allow MB uptake and luciferin diffusion. The MB-luciferin solution was not removed or refilled during the experiment. Each irradiation was always performed immediately after bioluminescence image acquisition. In each step, all three root canals were independently irradiated during 2 min (resulting in 6 min per tooth during each step). Samples were then promptly placed into the light-tight camera chamber, and bioluminescence imaging was performed to obtain the microbial reduction over time [1, 23–26]. Irradiation was performed during a total of 18 min/tooth (total of 6 min for each canal), resulting in a delivered energy of 108 J/tooth or 36 J/canal. A schematic figure of this procedure is presented in Fig. 1.
Fig. 1.
Schematic illustration of irradiation and bioluminescence quantification procedures
We also tested whether APDT could inactivate luciferin thereby reducing luminescence signal, regardless of cell viability. Thus, we irradiated the solution (MB-luciferin) prior to its incubation with cells, and no significant loss of intensity was observed in comparison to nonirradiated solution (data not shown).
Light distribution evaluation
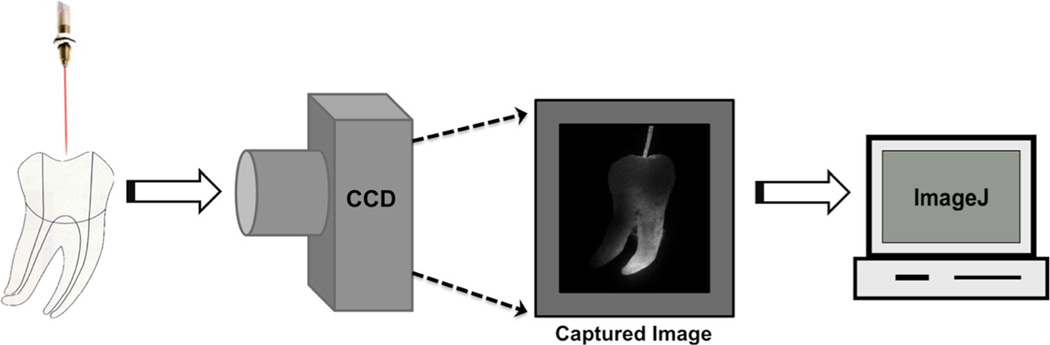
To analyze the light distribution inside the curved root canals provided by each light delivery method, standardized digital photography was evaluated by ImageJ software (National Institutes of Health, USA). A digital camera (Canon G10, 14.7 Mpx, ISO 80, magnification×0, exposure time 1/250 s, 600 DPI, F 7.1) was placed 5 cm orthogonal to the light beam path, and the intensity distribution of scattered light was photo graphed. The images were recorded as a bitmap with 32-bit resolution yielding a 256 Gy level image. A standard ImageJ plug-in (lookup tables) transforms each pixel in gray scale to fire scale false colors correspondent to the local light intensity. Values are given between the minimum of 0 for device threshold and 256 for light intensity saturation. The camera captured the side-scattered light, which is directly proportional to the local light intensity against the canal wall, so the images can correspond to a two-dimensional light intensity distribution model [22]. A schematic figure of this procedure is presented in Fig. 2.
Fig. 2.
Illustrative procedure employed for light distribution evaluation in root canals
Statistical analysis
Values are given as normalized means and standard deviation of the mean. Statistical comparisons intragroup and between groups were performed with t test using Origin software version 8.5 (OriginLab, Northampton, MA), after submitting data to Levenne and Shapiro-Wilk tests to confirm homogeneity and normality. Results were considered significant if p<0.05.
Results
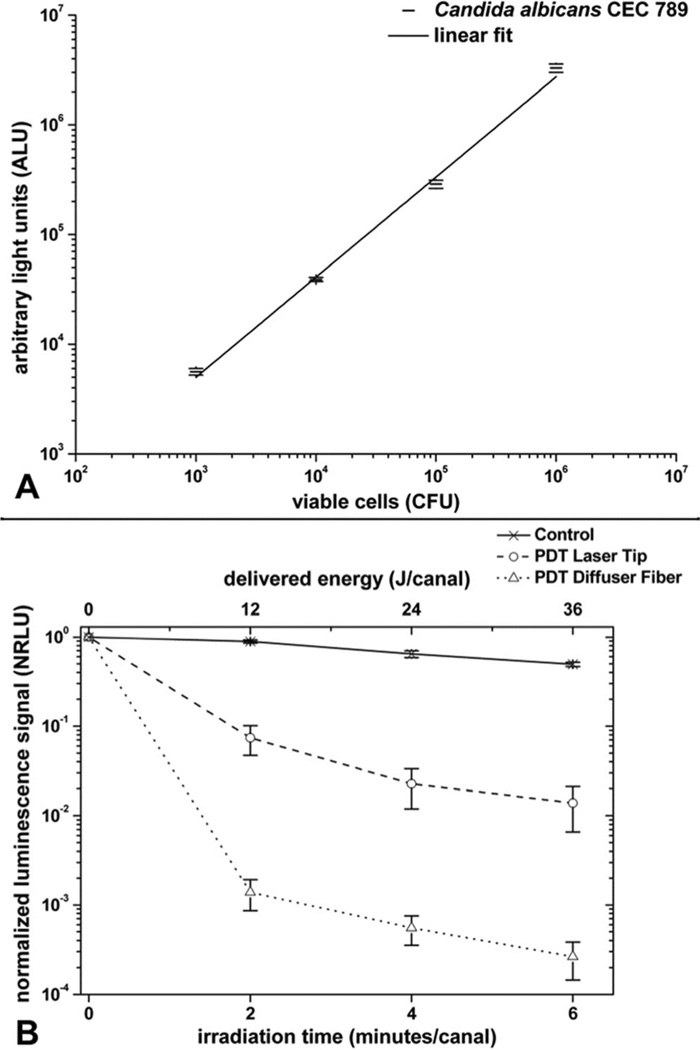
The fungal suspensions that were added into the root canal after 3 days of incubation reliably and reproducibly produced biofilms whose bioluminescence could be imaged through the width of the tooth material. The relationship between cell number measured by CFU and the light emission from the fungi incubated with 90 µM MB for 2 min was validated in a di-log correlation (Fig. 3a). The presence of a microbial biofilm in root canals rather than planktonic fungi was demonstrated by the failure of irrigation with saline to significantly diminish the luminescence signal (data not shown).
Fig. 3.
a Correlation between bioluminescence emission and colony-forming units (CFU) counts. b Luminescence signal in normalized relative light units (NRLU) versus irradiation time and delivered energy onto C. albicans biofilms adhered in root canals during endodontic PDT. Values are given as normalized means and standard deviation of the mean
To make quantified data explicit and clear for understanding we have composed figure 3b with mean normalized bioluminescence values and their respective standard errors. Also, representative bioluminescence images from all groups are displayed in Fig. 4. The bioluminescence reduction for the laser tip and diffuser fiber was always significantly greater than the control group. There was a substantial decrease in microbial load after the laser tip irradiation; nevertheless, when the irradiation was performed with the diffuser fiber, the reduction was significantly higher. The laser tip gave a mean reduction of 1 log10 after 2 min of APDT and almost 2 log10 after the total treatment, while the diffuser fiber luminescence reduction achieved was about 3 log10 after 2 min and over 3.5 log10 after the final irradiation. After irradiating with the laser tip for 6 min/canal, the microbial reduction was not as effective as 2 min of irradiation with the diffuser fiber, being insufficient to reach a satisfactory microbicidal action (considered as >3 log10 reduction). All experimental groups presented significant statistical differences (p<0.05) when compared to each other at the same irradiation time point, with exception to the initial point (irradiation time=0 min). However, other than in relation to the initial bioluminescence reading, no statistically significant difference was observed in respect to intragroup analysis. It means that irradiating each canal for more than 2 min failed to improve significantly the decontamination achieved in our experiment, and possibly, even shorter exposure times, not evaluated in this study, could present the same fungicidal effectiveness.
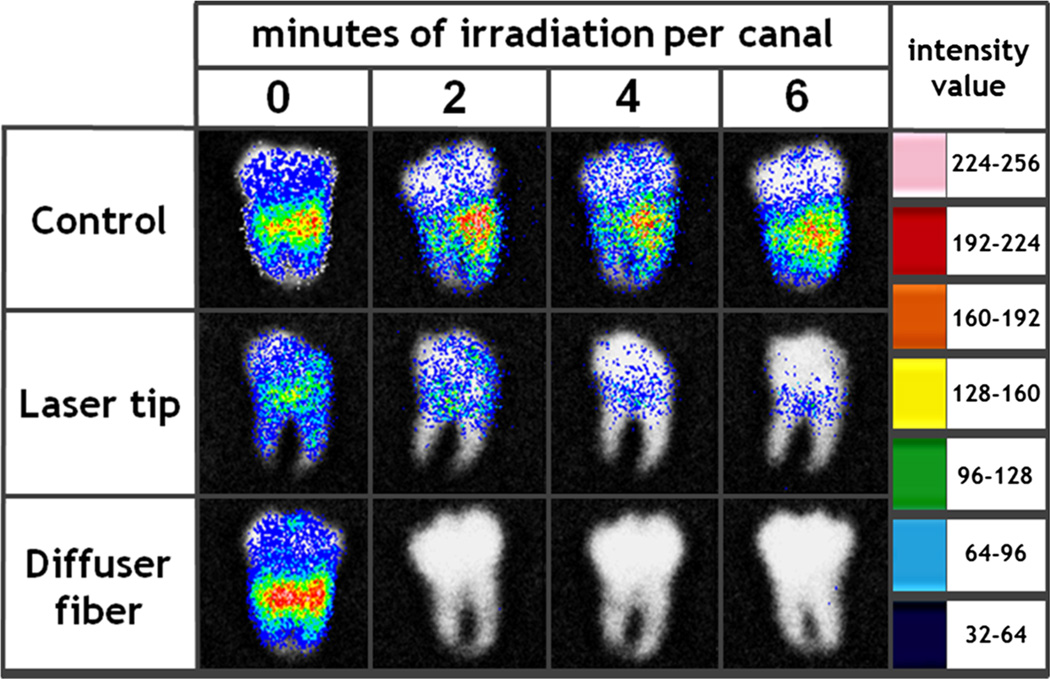
Fig. 4.
Representative bioluminescence images from control, laser tip, and diffuser fiber groups. It can be observed that after 2 min of irradiation per canal employing the diffuser fiber, only weak or null bioluminescence signals are detected, while the laser tip group is still considerably contaminated even after having been exposed to three times higher energies. Argus software transformed the grayscale photon count 8-bit image (0 for detection threshold and 256 for saturation) into false colors according to the light intensity between minimum blue and maximum pink values
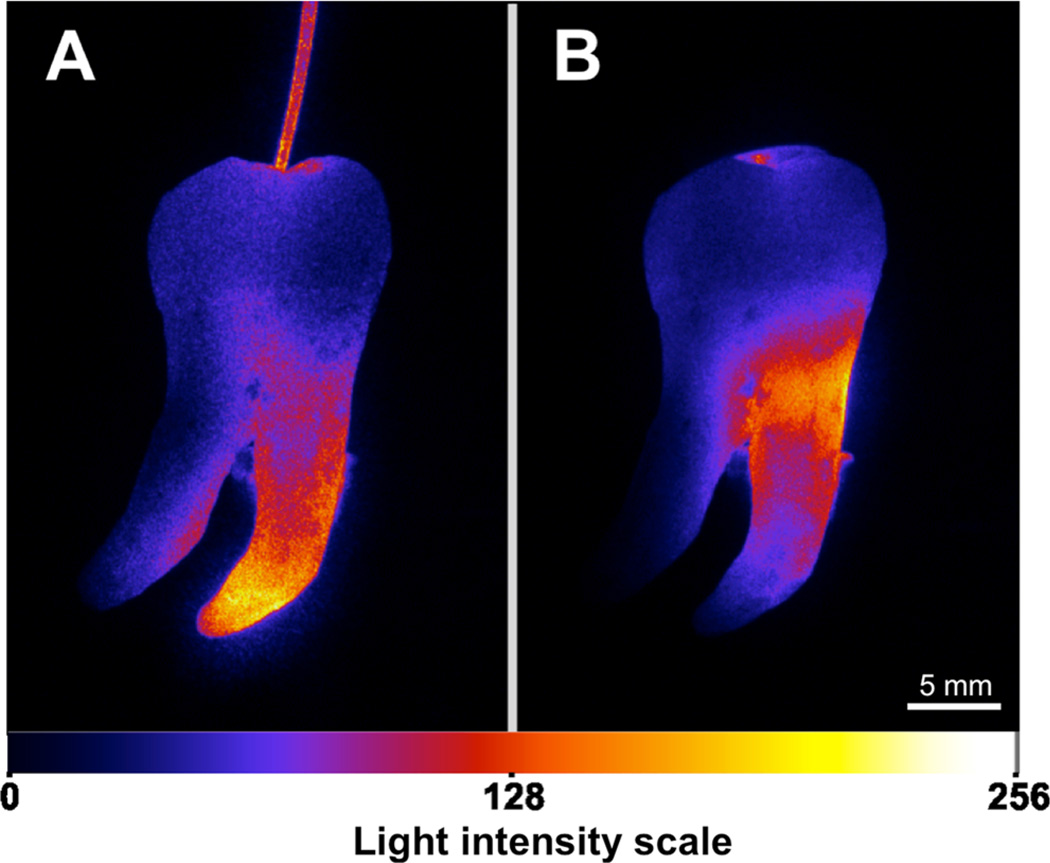
Figure 5 displays the light intensity distribution along a curved root canal when the irradiation was performed with or without the diffuser fiber. The yellowish color representing the maximum intensity clearly confirms that the light intensity along the canal and especially next to the apex is higher and more homogeneous when the diffuser fiber is used compared to the laser tip irradiation method.
Fig. 5.
Representative images of light intensity distribution along curved root canals for each irradiation method. a Light distribution with diffuser fiber and b positioning the laser tip over the pulp chamber. ImageJ software transformed the grayscale 8-bit image (0 for detection threshold and 256 for saturation) into false colors according to the light intensity between minimum blue and maximum yellow values. Note the light intensity reaching the apex region in a
Discussion
In this study, a C. albicans bioluminescent biofilm was successfully developed inside curved root canals allowing a realtime monitoring of APDT effect over the biofilm. Even though microbial colonization can be heterogeneous, there were minor variations from tooth to tooth [especially for nonirradiated samples (control group)] in the pattern of the normalized luminescence detected, and we obtained a well-behaved curve describing the bioluminescence reduction kinetics. The few divergences observed can most likely be attributed to variations in geometry and anatomy of the individual root canals that may lead to variable fungal burden in some areas and different light propagation pattern.
Regarding the microbial inactivation, an energy-dependent reduction in bioluminescence was observed until the delivered energy of 36 J/canal (6 min/canal) was reached. This irradiation time is based on parameters proposed by Garcez et al. where straight root canals were irradiated for 1, 2, 3, and 4 min to determine the ideal irradiation time [1]. Our current results showed that more than 12 J/canal of irradiation ceased to have a significant measurable effect, and 50 % of the diffuser fiber group samples presented null signal indicating luminescence emission below equipment detection threshold or possible total microbial eradication. Additionally, the heterogeneous distribution of microbial colonization mimics well what would be found in a clinical situation. Molar teeth have a heterogeneous anatomy, and root canals can be populated by different pathogens in several different and isolated sites. In this aspect, our results suggest that endodontic APDT can be an efficient strategy to inactivate high burdens of biofilms, when applied with the aid of a diffuser fiber.
MB combined with red light has been employed as a promising and safe alternative in several clinical dental procedures [4, 28, 29]. One simple and efficient way to increase the photodynamic reaction rate and, consequently, the microbial inactivation potential is to uniformly improve the light intensity reaching photosensitizer molecules nearby or in direct contact with cellular structures. Diffuser fibers have been shown to be a good approach for this strategy leading to enhancement of antimicrobial effects probably by increased ROS production at distinct sites [22].
Periapical lesions and biofilms located at the root canal system and surrounding bone tissue may be one of the main reasons for endodontic persistent infections [15]. Fungal biofilms are in general more resistant to oxidative stress than planktonic fungal cells due to limited drug (photosensitizer) diffusion and further cellular resistance mechanisms [10]. Sufficient illumination of the root canal system can be difficult to achieve. Therefore, the use of diffuser optical fibers can be recommended for this procedure as they provide better light distribution in the entire root canal system leading to sufficient reduction of microbial load at shorter irradiation protocols [22]. Our study showed successful C. albicans biofilm inactivation in challenging situations for chemical–mechanical preparation and light propagation, such as curved root canals [15, 19].
Polymeric diffuser fibers can be affordable and do not represent a significant increase in the therapy cost. Their cone shape presents dimensions that are consistent with root canal mean length and diameter, and they are also flexible enough to allow the passage through the curved canal geometry. Concerns about light distribution are not new. Fimple et al. have recommend to notch the fiber to produce better light diffusion along the root canal by local light scattering [20]. The authors stated that light could be uniformly distributed over 360° improving the APDT effect on microbial reduction. These features allow precise and easy irradiation and may be considered an important element for endodontic APDT. According to the light scattering analysis interpreted from Fig. 5 in comparison to data presented by Garcez et al. in a similar experiment about straight single root canals, we observed that light distribution in the root/conduits is fairly homogeneous regardless of root anatomy and dentinal tubules slope and angulation [22].
In order of comparing laser tip at the entrance of the root canal and the optical diffusor inside the root canal, the light interaction with dental tissues is of crucial importance. According to the work of Odor et al., red light penetrates fairly well in human teeth; therefore, if a good penetration of light is present, there would be no need of any device to irradiate inside the root canal [30]. Corroborating with that hypothesis, the work of Nunes et al. showed the same results with or without fiber optics for endodontic disinfection [31]. On the contrary, previous work of our group showed the need for a diffuser fiber [22]. So, the matter is still under investigation, and our present results added some new information over this topic using a more complex model of the curved molars’ root canals. Other devices as a radial tip may present the same and/ or even better results than the diffuser; therefore, new studies should be performed to elect the best endodontic irradiation model for APDT [32–34].
In fact, the red light distribution into teeth is a complex phenomenon. According to Fernandez-Oliveras and co-workers, enamel and dentin present a different behavior upon irradiation with red light (632.8 nm) [35]. Also, the samples’ thickness directly interferes with light distribution since the thicker they are the more side-scattered light will be. To reach the dental apex with the laser tip positioned at the pulp chamber entrance, a forward-directed scattering would be required. However, human dentin presents very strong side light-scattering properties since it consists of a complex inhomogeneous structure of collagen and hydroxyapatite allied to microtubules crossing a large portion of teeth in many different pathways [35].
For APDT, it is important that light reaches all the target area since our results as well as data presented in the literature shows a direct link between optical intensity and energy with microbial reduction [36]. Such parameters were proven to affect APDT-mediated eradication of periopathogenic biofilm and planktonic cells. Our results also pointed out the importance of the light being delivered in an appropriated manner since the same amount of energy did not present similar results depending on the delivery system.
The presence of sufficient concentration of triplet molecular oxygen (3O2) is one of the key aspects of APDT success. ROS are formed after direct energy transfer from the PS to 3O2 (type II reaction) and/or electron transfer from the PS to 3O2 and/or biomolecules present in the microenvironment forming free radicals (type I reaction). Due to 3O2 consumption during photodynamic reactions, we hypothesize that spiral movements performed with the fiber optics inside root canal may contribute to reoxygenation of the MB solution enhancing APDT efficiency. Baier et al. reported that APDT efficiency depends critically on the oxygen concentration and local oxygen consumption due to photooxidative process may be crucial for APDT success [37]. Furthermore, Tavares et al. showed the importance of type II PDT mechanism, whereas a direct transference of energy occurs from the photosensitizer to the molecular oxygen [38]. Nunez et al. also pointed out the importance of molecular oxygen availability for antimicrobial photodynamic damage [39]. Therefore, for predictable results in endodontic APDT, the oxygen tension inside all the anatomic features from different root canal areas should be also evaluated, and the method of irradiation may contribute to balance the oxygen consumption.
In addition, the spiral movements performed with the fiber optics may aid biofilm dislocation and MB diffusion inside the root canal. The gentle movements performed with the fiber may not dislodge biofilm at first, but once APDT starts destroying the extracellular matrix, the biofilm adherence may be modified and, in this situation, the movement although mild may aid the MB dislocation to deeper dentin tubules. In fact, the work of Sharma et al. suggests that APDT disrupts biofilm structure and in such case the fiber movement may be an interesting approach to enhance APDT efficiency [40].
According to our results and to the lack of side effects added to the simplicity of the APDT, execution encourages further research since a 2- to 6-min application, employing the diffuser optical fiber, provides a significant microbial reduction without using chemical compounds that might change the tooth structural strength and promote microbial resistance [41]. To eradicate persistent microbial load from root canals, the alternatives are the following: use of chemical irrigation solution, which depending on contact/time and concentration may significantly alter the dental structure microhardeness, elastic modulus, and flexural strength or to use systemic antibiotics which may increase the threat of microbial resistance selection [6, 42–44]. Additionally, even when APDT is unable to eradicate all C. albicans or E. faecalis cells, the remaining population exhibits inhibited virulence factors, including traditional antimicrobial drugs, leading to decreased recolonization risks [45–47]. In this way, the use of APDT seems to be an attractive alternative approach both form the microbiological point of view and from the dental structure maintenance standpoint.
Despite the good results reported by APDT in our study, some variables should be evaluated, so predictable clinical results could be obtained with this technique. The biofilm present in periapical lesions is a challenge that should be evaluated. Moreover, the best technique of irradiation and instrumentation has yet to be chosen. According to our results, we can state that instruments that favor the light distribution within the total length of the canal presented better antimicrobial effects; diffusors are one of the options, but radial tips and other devices that present the same or optimize characteristics should also be tested. Also, the oxygen concentration may play an important role on the therapy effects and methods of increase or at least maintain the oxygen levels in the entire canal area should also be investigated.
Conclusion
In summary, we developed a nondestructive bioluminescent C. albicans biofilm model inside curved root canals that can be used to evaluate microbial inactivation through the entire root canal extension. According to our model, the use of a diffuser fiber provides better light distribution inside curved root canals, and this is important for APDT-mediated disinfection. We conclude that endodontic APDT using diffuser fibers can be an effective way to inactivate biofilm embedded microorganisms inside curved canals using a technique that requires a relatively short time period.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant 2010/13313-9) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). MR Hamblin was supported by the US National Institutes of Health (NIH R01AI050875). The authors gratefully recognize J. Chibebe Junior (Department of Biosciences and Oral Diagnosis, Univ Estadual Paulista/UNESP, São José dos Campos, SP 12245–000, Brazil) for the experimental assistance and Christophe d’Enfert (Unité Biologie et Pathogénicité Fongiques, F-75015 Paris, France) for providing the luciferase-expressing strain of C. albicans.
Contributor Information
C. P. Sabino, Center for Lasers and Applications, IPEN-CNEN/SP, São Paulo, SP, Brazil Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA.
A. S. Garcez, São Leopoldo Mandic Dental Research Center, Campinas, SP, Brazil
S. C. Núñez, Center for Lasers and Applications, IPEN-CNEN/SP, São Paulo, SP, Brazil
M. S. Ribeiro, Center for Lasers and Applications, IPEN-CNEN/SP, São Paulo, SP, Brazil marthasr@usp.br
M. R. Hamblin, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA Department of Dermatology, Harvard Medical School, Boston, MA, USA; Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA.
References
- 1.Garcez AS, Ribeiro MS, Tegos GP, Nunez SC, Jorge AO, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcez AS, Nunez SC, Hamblin MR, Suzuki H, Ribeiro MS. Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: a preliminary report. J Endod. 2010;36:1463–1466. doi: 10.1016/j.joen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 3.George S, Kishen A. Influence of photosensitizer solvent on the mechanisms of photoactivated killing of Enterococcus faecalis . Photochem Photobiol. 2008;84:734–740. doi: 10.1111/j.1751-1097.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 4.Ng R, Singh F, Papamanou DA, Song X, Patel C, Holewa C, Patel N, Klepac-Ceraj V, Fontana CR, Kent R, Pagonis TC, Stashenko PP, Soukos NS. Endodontic photodynamic therapy ex vivo. J Endod. 2011;37:217–222. doi: 10.1016/j.joen.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S, Foschi F, Doucette S, Bammann LL, Fontana CR, Doukas AG, Stashenko PP. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Lim EJ, Oak CH, Heo J, Kim YH. Methylene blue-mediated photodynamic therapy enhances apoptosis in lung cancer cells. Oncol Rep. 2013;30(2):856–862. doi: 10.3892/or.2013.2494. [DOI] [PubMed] [Google Scholar]
- 7.Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74(5):656–669. doi: 10.1562/0031-8655(2001)074<0656:thopap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem PhotobiolSci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Denis TG, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, Tegos GP. All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2:509–520. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nature Prot. 2008;3:1909–1924. doi: 10.1038/nprot.2008.192. [DOI] [PubMed] [Google Scholar]
- 11.Arendorf TM, Walker DM. The prevalence and intra-oral distribution of Candida albicans in man. Arch Oral Biol. 1980;25:1–10. doi: 10.1016/0003-9969(80)90147-8. [DOI] [PubMed] [Google Scholar]
- 12.Siqueira JF, Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2004;97:632–641. doi: 10.1016/S1079210404000046. [DOI] [PubMed] [Google Scholar]
- 13.Vaghela DJ, Kandaswamy D, Venkateshbabu N, Jamini N, Ganesh A. Disinfection of dentinal tubules with two different formulations of calcium hydroxide as compared to 2 % chlorhexidine: as intracanal medicaments against Enterococcus faecalis and Candida albicans: an in vitro study. J Conserv Dent. 2011;14:182–186. doi: 10.4103/0972-0707.82625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calzavara-Pinton P, Rossi MT, Sala R, Venturini M. Photodynamic Antifungal Chemotherapy. Photochem Photobio. 2012;88:512–522. doi: 10.1111/j.1751-1097.2012.01107.x. [DOI] [PubMed] [Google Scholar]
- 15.Sunde PT, Olsen I, Debelian GJ, Tronstad L. Microbiota Periapical Lesions Refract Endod Ther. 2002;28(4):304–310. doi: 10.1097/00004770-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Kovac J, Kovac D, Slobodnikova L, Kotulova D. Enterococcus faecalis and Candida albicans in the dental root canal and periapical infections. Bratisl LekListy. 2013;114(12):716–720. [PubMed] [Google Scholar]
- 17.Narayanan LL, Vaishnavi C. Endodontic microbiology. J Conserv Dent. 2010;13(4):233–239. doi: 10.4103/0972-0707.73386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32:271–275. doi: 10.1016/0030-4220(71)90230-1. [DOI] [PubMed] [Google Scholar]
- 19.Wu MK, Wesselink PR. Efficacy of three techniques in cleaning the apical portion of curved root canals. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 1995;79:492–496. doi: 10.1016/s1079-2104(05)80134-9. [DOI] [PubMed] [Google Scholar]
- 20.Fimple JL, Fontana CR, Foschi F, Ruggiero K, Song X, Pagonis TC, Tanner AC, Kent R, Doukas AG, Stashenko PP, Soukos NS. Photodynamic treatment of endodontic polymicrobial infection in vitro. J Endod. 2008;34:728–734. doi: 10.1016/j.joen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcez AS, Nunez SC, Lage-Marques JL, Jorge AO, Ribeiro MS. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2006;102:93–98. doi: 10.1016/j.tripleo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Garcez AS, Fregnani ER, Rodriguez HM, Nunez SC, Sabino CP, Suzuki H, Ribeiro MS. The use of optical fiber in endodontic photodynamic therapy. Is it really relevant? Lasers Med Sci. 2013;28:79–85. doi: 10.1007/s10103-012-1073-8. [DOI] [PubMed] [Google Scholar]
- 23.Garcez AS, Nunez SC, Lage-Marques JL, Hamblin MR, Ribeiro MS. Photonic real-time monitoring of bacterial reduction in root canals by genetically engineered bacteria after chemomechanical endodontic therapy. Braz Dent J. 2007;18:202–207. doi: 10.1590/s0103-64402007000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, Vecchiarelli A, Brown AJ, d'Enfert C. A multifunctional, synthetic Gaussiaprinceps luciferase reporter for live imaging of Candida albicans infections. IfecImmun. 2010;77(11):4847–4858. doi: 10.1128/IAI.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedgley C, Applegate B, Nagel A, Hall D. Real-time imaging and quantification of bioluminescent bacteria in root canals in vitro. J Endod. 2004;30:893–898. doi: 10.1097/01.don.0000132299.02265.6c. [DOI] [PubMed] [Google Scholar]
- 26.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J PhotochemPhotobiol B. 2005;81(1):15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Souza SC, Junqueira JC, Balducci I, Koga-Ito CY, Munin E, Jorge AO. Photosensitization of different Candida species by low power laser light. J Photochem Photobiol B. 2006;83(1):34–38. doi: 10.1016/j.jphotobiol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Campos GN, Pimentel SP, Ribeiro FV, Casarin RC, Cirano FR, Saraceni CH, Casati MZ. The adjunctive effect of photodynamic therapy for residual pockets in single-rooted teeth: a randomized controlled clinical trial. Lasers Med Sci. 2013;28:317–324. doi: 10.1007/s10103-012-1159-3. [DOI] [PubMed] [Google Scholar]
- 29.Scwingel AR, Barcessat AR, Núñez SC, Ribeiro MS. Antimicrobial photodynamic therapy in the treatment of oral candidiasis in HIV-infected patients. Photomed Laser Surg. 2012;30:429–432. doi: 10.1089/pho.2012.3225. [DOI] [PubMed] [Google Scholar]
- 30.Odor TM, Chandler NP, Watson TF, Ford TR, McDonald F. Laser light transmission in teeth: a study of the patterns in different species. Int Endod J. 1999;32(4):296–302. doi: 10.1046/j.1365-2591.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 31.Nunes MR, Mello I, Franco GC, de Medeiros JM, Dos Santos SS, Habitante SM, Lage-Marques JL, Raldi DP. Effectiveness of photodynamic therapy against Enterococcus faecalis, with and without the use of an intracanal optical fiber: an in vitro study. Photomed Laser Surg. 2011;29(12):803–808. doi: 10.1089/pho.2011.2995. [DOI] [PubMed] [Google Scholar]
- 32.George R, Walsh LJ. Performance assessment of novel side firing flexible optical fibers for dental applications. Lasers Surg Med. 2009;41(3):214–221. doi: 10.1002/lsm.20747. [DOI] [PubMed] [Google Scholar]
- 33.George R, Walsh LJ. Performance assessment of novel side firing flexible optical fibers for endodontic applications. J Biomed Opt. 2011;16(4):048004. doi: 10.1117/1.3563637. [DOI] [PubMed] [Google Scholar]
- 34.Vesselov LM, Whittington W, Lilge L. Performance evaluation of cylindrical fiber optic light diffusers for biomedical applications. Lasers Surg Med. 2004;34(4):348–351. doi: 10.1002/lsm.20031. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Oliveras A, Rubino M, Perez MM. Scattering anisotropy measurements in dental tissues and biomaterials. Europ Opt Soc Rap Public. 2012;7:12016. [Google Scholar]
- 36.Street CN, Pedigo LA, Loebel NG. Energy dose parameters affect antimicrobial photodynamic therapy-mediated eradication of periopathogenic biofilm and planktonic cultures. Photomed Laser SurgSuppl. 2010;1:S61–S66. doi: 10.1089/pho.2009.2622. [DOI] [PubMed] [Google Scholar]
- 37.Baier J, Maisch T, Regensburger J, Loibl M, Vasold R, Bäumler W. Time dependence of singlet oxygen luminescence provides an indication of oxygen concentration during oxygen consumption. J Biomed Opt. 2007;12(6):064008. doi: 10.1117/1.2821153. [DOI] [PubMed] [Google Scholar]
- 38.Tavares A, Dias SR, Carvalho CM, Faustino MA, Tomé JP, Neves MG, Tomé AC, Cavaleiro JA, Cunha Â, Gomes NC, Alves E, Almeida A. Mechanisms of photodynamic inactivation of a gram-negative recombinant bioluminescent bacterium by cationic porphyrins. Photochem Photobiol Sci. 2011;10(10):1659–1669. doi: 10.1039/c1pp05097d. [DOI] [PubMed] [Google Scholar]
- 39.Núñez SC, Garcez AS, Kato IT, Yoshimura TM, Gomes L, Baptista MS, Ribeiro MS. Effects of ionic strength on the antimicrobial photodynamic efficiency of methylene blue. Photochem Photobiol Sci. 2014;13(3):595–602. doi: 10.1039/c3pp50325a. [DOI] [PubMed] [Google Scholar]
- 40.Sharma M, Visai L, Bragheri F, Cristiani I, Gupta PK, Speziale P. Toluidine blue-mediated photodynamic effects on staphylo-coccal biofilms. Antimicrob Agents Chemother. 2008;52(1):299–305. doi: 10.1128/AAC.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascon FM, Kantovitz KR, Soares LE, Santo AM, Martin AA, Puppin-Rontani RM. Morphological and chemical changes in dentin after using endodontic agents: Fourier transform Raman spectroscopy, energy dispersive x-ray fluorescence spectroscopy, and scanning electron microscopy study. J Biomed Opt. 2012;17(7):075008. doi: 10.1117/1.JBO.17.7.075008. [DOI] [PubMed] [Google Scholar]
- 42.Saleh AA, Ettman WM. Effect of endodontic irrigation solutions on microhardness of root canal dentine. J Dentistry. 1999;27(1):43–46. doi: 10.1016/s0300-5712(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 43.Sim TPC, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int End J. 2000;34(2):120–132. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 44.Dai T, Huang YY, Hamblin MR. Photodynamictherapy for localized infections—state of the art. Photodiagnosis Photodyn Ther. 2009;6(3–4):170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato IT, Prates RA, Sabino CP, Fuchs BB, Tegos GP, Mylonakis E, Hamblin MR, Ribeiro MS. Antimicrobial photodynamic inactivation inhibits Candida albicans virulence factors and reduces in vivo pathogenicity. Antimicrob Agents Chemother. 2013;57:445–451. doi: 10.1128/AAC.01451-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chibebe Junior J, Sabino CP, Tan X, Junqueira JC, Wang Y, Fuchs BB, Jorge AO, Tegos GP, Hamblin MR, Mylonakis E. Selective photoinactivation of Candida albicans in the non-vertebrate host infection model Galleria mellonella. BMC Microbiol. 2013;13:217. doi: 10.1186/1471-2180-13-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chibebe Junior J, Fuchs BB, Sabino CP, Junqueira JC, Jorge AO, Ribeiro MS, Gilmore MS, Rice LB, Tegos GP, Hamblin MR, Mylonakis E. Photodynamic and antibiotic therapy impair the pathogenesis of Enterococcus faecium in a whole animal insect model. PLoS One. 2013;8(2):e55926. doi: 10.1371/journal.pone.0055926. [DOI] [PMC free article] [PubMed] [Google Scholar]